Published online Apr 15, 1999. doi: 10.3748/wjg.v5.i2.147
Revised: January 14, 1999
Accepted: January 23, 1999
Published online: April 15, 1999
AIM To study the tumorigenicity of colorectal cancer cells transfected with B7 gene and the anti-tumor immunity induced by B7 gene modified colorectal cancer cells.
METHODS B7 gene was transfected into mouse colon cancer cell line CMT93. The transfectants were selected in DMEM containing 800 mg/L-G418, and B7 molecules were detected by immunohistochemistry. Experiments in vivo include: (1) 5 × 106 B7+ CMT93 cells were inoculated into the back of C57BL/6 mice subcutaneously to determine their tumorigenicity (n = 4). As control, wild type CMT93 cells were inoculated the same as the experimental group (n = 3). (4) The mice primed by B7+ CMT93 cells whose tumors vanished were rechallenged with wild type CMT93 to observe the immune protection of these mice against the wild type CMT93 (n = 4). Non-primed 4 native mice inoculated with wild type CMT93 were used as control. With in vitro cytotoxicity assay, the mice were immunized with B7+ CMT93 or the wild type CMT93 by intraperitoneal injection(n = 4 × 2). The spleen cells and the abdominal cavity infiltrating lymphocytes were obtained and cultured for two days. Cytotoxicity of these cells against the B7 gene modified or wild type CMT93 was detected by MTT assay.
RESULTS B7 high expression clones were obtained after the transfection of the B7 gene into CMT93 cells by electroporation. Immunohisto-chemistry results showed mainly membrance staining and partly cytoplasm staining in B7 gene transfected CMT93 cells. In vivo-experiments: (1) After the inoculation of the B7+ CMT93 cells into the back of C57BL/6 mice, they lost their tumorigenicity greatly (P < 0.01). All the small tumorsgrowing in the early period in the experimental group vanished in one month, and the tumors in control group grew progressively. (2) No tumors were found in all 4 mice primed by B7+ CMT93 cells after they were rechallenged with wild type CMT93. In the control group all mice had grown tumors (P < 0.05). In vitro-cytotoxicity assay, the CTLs induced by B7+ CMT93 had a higher cytotoxity against the wild type CMT93 than that induced by wild type CMT93 (P < 0.05), and the cytotoxity of CTLs induced by B7+ CMT93 against B7+ CMT93 cells was higher than that against wild type CMT93 cells (P < 0.05).
CONCLUSION The results suggest that the expression of costi mulation B7 molecules by colorectal cancer cells can decrease their tumorigenicity greatly, and the B7 molecule can augment the activation of the CTLs against colorectal cancer, and it plays an important role in CTL effector function as well.
-
Citation: Hu JY, Wang S, Zhu JG, Zhou GH, Sun QB. Expression of B
7 costimulation molecules by colorectal cancer cells reducestumorigenicity and induces anti-tumor immunity. World J Gastroenterol 1999; 5(2): 147-151 - URL: https://www.wjgnet.com/1007-9327/full/v5/i2/147.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i2.147
For efficient activation of T cells, two signals are required. The first antigen-specific signal is meditated by the T cell receptors after their interaction with peptide-MHC complexes. The second signal is antigen-nonspecific costimulatory signals, such as that delivered by the CD28 molecule on the T cells interac ting with B7 molecule on the APCs[1]. When tumor antigen is presented to T cells, there are three distinct pathways to deliver costimulatory signals: trans-costimulation, cis-costimulation and transfer cis-costimulation. Among them, the cis-costimulation in which two signals are delivered by one tumor cell simultaneously is the most efficient mode[2]. Usually, B7 molecule is not expressed on the surface of most tumor cells, so tumor antigen can not activate T cells by cis-costimulation. After the tumor cells were transfected with B7 gene however, efficient anti-tumor immunity can be induced by cis-cost imulatory mode. The mice primed with B7 gene modified tumor cells can reject not only B7 gene modified tumor cells, but also the wild type tumor cells[3,4]. Our experimental results showed that after the transfection of colorectal cancer cells CMT93 with B7 gene, the tumorigenicity of the B7 gene modified tumor cells decreased, and B7 molecules augmented the immunity against tumor cells.
Male or female C57BL/6 mice, 6.8 weeks old, were purchased from Shanghai Experimental Animal Center of Chinese Academy of Sciences. Colorectal cancer ce ll line CMT93 was bought from ATCC. Plasmid pLNSX containing mouse B7. 1 gene (p LNSXB7) was provided kindly by Dr. CHEN Lie-Ping et al[5]. Rat IgG anti-mouse B7 molecule was purchased from Pharmingen . Alkaline phosphatase-conjugated goat IgG anti-rat Ig was bought from Organon Teknik. G418 was obtained from Gibco.
