Published online Jun 15, 1999. doi: 10.3748/wjg.v5.i3.245
Revised: April 2, 1999
Accepted: April 26, 1999
Published online: June 15, 1999
AIM: To investigate the specific pathogenesis of O-polysaccharide (O-PS) which is on the outer membrane of lipopolysaccharides (LPS) from Shigella flexneri.
METHODS: The O-PS was isolated and purified from Shigella flexneri-5 M90T by enzymatic hydrolysis and gel chromatography. Effects of O-PS were observed by in vitro experiment, (HeLa cell culture), and in vivo experiment (rabbit ileal loop assay).
RESULTS: In vitro and in vivo experiments with the purified O-PS from Shigella flexneri-revealed that the O-PS alone was toxic to Hela cells and caused mucosal inflammation and hemorrhagic exudation in ileal loop of rabbit.
DISCUSSION: O-PS might be one of the factors causing diarrhea and its mechanism was different from endotoxin reaction of LPS. The molecular mechanism of O-PS need further studies.
- Citation: Zhong QP. Pathogenic effects of Opolysaccharide from Shigella flexneri strain. World J Gastroenterol 1999; 5(3): 245-248
- URL: https://www.wjgnet.com/1007-9327/full/v5/i3/245.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i3.245
Shigella flexneri is one of the pathogens which causes diarrhea and its pathogenic mechanism is still unclear, even though extensive investigations were carried out worldwide. It has been known that the invasive outer membrane protein encoded by its plasmid DNA plays a primary role in bacterial infection. The roles of other factors, especially lipopolysaccharides (LPS), were also better understood. The specific pathogenesis of O-polysaccharide (O-PS) which is on the outer membrane of lipopolysacchrides remains unclear. To understand the pathogen esis of the O-PS of Shigella flexneri, we isolated and purified the O-PS from Shigella flexneri- 5 M90T and observed the pathogenesis of O-PS in vitro and in vivo.
Strain Wild-type Shigella flexneri 5 M90T was provided by Pasteur Institute (French).
Medium Lauria-Bertani (LB) medium.
Reagents Proteinase K was purchased from Merck, Sephadex G50 from Sigma, fetal bovine serum (FBS) from Institute of Hematology, Chinese Academy of Medical Sciences, 1640 medium from GIBCO, and nuclease from Huamei.
Preparation of O-PS (1) Cultivation of bacteria and harvest. A single colony of Shigella flexneri-5 M90T was inoculated onto LB plates, incubated at 37 °C overnight and harvested into a clean centrifuge tube. The bacteria were washed with 20 mmol/L Tris-HCl (pH 7.4) buffer, lyophilised and ground to powder in a mortar. (2) Isolation of LPS by enzymatic hydrolysis and gel chromatography[1,2]. Bacterium powder (25 mg) was suspended in 1.0 mL ddH2O, boiled for 15 min and centrifuged to remove cell debrits . The supernatant was added with DNase (50 mg/L) and RNase (100 mg/L) and incubated at 37 °C for 1 h, then added with proteinase K (1mg), incubated at 60 °C for 1 h, boiled for 5 min and centrifuged for supernatant. The supernatant was then dialyzed against ddH2O overnight and centrifuged again. The supernatant, the crude LPS, was separated by a Sephadex G50 chromatography column with elution of ddH2O. The first peak was collected, lyophilized and stored at 4 °C. (3) Preparation of O-PS by acid hydrolysis[2,3]. LPS was hydrolyzed in 1% acetic acid at 100 °C for 2 h. The solution was then centrifuged and the supernatant was separated by gel chromatography and O-PS was collected as described above.
Quantification of carbohydrates by the sulfuric acid-phenol method[4] A proper amount of O-PS was dissolved in 2 mL ddH2O, mixed well with 1 mL 5% phenol and then added with 5 mL sulfuric acid. The mixture was incubated at room temperature for 10 min and transferred to 25 °C-30 °C water bath for 10 min-20 min. The carbohydrate content was quantified by mea suring absorbance at 480 nm with 10 mg/L-100 mg/L rhamnose as standard.
