Published online Jun 15, 1999. doi: 10.3748/wjg.v5.i3.213
Revised: April 14, 1999
Accepted: April 28, 1999
Published online: June 15, 1999
AIM: To observe the nitric oxide synthase (NOS) distribution in the esophageal mucosa and hemodynamic changes in cirrhotic rats.
METHODS: NOS distribution in the lower esophagus of rats with carbon tetrachloride-induced cirrhosis was assessed by using NADPH-diaphorase (NADPH-d) histochemical method. Concentration of NO in serum were measured by fluorometric assay. Mean arterial pressure (MAP), cardiac output (CO), cardiac index (CI), splanchnic vascular resistance (SVR), and splanchnic blood flow (SBF) were also determined using 57Co-labled microsphere technique.
RESULTS: Intensity of NOS staining in the esophageal epithelium of cirrhotic rats was significantly stronger than that in controls. There was a NOS-positive staining area in the endothelia of esophageal submucosal vessels of cirrhotic rats, but the NOS staining was negative in normal rats. NO concentration of serum in cirrhotic rats were significantly higher in comparison with that of controls. Cirrhotic rats had significantly lower MAP, SVR and higher SBF than those of the controls.
CONCLUSION: Splanchnic hyperdynamic circulatory state was observed in rats with cirrhosis. The endogenous NO may play an important role in development of esophageal varices and in changes of hemodynamics in cirrhosis.
- Citation: Huang YQ, Xiao SD, Zhang DZ, Mo JZ. Nitric oxide synthase distribution in esophageal mucosa and hemodynamic changes in rats with cirrhosis. World J Gastroenterol 1999; 5(3): 213-216
- URL: https://www.wjgnet.com/1007-9327/full/v5/i3/213.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i3.213
Cirrhosis with portal hypertension is associated with hyp erdynamic ciculation characterized by generalized vasodilation and increased cardiac output and splanchnic regional blood flows. Endogenous NO, a very potent vasodilator factor, may play a very important role in the pathogenesis of hemodynamic changes in cirrhosis. It is unclear whether NO is involved in the pathogenesis of esophageal varices as one of severe complications of hepathic cirrhosis. The present study was aimed at investigating the effects of endogenous NO on esophageal varices and hemodynamic changes in cirrhotic rats.
Male Sprague-Dawley (SD) rats (supplied by the Shanghai Laboratory Animal Center of Chinese Academy of Sciences) weighing between 250 g and 300 g were used. Cirrhotic rat model was induced by injection of 60% CCl4 oily solution twice weekly subcutaneously (0.3 mL/100 g, first time 0.6 mL/100 g) for two months [1].After the model was established, there were 8 cirrhotic rats in experimental group and 8 normal SD rats served as controls.
Under Ketamine anesthesia (100 mg/kg intramuscularly), the right femoral artery and the femoral vein were cannulated with a polyethylene 50 catheters, which went forward respectively to the abdominal aorta and inferior vena cava. The left ventricle was catheterized under pressure monitoring through the right carotid artery with a polyethylene 50 catheter for injection of 57Co-labeled microspheres. All catheters were connected to highly sensitive pressure transducers that were calibrated before each study, and blood pressures were registered on a multichannel recorder (Lifescope 6). An abdominal incision (1.5 cm-2.0 cm) was performed, and the portal pressure (PP) was indirectly measured thro ugh puncture of spleen with a No.4 needle[3]. Cardiac output and splanch nic blood flows were measured by using 57Co-labeled microspheres technique. A reference blood sample was obtained from the femoral artery catheter for 70 seconds at a constant rate of 1 mL/min with a continuous-withdrawal pump (model WZ-50, Zhejiang Medical University, China) . Meanwhile, approximately 50000 microspheres labeled with 57Co-labeled microspheres (diameter, 15 µm ± 0.6 µm, Du Pont Co., USA) were injecte d into the left ventricle 10 seconds after the start of blood withdrawal. Then 2 mL blood sample was withdrawn from the right femoral vein and stored at -70 °C for determination. The rats were then killed, the lower esophagus was quickly excised and quick-frozen in nitrogen solution for NADPH-d histochemical staining, and then liver specimens were fixed in 10% formalin for histologic examination. Other abdominal organs and mesentery were also taken out, weighed, and cut into small pieces, and placed in γ counter (model GP1, Shanghai Electronic Apparatus Co., China) for determining the radio-activity (cpm).