Plasmid pLNSX-mB7 was constructed by cloning murine B7 PCR product into the PCRTM II vectors and then subcloning it into retroviral vector pLNSX [5]. Plasmid was transfected into colorectal cancer cells CMT93 by electroporation. After transfection, the CMT93 cells were selected in DMEM containing 800 mg/L-G418. Two weeks later, the G418-resistant cells were cultured in DMEM containing 400 mg/L-G418.
Colorectal cancer CMT93 cells were digested and cultured in 8-room Slide Chamber (Costar). The immunohistochemistry analysis was performed when the cells were confluent to 50%-60%. Briefly, CMT93 cells were fixed with 0.15% glutaraldehyde solution for 15 min at room temperature, and rinsed 3 times with PBS (0.01 M, pH 7.2). Then the cells were incubated for 2 h at 37 °C with rat IgG anti mouse B7 molecule antibody (1:200 dilution). After rinsed with PBS for 3 times, the cells were incubated for 1 h at 37 °C with alkaline phosphatase-conjugated goat IgG anti-rat Ig (1:50 dilution). The cells were rinsed with PBS 3 times again, and the staining substrate NBT/BCIP (10:1) was added. The cells were stained for 30 min at room temperature. Then the cells were rinsed by PBS to terminate the reac-tion.
The B7+ CMT93 (CMT93-B7) or wild type CMT93 cells were digested when they grew confluent to 95%. Four mice were inoculated with 5 × 106 CMT93-B7 cells in their backs. Three mice were inoculated with 5 × 106 wild type CMT93 cells as control. Ten days later, the grown tumors were observed, and the long and short diameters of the tumors were measured by caliper (0.02 cm) every two days.
Four mice rejecting the initial inoculation of CMT93-B7 tumor cells were rechallenged with 2.5 × 106 wild type CMT93 cells in their backs by subcu tanous injection. Four non-primed mice were inoculated with 2.5 × 106 wild type CMT93 cells in their backs as control. After five days, when the tumors in control group became palpable, all mice were examined to see whether the tumors existed or not, and the long and short diameters of the tumors were measured by caliper every three days. One month later, when the tumor size was approximately 1.5 cm × 1.5 cm, all mice were killed.
Four mice were primed with 5 × 106 CMT93-B7 cells by intraperitoneal injection. Another four mice were primed with 5 × 106 wild type CMT93 cells as control. Seven days after injection, all mice were killed with their abdominal cavities rinsed with 5 mL-Hank's solution. All cells taken from the abdominal cavities were cultured in RPMI 1640 medium supplemented with 10% FCS. After two days of culture, the macrophages and the non-rejected tumor cells were removed by adherence, and the cytotoxicity of CTL from abdominal cavities against CMT93-B7 cells and wild type CMT93 cells were detected by MTT assay[6]. In ad dition, the spleen cells were prepared from all CMT93-B7 or wild type CMT93 primed m ice, and their cytotoxicity against CMT93-B7 cells and wild type CMT93 cells were detected as well.
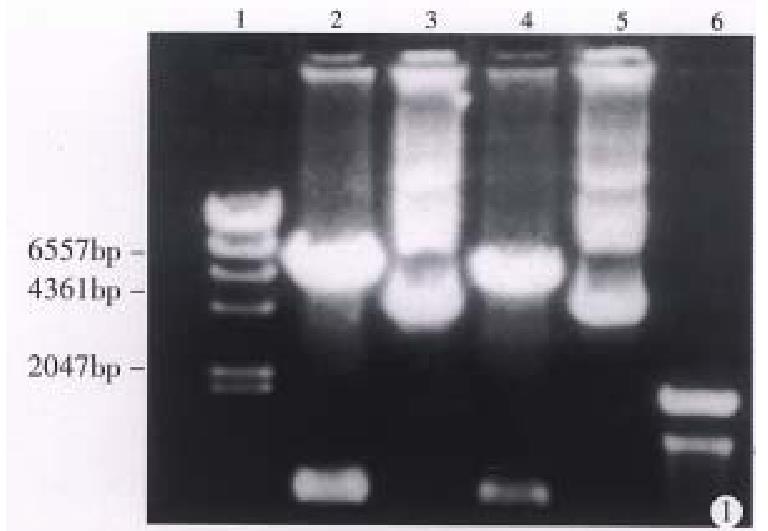
Plasmid pLNSX is a retroviral vector[3]. There is a Hind III site in front of and behind the inserted B7 DNA fragment respectively. Figure 1 shows that pLNSX-B7 was cleavaged into two fragments after the digestion with Hind III. They represented plasmid DNA and B7 DNA respectively.
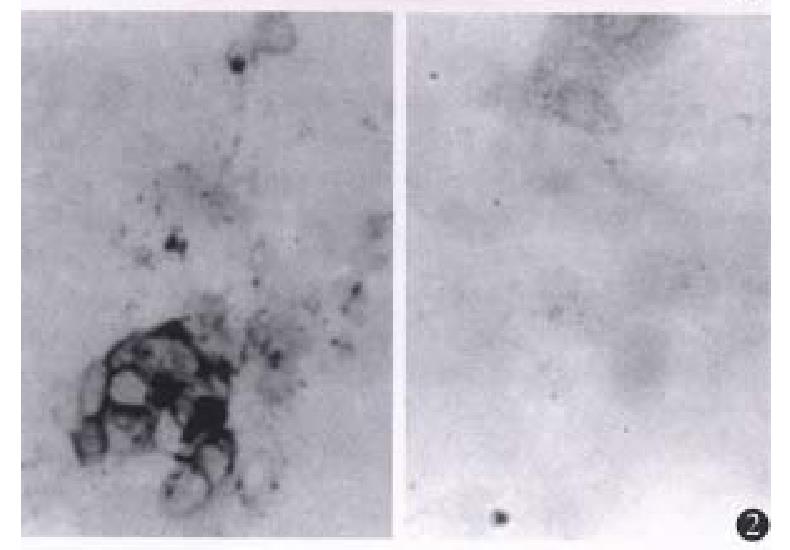
Positive staining was found in G418-resistant CMT93-B7 cells. The positive staining was mainly located in the cell membrane, and some plasma staining was found as well (Figure 2). The percen tage of staining of CMT93-B7 cel ls was 62%. The percentage of staining of wild type CMT93 cells was less than 5% (Figure 2), so we considered this wild type cells did not express B7 costimulation molecules.
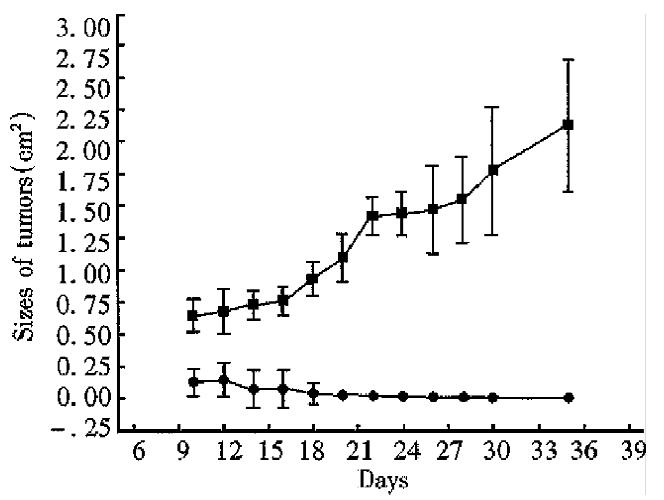
After inoculattion with 5 × 106 tumor cells, all mice grew palpable tumors on the seventh day. Then the tumors of the mice in the control group grew progressively. Yet the tumors of the mice in the experimental group shrank gradually from the second week. The tumors of the four mice in the experimental group disappeared on day 12, day 14, day 14 and day 32 respectively. The size of the biggest tumor was 0.996 cm × 0.302 cm (No 4, on day 12). By unpaired Student’s t test, significant difference was found between the data from the experimental group and the control group in each measurement (P < 0.01, Figures 3 and 4).
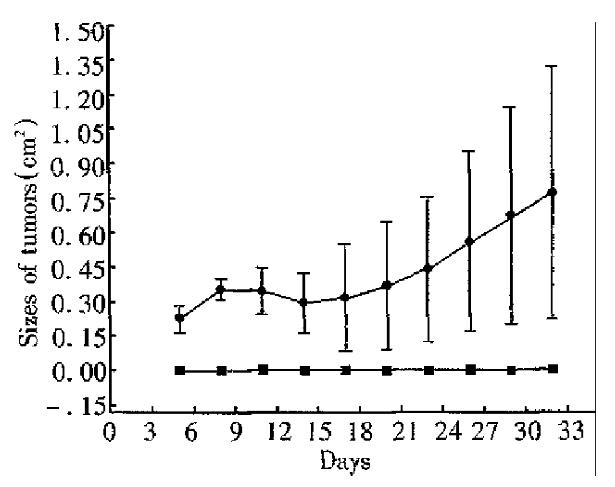
Four mice primed with CMT93-B7 were rechallenged with 2.5 × 106 wild type CMT93. From day 1 to day 32 after rechallenge, no tumor was found in them. In the control group, however, tumors were found in all the four mice injected with 2.5 × 106 wild type CMT93 cells on day 7. In the second week, the tumors in the control group shrank partly. Among them, one tumor was rejected completely on day 16, but the others grew progressively from the third week. By unpaired Student’s t test, significant difference was found between the data from experimental group and control group in each measurement (P < 0.05, Figure 5).
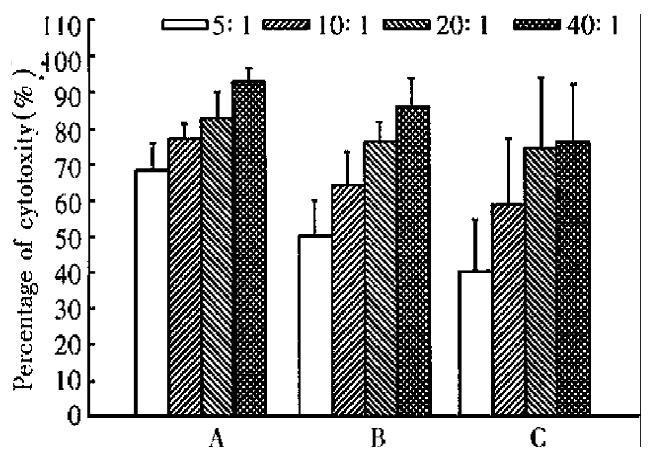
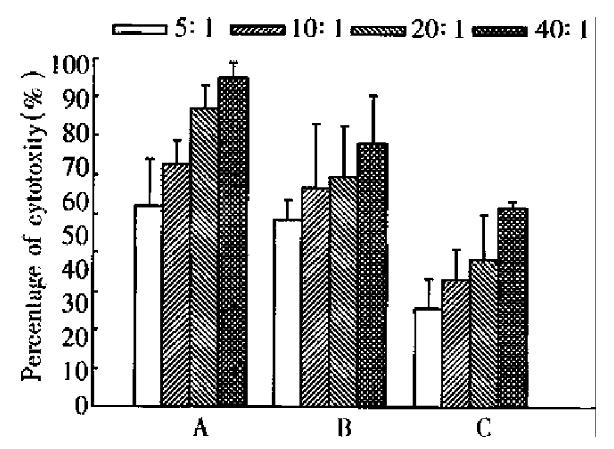
Inoculation of CMT93 or CMT93-B7 cells in the mice by intraperitoneal injection induced infiltration of lymphocyte in abdominal cavities, but the infiltrating lymphocytes induced by inoculation of CMT93 were less than that induced by ino culation of CMT93-B7 (data not shown). The cytotoxicity assay showed that the a bdominal cavity infiltrating lymphocytes induced by CMT93-B7 were more cytotoxic against CMT93 than that induced by wild type CMT93. The results of the spleen cells were the same as the abdominal cavity infiltrating lymphocytes (Figure 6). Moreover, the cytotoxicity of abdominal cavity infiltrating lymphocytes induced by CMT93-B7 against CMT93 - B7 was higher than that against wild type CMT93. These results suggested that B7 molecule might play an important role in CTL cytotoxicity.
Tumor-bearing host can not reject tumor cells efficiently. The unresponseness of T cells to tumor antigens should be considered as ignorance rather than tolerance[7]. Three critical parameters contribute to the ignorance of the immune system. They are low levels of antigen presentation, lack of availability of costimulation signals, and a relatively low frequency of relevant CTL precursors[7]. These factors may play a role singly or simultaneously. B7 molecules deliver costimulation signal in three different ways. Among them, the cis-costimulation is the most efficient mode[2].
Tumor cells expressing B7 molecules activate T cells by cis-costimulation. In this process, tumor’s antigenicity is required. The tumor cells without antigenicity or with poor antigenicity can not induce efficient anti-tumor immunity though they express B7 costimulation molecules[5]. CMT93 is a weak immunogenicity cell line. We found that its tumorigenicity decreased greatly after the transfection with B7 gene . The inoculated CMT93-B7 tumor cells proliferated and formed some palpable tumors in the early stage after inoculation. But a short time later the tumors disappeared completely. Yet in the control group, all tumors grew progressively (Figure 4). The reason why CMT93-B7 cells formed some small tumors in the early stage after inoculation is that the immune system of the host needs a short time to recognize the tumor antigen and to produce immune response to the tumor cells.