Antigenicity measurement of O-PS by ELISA Rabbit antisera against-Shigella flexneri-5 M90T and enzyme-conjugated goat antirabbit IgG were added to the plate coated with 50 mg/L O-PS. The antigenicity of O-PS was determined by color reaction density after adding TMB.
Effects of O-PS on HeLa cell With 1640 medium containing 10% bovine sera, penicillin and streptomycin, HeLa cells were cultured into monolaye r and transferred into a 12 hole tissue culture plate. When cells grew onto the plate walls and formed small clumps, the medium was replaced by fresh one. O-PS was added to each hole to reach a final concentration of 100, 200, 500 and 1000 μg/L respectively and incubated under 5% CO2 at 37 °C. Growth and morphology of the HeLa cells were observed every 24 h.
Rabbit ileal loop assay Rabbits were fasted for 24 h. The rabbit ligated ileal loops (5 cm) were prepared in rabbits (weight 2 kg) anesthesized with procaine hydrochloride by local infiltration. Twenty μg O-polysaccharides in 0.5 mL saline was injected into the loop. Rabbits were sacrificed 24 h later. Portions of tested loops were taken and fixed in 10% buffered formalin immediately. The pathologic slices of the specimen were prepared with standard procedures.
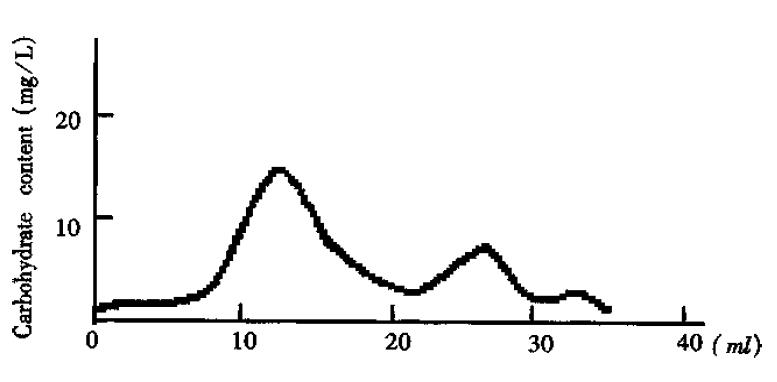
Separation of the crude O-PS by Sephadex G50 showed three major peaks of carbohydrates measured with the sulfuric acid- phenol method (Figure 1). According to references[3] and the molecular weight, the peak I was thought to be the long-chain O-PS. This was confirmed by ELISA analysis which gave rise to positive results with peak I by using antisera against-Shigella flexneri-5 M90T. On the contrast, peak II yielded negative results with ELISA analysis. Peak III was not analyzed due to its small amount and low molecular weight. Therefore, it is plausible that peak II and III were the fragments generated in acid-hydrolysis. Peak I is the O-PS based on its molecular weight and antigenicity.