Cardiac output (CO) (mL/min) = injected 57Co (cpm) × reference blood sample (mL/min)/reference blood 57Co (cpm).
Cardiac index (CI) (mL·min-1·100 g-1 BW) = CO (mL/min)/100 g BW.
Splanchnic blood flow (SBF) (mL/min) = organ 57Co (cpm) × reference blood sample (mL/min)/reference blood 57Co (cpm).
Portal vein blood flow (PVF) (mL/min) = the sum of gastric, splenic, intestinal, mesenteric and pancreatic venous flows.
Splanchnic vascular resistance (SVR) (kPa·mL-1·min-1) = [MAP-PP (kPa)]/PVF (mL/min).
Esophageal samples were fixed in 4% paraformaldenyde and 0.4% picric acid in 0.16 mol/L sodium phosphate buffer, pH 6.9, for 4 h. Then they were transferred to 10% sucrose in 0.1% mol/L sodium phosphate buffer, pH 7.2, at 4 °C for 24 h. Cryostat sections (10 µm thick, -20 °C Minotome cryosant, USA) were immersed for 10 min in 0.01 mol/L phosphate buffer, pH 8.0, and were incubated for 40 min at 37 °C in prewarmed solution consisting of 0.01 mol/L phosphate buffer, pH 8.0; 0.3% Triton X-100; 0.5 mmol/L nitroblue tetrazolium (NBT, Sigma, USA); and were 1.0 mmol/L NADPH (Sigma, USA). After washing in 0.01 mol/L phosphate buffer, pH 7.4, the sections were dehydrated with graded alcohol and mounted on microscopic glass slides.
This assay is a modification of the method of Damiani and Burini[5] for the fluorometric determination of nitrite. Briefly, the serum sample is added with 20% sodium sulfosalicylic acid to remove protein. After centrifugation, the filtrate is added with 0.01 mol/L EDTA and 2, 3-Diaminonaphthalene (DAN, Fluka, Switzerland) hydrochloric acid solution. The reaction is terminated after 10min at 20 °C by addition of 2.8 mol/L-NaOH solution. The intensity of fluorescent signal of 1(H) naphthotriazole in the serum sample were obtained in a luminescence spectrofluorometer (Model F-4000, Hitachi, Japan) with excit ation at 365 nm, emission at 420 nm and slits at 3 nm. Nitrite levels in samples were then calculated as a standard curve for nitrite.
The liver samples were fixed in 10% formalin, processed routinely, and embedded in paraffin. The sections were stained with HE and were then observed using a microscope.
The results were expressed as -x±s, and were analyzed with Student’s t test. P < 0.05 was regarded as of statistical significance.
The liver histology of all the animals treated with CCl4 in the study had a granulated surface, and histological examination showed the characteristic features of cirrhosis.
