Published online Apr 15, 1999. doi: 10.3748/wjg.v5.i2.152
Revised: January 12, 1999
Accepted: January 24, 1999
Published online: April 15, 1999
AIM To study the significance of C-erbB-2 oncogene amplification in gastric cancer.
METHODS C-erbB-2 oncogene amplification was examined by using differential polymerase chain reaction (dPCR) in surgical and endoscopic specimens of 83 cases of gastric cancer and 101 metastatic lymph nodes.
RESULTS C-erbB-2 amplification was found in 28.9% (24/83) surgical specimens and 20.5% (17/83) endoscopic ones of gastric cancer patients. The amplification was significant in both types of specimens of advanced cancer cases (P < 0.05) and surgical specimens with lymph node metastasis (P < 0.01). The incidence of C-erbB-2 amplification in lymph nodes with metastasis was higher than in primary sites (surgical specimens, P < 0.05). The patients with amplification tumors had poorer 5-year survival rates than those with unamplification ones in the early cancers and well to moderately differentiated adenocarcinomas (P < 0.05). The same surgical samples were tested again by Southern blot hybridization to ascertain C-erbB-2 amplification, and the positive rate of C-erbB-2 amplification (15.7%) was lower than that of dPCR (28.9%, P < 0.05).
CONCLUSION Examining C-erbB-2 amplification by dPCR is a quick, simple, reliable and independent method, and is helpful in predicting prognosis and metastatic potential of gastric cancer.
- Citation: Ji F, Zhan QBPJB, Li YM. Study of differential polymerase chain reaction of C-erbB-2 oncogene amplification in gastric cancer. World J Gastroenterol 1999; 5(2): 152-155
- URL: https://www.wjgnet.com/1007-9327/full/v5/i2/152.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i2.152
Human proto-oncogene, C-erbB-2, has been showed to share homology with the closely related V-erbB oncogene and with the epidermal growth factor receptor (EGFR) by DNA sequence analysis. The amplification of C-erbB-2 gene is frequen tly found in many cancers and has relation to the gene protein overexpression in cancer cells. In breast cancer, the C-erbB-2 amplification or protein over expression of C-erbB-2 is a signal of poor prognosis and in gastric cancer, the overexpression of C-erbB-2 protein examined by immunohistochemistry has relation to differentiation, progress and metastasis of tumor[1]. The current study was designed to examine the possible association between the amplification of C-erbB-2 gene which was examined by differential polymerase chain reaction (dPCR) and clinicopathologic features of gastric cancer. We compared the dPCR with Southern blot analysis, in order to demonstrate its superiority, reliabilit y and practical value.
The surgical and endoscopic biopsy specimens and 101 metastatic lymph nodes in 83 patients with resected gastric cancers diagnosed pathologically were collected. All specimens and normal gastric mucosa for control were immediately washed to remove blood and fat, and stored in liquid nitrogen.
Taq DNA polymerase and a C-erbB-2 probe were obtained from Promega (U.S.A.) and Wako Pure Chemical Industries (Japan) respectively. The human C-erbB-2 primers were:A:5’-ACGTCTAAGATTTCTTTGTT-3’, B:5’-ACCTTGGCAATCTGCATACA-3’. The β-actin primers were: A: 5’-CAAAGACCTCATACGCTTACA3’ B: 5’-ATAAGCCATGCCAAAGTGATC-3’. The amplifying DNA fragments of above two pairs of primers were 104 bp and 296 bp respectively and all primers were synthesized in the Cell Biology Research Institute of Shanghai.