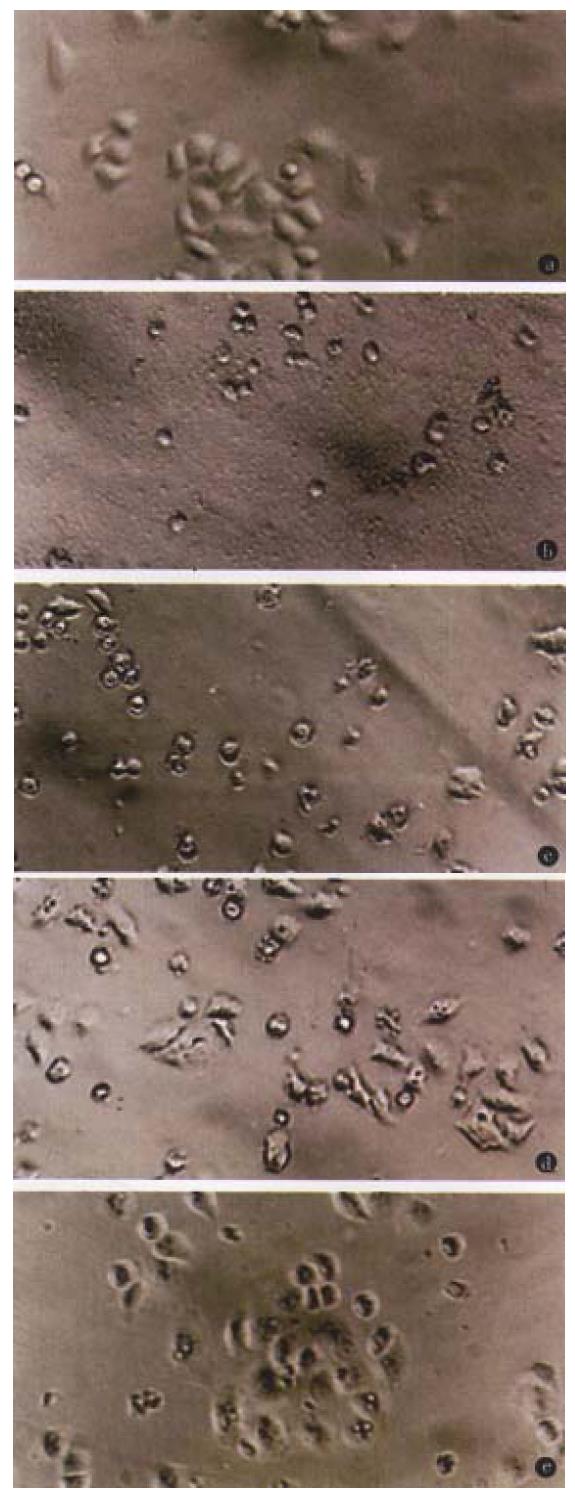
The toxicity of O-PS to HeLa cells demonstrated as cell shrinking and falling off from plate walls within 48 h. The cytopathic effect of HeLa cells was positively related to the concentration of O-PS in medium (Figure 2).
After 24 h of injection of 20 μg O-PS into ileal loop of rabbit, mucosal inflammation and hemorrhagic exudation in ileal loop of rabbit could be observed. When observing the pathologic slice of the specimen under light microscope, we could see inflammatory reaction, the mucosa and submucosa had moderate to severe hyperemia and infiltration of neutrophils and lymphocytes, the muscularis and serosa had mild hyperemia and infiltration of white blood cells. There were necrotic substances and hemarrhage in the lumen.
Shigella flexneri is an intracellular pathogen which causes acute inflammation by infection of the mucosal membrane of the large intestine and damages the mucosal epithelial cells, leading to ulcer. It has been shown that the infection of Shigella flexneri is accomplished by releasing the across membrane signals which induce aggregation of actin and polymerization of myosin to trigger the endocytosis . This will bring about the breakdown of membrane and allow bacteria to get into cells, proliferate and move in cells, diffuse into surrounding cells and eventually kill the cells infected by inhibiting respiration[5]. These functions were dependent on the presence of O antigen and involved in form a “tail-like” actin structure on the bacterium surface as a motile appratus. As for the infection of Shigella flexneri, LPS plays an important role in ind ucing inflammation. For example, antibodies against LPS showed protective effects for inflammatory reaction and the mutants defective in O-antigen can not cau se keratitis in guinea pigs, although it is able to invade HeLa cells. However, little is known about the roles and mechanisms of LPS played in the infection of cells.
LPS of gram negative bacteria is composed of lipoid A, core polysaccharide and O-PS. Among them, lipoid A, the principal component of LPS, causes pathological or physiological damages by inducing some cellular factors[6]. But O-PS is generally thought to determine the antigenicity of the bacterium and its res istance to the defendance of the host cells[7]. In recent years, the specific pathogenesis of O-PS has received increasing attention worldwide[8,9]. There are reports using whole baterium in the research, but the role played by each component of LPS has not been distinguished. For example, enteroinvasive Escherichia coli (EIEC) can be strongly recognized by the antisera against O-PS, suggesting that the structure of O-PS may be related to its toxicity. The Shigella vaccine of the same serotype was efficient; the mAb against O-PS of Shigella flexneri could inhibit contact hemolytic activity[10]. All these indicate the importance of O-PS in pathogenesis of Shigella flexneri. Therefore, it is important to investigate the pathogenesis by using purified O-PS.