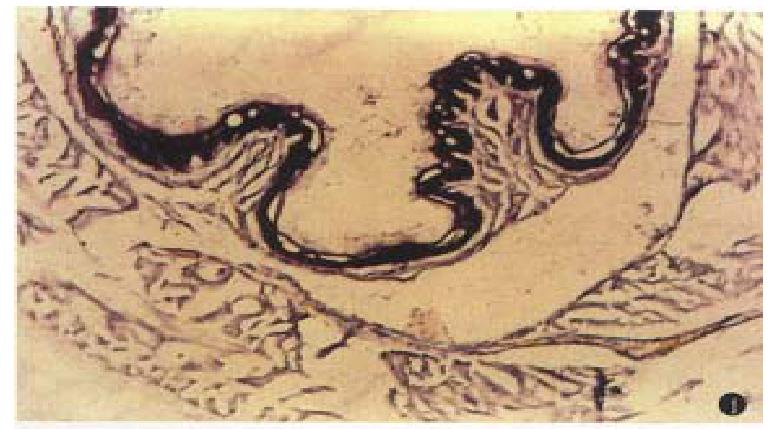
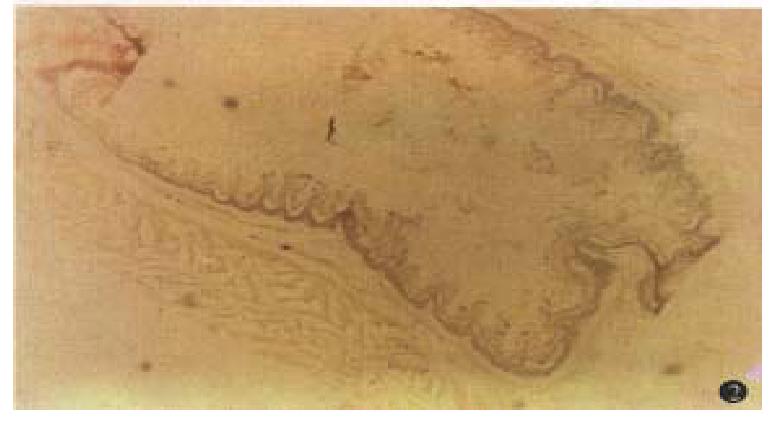
Areas with a positive reaction for NADPH diaphorase were stained dark blue. The reaction was negative in specimens stained without NADPH. NADPH-diaphorase hist ochemical staining showed that intensity of NOS staining in lower esophageal epithelium of cirrhotic rats was significantly stronger than that in normal SD rats. There was a NOS positive staining area in the endothelium of esophageal submucosal vessels, but the NOS staining was negative in normal controls (Figures 1 and 2).
Hyperdynamic circulatory status associated with portal hypertension was observed in all rats with cirrhosis (Tables 1 and 2). Serum NO level in cirrhotic rats were significantly higher than that in normal controls (4.204 µmol/L ± 1.253 µmol/L vs 0.532 µmol/L ± 0.257 µmol/L, P < 0.01).
Lower esophageal varices are the main clinical manifestation and the cause of upper gastrointestinal hemorrhage in cirrhosis associated with portal hypertension. Cirrhosis with portal hypertension is often associated with hyperdynamic circulation characterized by genealized vasodilation and increased cardiac output and splanchnic regional blood flows. However, the mechanisms responsible for the development of lower esophageal varices and the hyperdynamic circulatory status are still unclear.
Our study showed that intensity of NOS staining in esophageal epithelium of cirrhotic rats associated with portal hypertension was significantly stronger than that in normal SD rats. There was a NOS positive staining area in the endothelium of esophageal submucosal vessels, but the NOS staining was negative in normal rats. In addition, we also found that the levels of serum NO were all significantly elevated in cirrhotic rats as compared to normal rats. The hyperdynamic circulatory state of cirrhosis with portal hypertension could provide continuous stimuli (such as a progressive increase in blood flow, high oxygenation in portal blood, or endotoxemia) for nitric oxide synthase (NOS) induction in the portal collateral bed[6]. Our findings suggest that NO may play an important role in the collateralization of the portal system because inhibition of NO synthesis reduces portal systemic shunting without affecting portal pressure in cirrhotic rats[7]. Therefore, overexpressed NOS in the mucosa of the lower esophagus of cirrhotic rats significantly shows a mechanism for the predisposition of collaterals to develop at this site by enhancing NO production. Therefore, greater NOS content in the lower esophageal mucosa of cirrhotic rats would produce increased amounts of NO, adding to the hyperdynamic circulation in the resion.
