Published online Apr 15, 1999. doi: 10.3748/wjg.v5.i2.172
Revised: February 22, 1999
Accepted: February 28, 1999
Published online: April 15, 1999
- Citation: Xu ZK, Liu XL, Zhang W, Miao Y, Du JH. Establishment of a pig model of combined pancreas-kidney transplantation. World J Gastroenterol 1999; 5(2): 172-174
- URL: https://www.wjgnet.com/1007-9327/full/v5/i2/172.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i2.172
We studied the recipient and graft pathophysiologic changes after transplantation, the inducement of immunotolerance, the regularity of chronic rejection and its prophylactico-therapeutic measures by establishing a model of pancreas-kidney transplantation in large animals.
Twenty-six local healthy hybrid pigs, male or female, weighing 18.4 kg ± 2.8 kg used as donors and recipients, were provided by Experimental Animals Centre of Jiangsu Province and fasted for 24 h.
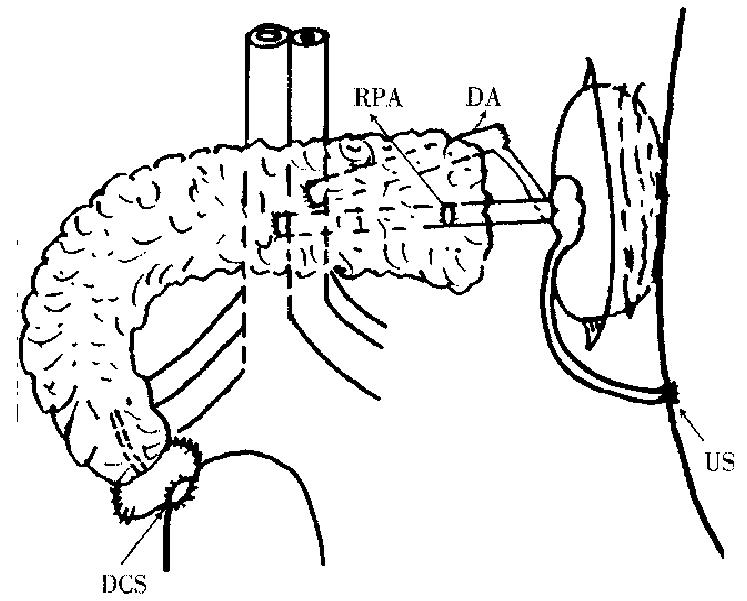
Ketamine (15 mg/kg) was intramuscularly injected 15 min before anesthesia. A trocar was placed in the auricular vein for fluid infusion. The anesthesia was maintained with 30 g/L pentobarbital sodium. Ventilation was provided by tracheal inturbation. (1) donor operation: the whole stomach was excised. A fter dissection of the hepatoduodenal ligament, the portal vein was isolated. The proper hepatic artery and common bile duct were then ligated and divided. The jejunum was transected 5 cm distal to the ligament of Treitz. The superior mesenteric artery and vein were identified. The uncinate lobe, body and tail of the pancreas were mobilized. At this point, the pancreas was only attached to its arterial blood supply (consisting of the celiac axis and superior mesenteric artery and the portal vein). The aortic cannula was placed, through which 200 mL blood was drawn and kept for use. In situ flushing with 4 °C hyperosmo tic citrate adenine (HCA) containing 12 mL/L of 20 g/L ligustrazin hydrochloride was performed. The perfusate pressure was about 80 cm high of water column. The aortia was clamped, and the portal vein and inferior vena cava were divided. Perfusion was stopped when the effluent from the portal vein was clear, and pancreas, duodenum, two kidneys became blanched. About-300 mL of cold perfusate solution was used . The entire pancreas and attached duodenum, spleen, two kidneys and ureters were removed in continuity with abdominal aorta, inferior vena cava and portal vein. They were placed in 0 °C-4 °C HCA soon afterthey had been removed. Then the preparation of the graft was made. The abdom inal aorta was ligated and divided 1 cm distal to the left renal artery. The right kidney was removed. The distal of the portal vein and the left renal vein were immobilized. The issue whether the left renal vein should be transected or hold an inferior vena cava button was decided in accordance with the diameter of the distal end of the portal vein. The two veins were anastomosed in an end to end fashion. Reperfusion was done through the aortic cannula with cold perfusate solution to check the anastomotic stoma. The duodenum was rinsed with metronidazole and then its ends were closed. (2) Recipient operation: After anest hesia worked, a cannula was placed in the external jugular vein and was immobili zed behind the earthrough the tunnel under the skin. The recipient pig received 1.0 g cephradine, 100 mL of 5 g/L metronidazol e and fluid infusion. A cannula was placed in the left femoral artery to detect the average arterial pressure. After entering into the abdomen, the entire pancreas was removed. The abdominal aorta between the common iliac artery and renal artery was freed, and the lumber arteries were ligated and divided. The inferior vena cava was also freed by ligating and dividing all the branches. The proximal aorta was clamped, 30 mL of normal saline containing 1 mg/kg of heparin was injected from the distal aorta. Then, the distal end of the abdominal aorta was clamped. An oval opening was made on the anterior wall of the recipient abdominal aorta, its caliber was similar to the diameter of the proximal end of the donor abdominal aorta. The lacuna was rinsed with normal saline co ntaining heparin. The donor aorta anastomosis was performed in an end to side fashion to the recipient aorta, and the portal vein was anastomosed to the inferior vena cava (Figure 1). The graft was covered with ice bag during the procedure of vascular anastomosis. Soon after circulation to the graft was restored, the graft became pink with its arteriopalmus and peristalsis recovered. Urine overfl owing from the ureter graft was perceived. The duodenum allograft was then anastomosed to the host’s bladder in a side to side fashion. The ureterostomosis of the graft was performed . The graft was fixed to the posterior side of the abdominal wall. The donor’s spleen was removed. Just before closing the incision, a drainage-tube was placed in the left iliac fossa. The amount and kinds of fluid infusion depended on the monitoring results during the operation. However, 200 mL of blood was regularly transfused intravenously.
The graft function was intensively monitored by urine amylase, plasma glucose, urine volume of kidney allograft. All recipients received 1500 mL-2000 mL fluid infusion intravenously per day in the first few postoperative da ys containing 500 mL of low molecular dextran. 1.0 g of cephradine and 100 mL of 5 g/L metronidazole. The drainage-tube was extracted on the third postoperative day. The recipients were allowed to eat on the fourth or fifth postoperative day. The fluid infusion was then decreased or stopped. No immunosuppression was administered to the pigs.
There was no warm ischemia, the cold ischemia of the transplant was 151.4 min ± 15.7 min. The vascular anastomosis was 55.6 min ± 4.9 min.
The survivors usually began to defecate 3 d-4 d after surgery, and then were allowed to eat. Listlessness, hypodynamia and anorexia were found 7 d-9 d after surgery with rapid weight loss. The survivors died 1 d-2 d after a lump could be palpated in the abdomen, due to disturbance of internal environment and anastomosis bleeding. The other 11 pigs survived a mean period of 9.1 d ± 2.4 d. Among them, number 9603 and 9610 were killed on the seventh day and ninth day respectively becau se of obvious decrease in urine amylase and in urine volume of the kidney graft and increase in fasting blood glucose . The graft turned dark, and necrotic areas were noted. The histopathology showed acute rejection. The destruction of the graft induced by acute rejection was evaluated by urine amylase, blood glucose and urine volume of the kidney graft. The urine amylase concentrations usually began to decline 5 d-6 d and became obvious 2 d-3 d before the pig died. The urine volume of the kidney allograft decreased rapidly 4 d-5 d before the death of the pig. The fasting blood glucose elevated significantly 1 d-2 d before the pig died.
The transplantation technique was improved on the basis of the old one as follows[1,2]: (1) the donor aortic segment and the recipient abdominal aorta were anastomosed in an end-to-end fashion; (2) a renoportal end-to-end anastomosis was performed between the left renal vein and the distal end of portal vein before the end-to-side anastomosis of the proximal end of portal vein to the recipient inferior vena cava; (3) the donor duodenum was anastomosed to the host bladder in a side-to-side fashion; and (4) the ureterostomosis of the graft was performed. The present technique has the following advantages: (1) Iliac blood vessels are too slender to be operated, whereas it is simple to anastomose the donor abdominal aorta to the host abdominal aorta in an end-to-side fashion and the donor portal vein to the host inferior vena cava in an end-to-side fashion. This technique enjoys a high success rate. (2) The kidney allograft func tion can be monitored in ureterostomosis. (3) Only two vascular end-to-side anastomoses were performed, which shortened the interruption time of blood flow. It is important to modify the disturbance of the- recipient’s -physiological process and maintain the graft function. (4) The pancreas allograft function is easy to monitor by urine amylase when the pancreas exocrine secretion drainage is established with duodenocystostomy.
(1) The spleen instead of pancreas allograft was ha rvested, prepared and implanted in order not to damage the pancreas allograft and the circulation of the pancreas allograft was monitored. (2) Since pig’s systema lymphaticum is very abundant, when the recipient abdominal aorta and the infe rior vena cava are isolated, the lymph-vessels should be ligated carefully in order to avoid lymph extravasation after operation. (3) The recipient’s internal environment should be kept stable. Number 9601 pig’s tracheal intubation could not be pulled out, and the pig died 8 h after operation. The amount and variety of fluid infusion depended on the monitoring results of average arterial pressure, blood gas, electrolytes, blood glucose and urine volume. Two hundred mL donor blood was regularly transfused to the recipient. The interruption time of blood flow was shortened. The left renal vein and the distal end of portal vein were anastomosed first. Then, only one donor vein should be anastomosed to reci pient vein. After the above-mentioned measures were adopted, satisfactory transplantation results were achieved.
Edited by WANG Xian-Lin
| 1. | Gänger KH, Mettler D, Böss HP, Ruchti C, Stoffel M, Schilt W. Experimental duodeno-pancreatico-renal composite transplantation: a new alternative to avoid vascular thrombosis. Transplant Proc. 1987;19:3960-3964. [PubMed] |
| 2. | Gruessner RW, Nakhleh R, Tzardis P, Schechner R, Platt JL, Gruessner A, Tomadze G, Najarian JS, Sutherland DE. Differences in rejection grading after simultaneous pancreas and kidney transplantation in pigs. Transplantation. 1994;57:1021-1028. [PubMed] |