Total DNA from cancer, normal mucosa and metastatic lymph node cells were extracted by using the phenol-chloroform method. The purity and content of DNA extracted were examined by spectrophotometer analysis. The dPCR was manipulated according to the article[2] in detail. A total amount of 25 μL-reaction solution contained 0.1 μg DNA sample and 0.05 nmol/L-of each pair primers. The amplification of C-erbB -2 and β-actin was carried out in one reaction system and PCR procedure was as follows: predenaturation at 95 °C for 5 min, addition of Taq DNA polymerase, denat uration at 94 °C for 60 s, annealing at 52 °C for 60 s and extension at 72 °C for 50 s, and the thermal cycles from denaturation at 94 °C to extension were repeated 30 times. Then 10 μL DNA amplificating product was subjected to electrophoresis in 4% agarose gel, stained with ethidium bromide and observed under ultraviolet light. The level of amplification was determined by densitometry (Beckman CD2000). The number of gene copy was calculated according to the ra tio of the OD value of C-erbB-2 and that of β-actin and the ratio × 296/104 was thought to be corrected number of gene copy.
DNA from surgical specimens was digested with EcoR-I, and 10 μg of completely digested DNA was subjected to electrophoresis in 0.8% agarose gel and then denatured, neutralized, and transferred to nylon filters. According to classical Southern blot hybridization[3], the filters were hybridized with 32P-labled C-erbB-2 cDNA probes. DNA from normal gastric mucosa was used as a control. After hybridization, the filters were washed and exposed to XAR-5 film. The level of amplification was determined by densitometry, and the densitometric signals of all the bands were summed up.
The significance of differences among groups was determined by the χ2 test. Five-year survival rate was calculated by Kaplan- Meier method and tested with Log-rank method.
| Variable | Case number | C-erbB-2 amplification (%) | |
| Surgical specimen | Endoscopic specimen | ||
| Age (yr) | |||
| < 60 | 16 | (31.3) | 4 (25.0) |
| ≥ 60 | 67 | 19 (28.4) | 13 (19.4) |
| Sex | |||
| Male | 51 | 15 (29.4) | 10 (19.6) |
| Female | 32 | 9 (28.1) | 7 (21.9) |
| Gross appearance | |||
| Borrmann1 | 8 | 3 (37.5) | 2 (25.0) |
| Borrmann 2 | 37 | 9 (24.3) | 7 (18.9) |
| Borrmann 3 | 32 | 10 (31.2) | 7 (21.9) |
| Borrmann 4 | 6 | 2 (33.3) | 1 (16.7) |
| Depth of invasion | |||
| Early cancer | 28 | 4 (14.3)a | 2 (7.1)a |
| Advanced cancer | 55 | 20 (36.4) | 15 (27.3) |
| Differentiation | |||
| Well-mod | 45 | 17 (37.8) | 12 (26.7) |
| Poor | 38 | 7 (18.4) | 5 (13.2) |
| Lymph node metastasis | |||
| Positive | 21 | 11 (52.4)b | 7 (33.3) |
| Negative | 62 | 13 (21.0) | 10 (16.1) |
| Liver metastasis | |||
| Positive | 13 | 6 (46.2) | 4 (30.8) |
| Negative | 70 | 18 (25.7) | 13 (18.6) |
Within the extent of 0.17-0.49 (99% convincing range) was the numbers of gene copy of C-erbB-2 in 30 control cases (normal gastric mucosa). After multiplic ating 296/104, the extent of the number was corrected to be 0.48-1.39. Every case with the number of gene copy of C-erbB-2 over 1.39 was thought to be of gene amplification. Twenty-four surgical and seventeen endoscopic specimens of 83 cases with gastric cancer showed a positive gene amplification and the multiples of amplification were within the extent of 2-33 and 2-29 in the two types of specimens respectively. No difference in the incidence of positive rate of amplification was found between surgical and endoscopic specimens.
The cases of gastric cancer with positive C-erbB-2 amplification in endoscopic specimens were all associated with positive amplification in their surgical ones. The amplification of C-erbB-2 was correlated with the depth of invasion (in the both types of specimens, P < 0.05) and lymph node metastasis (only in surgical specimens, P < 0.01), but not correlated with the age, sex, gross appearance of tumor and liver metastasis in cases with gastric cancer. C-erbB-2 amplification was detected more often, but not to a significant extent, in well-moderately differentiated adenocarcinoma ( χ2 = 3.75, P > 0.05).