In this paper, we studied Shigella flexneri pathogenesis by using purified O-PS both in vitro and in vivo. We have modified Eidhin’s method to improve the preparation of crude LPS by conducting additional steps of nuclease digestion and Sephadex G50 gel chromatography. The experiment showed that M90 O-PS alone was toxic to HeLa cells and caused mucosal inflammation and hemorrhagic exudation of ileal loop in rabbits. It appears that the toxicity to HeLa cells and ileal loop pathogenesis resulted from a common factor. Our experiments showed that Shigella flexneri O-PS may also be one of the factors which cause diarrhea. However, its molecular mechanism is still nuclear and need further investigations. The methods and results presented in this report provide a basis to study diarrhea pathogenesis and a new thought to develop diarrhea vaccines.
We are grateful to Professor Chen En-Lin for his overall guide to this paper, to Mr. He Jian-Min for his technical assistanc e in preparaing LPS, to Dr. Li Fang-Tao, Li Bao-Yan and Kang Ning for paoticip ating in the animal experiment, and Department of Pathology for pathological diagnosis.
Project supported by the National Natural Science Foundation of China, No. 39370040.
Edited by Jing-Yun Ma
| 1. | Eidhin DN, Mouton C. A rapid method for preparation of rough and smooth lipopolysaccharide from Bacteroides, Porphyromonas and Prevotella. FEMS Microbiol Lett. 1993;110:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Xu XP, Chen ZH, Su X. Study on immunogenicity of S. sonnei polysaccharide-protein conjugate. Chin J Microbiol Immunol. 1992;12:141-144. |
| 3. | Müller-Seitz E, Jann B, Jann K. Degradation studies on the lipopolysaccharide from E. coli 071: K: H12. Separation and investigation of O-specific and core polysaccharides. FEBS Lett. 1968;1:311-314. [PubMed] |
| 4. | Le Blay K, Caroff M, Richards JC, Perry MB, Chaby R. Specific and cross-reacting monoclonal antibodies to Bordetella parapertussis and Bordetella bronchiseptica lipopolysaccharides. Microbiology. 1994;140:2459-2465. [PubMed] |
| 5. | Sansonetti PJ. Genetic and molecular basis of epithelial cell invasion by Shigella species. Rev Infect Dis. 1991;13 Suppl 4:S285-S292. [PubMed] |
| 6. | Jiao BH. Molecular endotoxicology. Shanghai: Shanghai Scientific and Technological Publishing House. 1995;151-156, 232-253. |
| 7. | Lindberg AA, Kärnell A, Weintraub A. The lipopolysaccharide of Shigella bacteria as a virulence factor. Rev Infect Dis. 1991;13 Suppl 4:S279-S284. [PubMed] |
| 8. | Rajakumar K, Jost BH, Sasakawa C, Okada N, Yoshikawa M, Adler B. Nucleotide sequence of the rhamnose biosynthetic operon of Shigella flexneri 2a and role of lipopolysaccharide in virulence. J Bacteriol. 1994;176:2362-2373. [PubMed] |
| 9. | Sandlin RC, Lampel KA, Keasler SP, Goldberg MB, Stolzer AL, Maurelli AT. Avirulence of rough mutants of Shigella flexneri: requirement of O antigen for correct unipolar localization of IcsA in the bacterial outer membrane. Infect Immun. 1995;63:229-237. [PubMed] |
| 10. | Mei Y, Su X, Li H, Xu XP. Analysis of biological characteristics of monoclonal antibodies to Shigella flexneri 2a O-side chain of LPS. Chin J Microbiol Immunol. 1992;12:322-323. |