In order to determine NOS, we used a kind of histochemical staining method depended on the presence of diaphorase. The technique of “diaphorase” staining is based on the ability of the C-terminal portion of nitric oxide synthase to transfer electrons from NADPH to nitroblue tetrazolium (NBT) reducing the substrate NBT to an insoluble purple fomazan product giving the characteristic “diaphorase” reaction[8]. Other studies showed that the overpressed of NOS visualized by immunohistochemical staining and of NADPH-d staining in brain and periph eral tissues were identical[9,10]. It seems that the NADPH-d staining technique is a useful and simple method to determine the expression of NOS[11]. In the present study, we found a strong expression of NADPH-d activity in the lower esophagus, reflecting NOS. The findings are identical to Tanoue’s report[4]. Tanoue et al found that expression of NOS proteins in endothelia of submucosal veins was markedly higher in portal-hypertensive rats than in controls. We postulate that because NO is a very potent vasodilator factor, overpression of NOS may be an important cause of esophageal varice rupture to give rise to hemorrhage.
In the present study, increment of splanchic blood flow associated with portal hypertension was observed in all 8 rats with cirrhosis except that splenic vein flow was lower than controls . Moreover, there were 6 cirrhotic rats with ascites in the 8 rats with cirrhosis. Although the cause of this hyperdynamic circulation is still a matter of controversy, it seems that vasodilatation, induced by increased activity of endothelia-independent and endothelia-dependent vasodilators, initiates the hyperdynamic state. Recently, a role of endogenous nitric oxide in the regulation of blood flow and vascular tone of the systemic and splanchnic circulations in portal hypertension has been suggested by several in vivo and in vitro studies, implying that excessive synthesis of NO could be responsible for the these circulatory abnormalities. Our previous studies had suggested that an excessive release of NO may be involved in the splanchnic hyperemia[12].
In conclusion, the results of the present study show that endogenous NO may play an important role in development of esophageal varices and in changes of hemodynamics pattern in cirrhosis.
Edited by Jing-Yun Ma
| 1. | Huang YQ, Zhang DZ, Mo JZ, Xiao SD, Li RR. Effects of nitric oxide on hemodynamics in cirrhotic rats. Chin J Hepatol. 1997;5:153-155. |
| 2. | Nagasawa M, Kawasaki T, Yoshimi T. Effects of calcium antagonists on hepatic and systemic hemodynamics in awake portal hypertensive rats. J Gastroenterol. 1996;31:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Benoit JN, Womack WA, Hernandez L, Granger DN. "Forward" and "backward" flow mechanisms of portal hypertension. Relative contributions in the rat model of portal vein stenosis. Gastroenterology. 1985;89:1092-1096. [PubMed] |
| 4. | Tanoue K, Ohta M, Tarnawski AS, Wahlstrom KJ, Sugimachi K, Sarfeh IJ. Portal hypertension activates the nitric oxide synthase genes in the esophageal mucosa of rats. Gastroenterology. 1996;110:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Damiani P, Burini G. Fluorometric determination of nitrite. Talanta. 1986;33:649-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Miller VM, Aarhus LL, Vanhoutte PM. Modulation of endothelium-dependent responses by chronic alterations of blood flow. Am J Physiol. 1986;251:H520-H527. [PubMed] |
| 7. | Lee FY, Albillos A, Colombato LA, Groszmann RJ. The role of nitric oxide in the vascular hyporesponsiveness to methoxamine in portal hypertensive rats. Hepatology. 1992;16:1043-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 106] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Norris PJ, Charles IG, Scorer CA, Emson PC. Studies on the localization and expression of nitric oxide synthase using histochemical techniques. Histochem J. 1995;27:745-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci USA. 1991;88:7797-7801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1120] [Cited by in RCA: 1168] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 10. | Hope BT, Michael GJ, Knigge KM, Vincent SR. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci USA. 1991;88:2811-2814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1183] [Cited by in RCA: 1229] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 11. | Ward SM, Xue C, Shuttleworth CW, Bredt DS, Snyder SH, Sanders KM. NADPH diaphorase and nitric oxide synthase colocalization in enteric neurons of canine proximal colon. Am J Physiol. 1992;263:G277-G284. [PubMed] |
| 12. | Huang Y, Xiao S, Zhang D. [Effects of erythropoietin or nitric oxide synthesis inhibitor on hyperdynamic circulatory state in cirrhotic rats]. Zhonghua Yixue Zazhi. 1998;78:139-142. [PubMed] |