Regardless of the presence or absence of C-erbB-2 amplification at the primary site, the rate of C-erbB-2 amplification in lymph nodes with metastasis (44.6%) was significantly higher than that at primary sites (28.9%, P < 0.05).
The rate of C-erbB-2 amplification (15.7%, 13/83) examined by Southern blot was significantly lower than that (28.9%) examined by dPCR in surgical specimens of gastric cancer (P < 0.05). C-erbB-2 amplification was detected by using dPCR in 12 of 13 amplification cases examined by Southern blot.
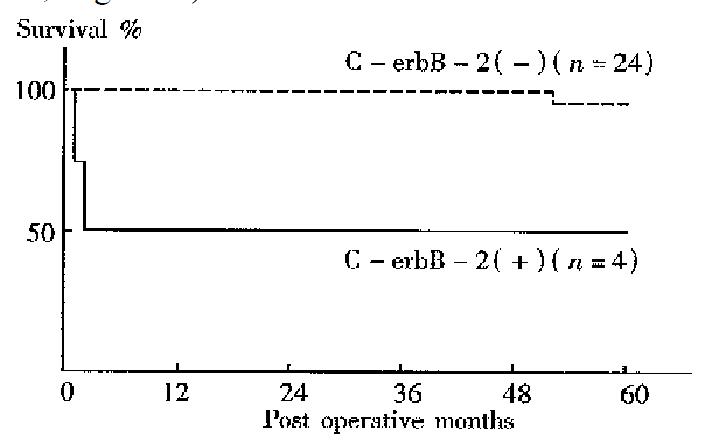
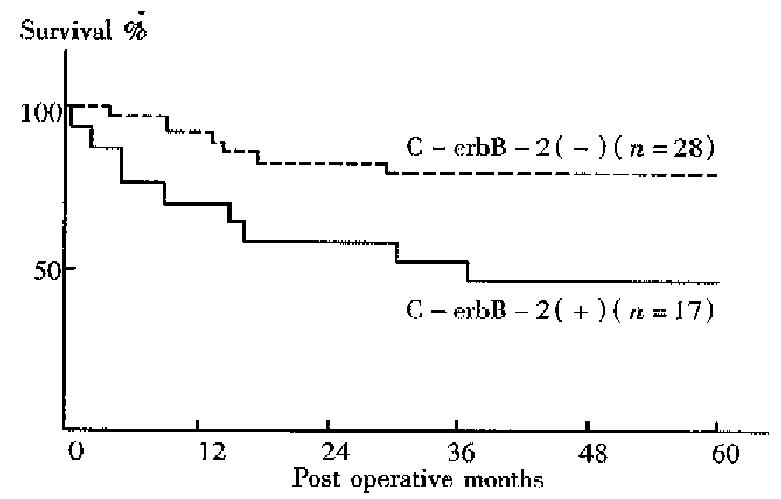
No difference between the 5-year survival rates of gastic cancer patients with C-erbB-2 amplification and those without was found. The 5-year survival rates of patients with the gene amplification tumors and those without were 50.0% and 95.8% respectively for early cancers, 47.1% and 78.6% respectively for well to moderately differentiated adenocarcinomas . Thus, the prognosis was significantly worse among patients with C-erbB-2 amplification tumors than among those without amplification in the early cases (P < 0.05, Figure 1) and in the well to moderately differentiated cancers (P < 0.05, Figure 2).