B7 gene transfected tumor cells can induce a strong anti-tumor immunity. The mechanism is that in the presence of B7 costimulation, the CTL clones against the domain epitopes of tumor cells are amplified more greatly than ever, and more importantly the CTL repertoire against subdomain epitopes of tumor antigen is expanded. Chen[2] considered that besides providing a unique signal for T cell activation, B7 costimulation may also reduce the threshold of interaction between peptide-MHC complex and TCR which is required for CTL activation. So the subdomain epitopes which usually can not activate T cells induce T cell activation. In this study, we found that the tumors growing initially after the inoculation of CMT93-B7 vanished in a short time, and the mice primed with CMT93-B7 rejected the rechallenge of wild type CMT93 completely. It suggested that when wild type tumor cells rechallenged the mice primed with B7 expressing tumor cells, T cells had already been activated, so the rechallenged wild type tumor cells could be rejected quickly and completely[8].
B7 costimulation signal is needed for T cell activation. During T cells displaying their effector function, this signal is considered no longer required, but few exceptions have been reported[9]. In the rechallenge experiment, the mice primed with CMT93-B7 rejected the rechallenged wild type CMT93 cells completely. The activated CTL from primed mice can kill the wild type tumor cells. In cytotoxicity experiment in vitro, the CTL induced by CMT93-B7 had a higher cytotoxicity against CMT93-B7 than against wild type CMT93 (Figure 6, Figure 7). This phenomenon showed that B7 molecules play an important role in effector function. The mechanism may be that the interaction of B7 with CD28 promoted the lymphocyte adherence to target cells, and that the interaction of B7 with CD28 promoted the combination of peptide-MHC with TCR, i.e., B7 costimulation reduced the threshold of interaction between peptide-MHC complex and TCR. Acco rding to the above results, we consider that the CTL induced by B7 positive tumor cells can kill wild type tumor cells, and the B7 molecules can augment the cyt otoxicity of CTL against tumor cells.
Presented at the 6th Meeting of Colorectal Cancer of China and the First China-Japan-Korea International Congress of Colorectal Cancer, Haerbin, 17-20, September 1998
Edited by Xian-Lin Wang
| 1. | Chen L, Linsley PS, Hellström KE. Costimulation of T cells for tumor immunity. Immunol Today. 1993;14:483-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 231] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Chen L. T cell costimulation and tumor immunity.. Chin J Cancer Biother. 1996;3:86-95. |
| 3. | Baskar S, Ostrand-Rosenberg S, Nabavi N, Nadler LM, Freeman GJ, Grimcher LH. Constitutive expression of B7 restores immu-nogenicity of tumor cells expressing truncated MHC class II molecules.. Proc Natl Acad Sci USA. 1993;90:5676-5690. [RCA] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 200] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Li Y, McGowan P, Hellström I, Hellström KE, Chen L. Costimulation of tumor-reactive CD4+ and CD8+ T lymphocytes by B7, a natural ligand for CD28, can be used to treat established mouse melanoma. J Immunol. 1994;153:421-428. [PubMed] |
| 5. | Chen L, McGowan P, Ashe S, Johnston J, Li Y, Hellström I, Hellström KE. Tumor immunogenicity determines the effect of B7 costimulation on T cell-mediated tumor immunity. J Exp Med. 1994;179:523-532. [PubMed] |
| 6. | Heo DS, Park JG, Hata K, Day R, Herberman RB, Whiteside TL. Evaluation of tetrazolium-based semiautomatic colorimetric assay for measurement of human antitumor cytotoxicity. Cancer Res. 1990;50:3681-3690. [PubMed] |
| 7. | Melero I, Bach N, Chen L. Costimulation, tolerance and ignorance of cytolytic T lymphocytes in immune responses to tumor antigens. Life Sci. 1997;60:2035-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Yang G, Hellström KE, Hellström I, Chen L. Antitumor immunity elicited by tumor cells transfected with B7-2, a second ligand for CD28/CTLA-4 costimulatory molecules. J Immunol. 1995;154:2794-2800. [PubMed] |
| 9. | Ramarathinam L, Castle M, Wu Y, Liu Y. T cell costimulation by B7/BB1 induces CD8 T cell-dependent tumor rejection: an important role of B7/BB1 in the induction, recruitment, and effector function of antitumor T cells. J Exp Med. 1994;179:1205-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 128] [Article Influence: 4.1] [Reference Citation Analysis (0)] |