In recent years, some studies have revealed a correlation of C-erbB-2 amplific ation with poor prognosis in breast or gastric cancers. However, there are few systematic studies about C-erbB-2 changes in gastric cancer in China. In the current study, the C-erbB-2 amplification were observed in 24 surgical specimens of 83 cases by dPCR. High rates of the gene amplification in advanced cancers showed a tendency to deep invasion in such cancers. C-erbB-2 amplification seemed to exist more often in cancers with nodal involvement than in those without. T herefore, cancers with the amplification might have a tendency to show a greater degree of lymph node metastasis. Recent studies have showed that p185, the protein products of C-erbB-2, which is located in microvillus and pseudopodia of cancer cells is able to accelerate cell movement. Amplification of C-erbB-2 mig ht therefore be related to the regulation of cancer cell invasion and metastasis[4]. In the present study C-erbB-2 amplification was found more often in well to moderately differentiated cancers, suggesting its correlation with cell differentiation; such a result was similar to the report of Yokota[5]. Our result also showed that the incidence of C-erbB-2 amplification in metas tatic lymph nodes was higher than that at the primary sites. Therefore, the gene amplification might be related to nodal involvement of gastric cancer, and the oncogene possibly allowed the cancer cells to spread from the primary site to lymph nodes. The activated C-erbB-2 gene has been indicated to enhance the metastatic potential of colon cancer cells, suggesting participation of the gene in the metastasis process[6]. In a 5-year follow-up of all cases, we found that there was no difference in prognosis between cases with C-erbB-2 amplification and those without. But in cases of early and well to moderately different iated gastric cancer, patients with C-erbB-2 amplification tumors were found to have a worse prognosis than those without amplification, perhaps because of the greater degree of deep invasion and lymph metastasis in C-erbB-2 amplificati on tumors.
The dPCR in the current study is a semi-quantitative analysis which can be used to detect gene changes rapidly and sensitively without using isotopic technique[7]. The basic property of dPCR is that both the target gene samples to be tested and the monocopy reference gene in PCR are run in one reactive system. The amplification copy number of the sample gene DNA is calculated on the basis of the ratio of OD values of amplifying products of the sample and reference genes. As compared with Southern blot hybridization, dPCR is simpler, needs less sample or DNA and no isotope, and causes less error. Although less amount of DNA from endoscopic specimens was used in dPCR, the rate of C-erbB-2 amplification reached 20.5% and was not significantly lower than that done with surgical specimens. Our results indicated that the prognosis of gastric cancer might be predicted preoperatively by using dPCR to examine C-erbB-2 amplification. The rate of C-erbB-2 amplification was significantly higher by using dPCR than using Southern blot hybridization in surgical specimens. These results prove that dPCR is a highly sensitive method. Therefore examining C-erbB-2 amplification by dPCR might be helpful in predicting prognosis and metastatic potential of gastric cancer, especially early and well to moderately differentiated cancers.
Project supported by the Zhejiang Natural Science Fundation, No.925006.
Edited by Han-Ming Lu
| 1. | Uchino S, Tsuda H, Maruyama K, Kinoshita T, Sasako M, Saito T, Kobayashi M, Hirohashi S. Overexpression of c-erbB-2 protein in gastric cancer. Its correlation with long-term survival of patients. Cancer. 1993;72:3179-3184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Neubauer A, Neubauer B, He M, Effert P, Iglehart D, Frye RA, Liu E. Analysis of gene amplification in archival tissue by differential polymerase chain reaction. Oncogene. 1992;7:1019-1025. [PubMed] |
| 3. | Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503-517. [PubMed] |
| 4. | De Potter CR, Quatacker J. The p185erbB2 protein is localized on cell organelles involved in cell motility. Clin Exp Metastasis. 1993;11:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Yokota J, Yamamoto T, Miyajima N, Toyoshima K, Nomura N, Sakamoto H, Yoshida T, Terada M, Sugimura T. Genetic alterations of the c-erbB-2 oncogene occur frequently in tubular adenocarcinoma of the stomach and are often accompanied by amplification of the v-erbA homologue. Oncogene. 1988;2:283-287. [PubMed] |
| 6. | Yusa K, Sugimoto Y, Yamori T, Yamamoto T, Toyoshima K, Tsuruo T. Low metastatic potential of clone from murine colon adenocarcinoma 26 increased by transfection of activated c-erbB-2 gene. J Natl Cancer Inst. 1990;82:1633-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |