Published online Apr 15, 1999. doi: 10.3748/wjg.v5.i2.135
Revised: January 20, 1999
Accepted: January 28, 1999
Published online: April 15, 1999
AIM To establish an extracorporeal bioartificial liver support system (EBLSS) using cultured human liver cells and to study its support effect for fulminant hepatic failure (FHF).
METHODS The liver support experiment of EBLSS consisting of aggregates cultured human liver cells, hollow fiber bioreactor, and circulation unit was carried out in dizhepatic dogs.
RESULTS The viability of isolated hepatocytes and nonparenchymal liver cells reached 96%. These cells were successfully cultured as multicellular spheroids with synthetic technique. The typical morphological appearance was retained up to the end of the artificial liver experiment. Compared with the control dogs treated with EBLSS without liver cells, the survival time of artificial liver support dogs was significantly prolonged. The changes of blood pressure, heart rate and ECG were slow. Both serum ammonia and lactate levels were significantly lowered at the 3rdh and 5thh. In addition, a good viability of human liver cells was noted after 5h experiment.
CONCLUSION EBLSS playing a metabolic role of cultured human hepatocytes, is capable of compensating the function of the liver, and could provide effective artificial liver support and therapy for patients with FHF.
- Citation: Wang YJ, Li MD, Wang YM, Nie QH, Chen GZ. Experimental study of bioartificial liver with cultured human liver cells. World J Gastroenterol 1999; 5(2): 135-137
- URL: https://www.wjgnet.com/1007-9327/full/v5/i2/135.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i2.135
Despite the progress of intensive therapy for patients with fulminant hepatic failure (FHF), their death rate is still up to 90%. Liver transplantation is the only curative treatment for FHF at present. Unfortunately, it is limited by a severe shortage of donor organs and the short time available to find a suitable liver. Since some patients with FHF are potentially reversible, it is believed that if liver support system could be provided, their natural liver tissue could regenerate with the liver function maintained stable until an appropriate organ was obtained. Over the past 30 years, various liver support systems have been investigated, such as cross-circulation, whole liver perfusion, hemadsorption, charcoal hemoperfusion, hemodialysis, plasma exchange, total body washout, etc. However, a lot of clinical trials showed that these detoxification systems could promote the recovery of consciousness in some patients, but the survival rate has not been improved[1], and none of these therapeutic procedures succeeded in gaining wide clinical acceptance. Recent development of bioartificial liver support system containing living hepatocytes is an important advance in this field. Because this system has potential metabolic and synthetic functions of liver, it may provide certain essential nutrients and factors necessary for regeneration of the liver tissue[2,3]. Further development of the bioartificial liver is expected to provide very interesting and valuable results in the treatment of FHF[4].
The purpose of our experimental study was to establish an optimal extracorporeal bioartificial liver support system (EBLSS) with cultured human hepatocytes and to furnish a new therapeutic method for these patients.
Human liver obtained from normal donors was transported in ice-cold physiological saline. Liver cells were isolated by the two-step perfusion method. The perfusion solution was Ca2+/Mg2+ free Hanks balanced salt solution and 0.05% collagenase (all chemical and cell culture reagents were purchased from Sigma Chemical CO). The hepatocytes and nonparenchymal liver cells were harvested by centrifugation at 50 × g for 3 min and at 500 × g for 3 min. The viability of the isolated liver cells was de-termined by the trypan blue exclusion test.
Freshly isolated liver cells were suspended in hepatocytes and nonparenchymal liver cells of about 4 × 105 and 2 × 105 viable cells/ mL. After 1 h of incubation at 37 °C, they were seeded in culture flasks precoated with poly (2-hydroxyethyl methacrylate) and cultured with hormonally defined medium (HDM) in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. In order to suppress attachment on their wells and promote their aggregation, the flasks were rotated at regular intervals. Cell morphology in the aggregate culture was observed by an Olympus phase contrast microscope.
The bioartificial liver consisted of aggregate liver cells (about 1 × 108), circulating and hollow-fiber bioreactor containing 200 cellulose nitrate/cellulose acetate porous fibers (pore size of 0.2 μm, fiber lenght of 21 cm, fiber inside diameter of 660 μm). The extrafiber volume was about 40 mL while the intrafiber volume was about 45 mL. The external fiber surface area was 0.34 m2.
Mongrel dogs (provided by Experimental Animal Center of Third Military Medical University), weighing between 11 kg and 14 kg, were used in the study. Under pentobarbital (30 mg/kg, intravenously) anesthesia, all dogs received side to side portocaval shunts with their portal vein and hepatic artery tied off to form dizhepatic model.
Animals were divided into two groups: group 1 (n = 5) was treated by EBLSS without liver cells and group 2 (n = 5) was supported by EBLSS with spheroids liver cells. After cannulation of their femoral arteries and veins, they received 5 h successive hemoperfusion with EBLSS at a rate of 30 mL/min to 50 mL/min. Heparin was administered at 50 U/kg. Perfusion was carried out for 5 h. Blood samples were obtained at 0, 1, 3 and 5 h. Ammonia and lactate were determined in our laboratory using standard techniques. The cell viability and aggregate adherence rate of EBLSS were observed under phase contrast microscope.
The total isolated hepatocytes and nonparenchymal liver cells by the simplified two step perfusing method were 13.6-14.7 × 107 cell/100 g of liver. Their estimated viability judged by the trypan blue test was 92%-96%.
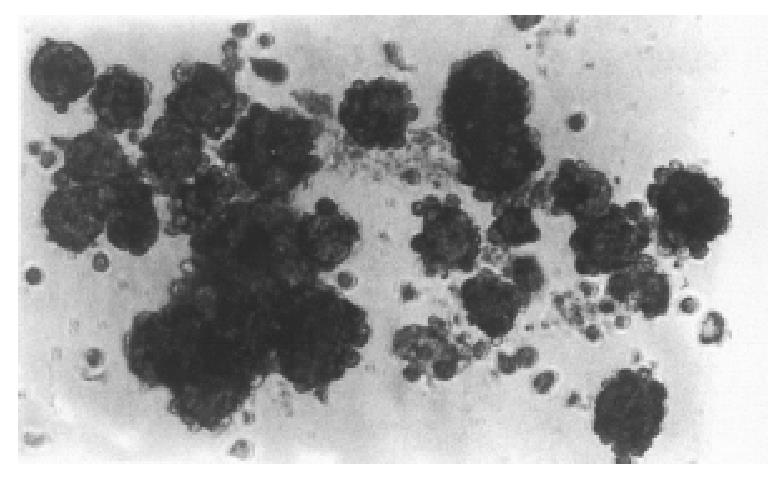
Under the defined culture environment and by gently shaking repeatedly, the freshly isolated hepatocytes were attached to each other within 4 h-8 h, multiple aggregates in different sizes were loosely formed, and the multiplicity of aggregates increased with the lapse of time. Up to 48 h a lot of regular spheroid aggregates were found in flasks. The aggregate hepatocyte spheroids appeared tight and dense in the center and on the surface of the spheroids attached to a lot of single cells. By the action of single cell, some spheroids were also attached to each other. The characteristics of multicellular spheroid aggregates can be maintained up to the end of the bioartificial liver support experiment (Figure 1).
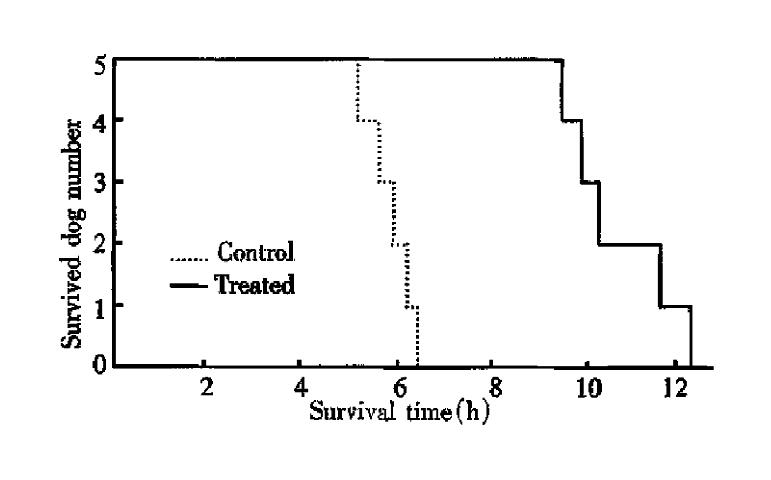
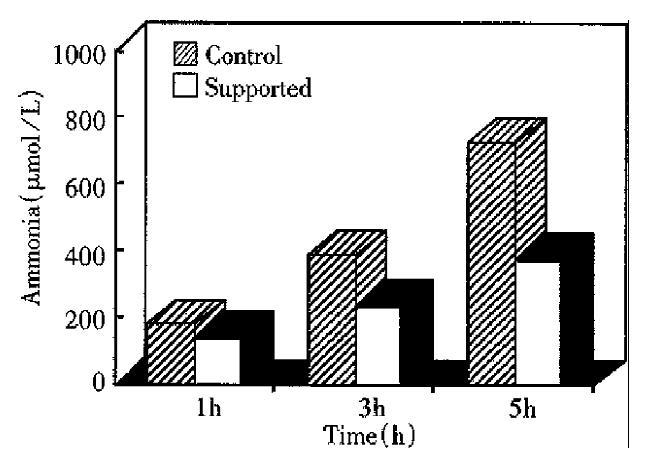
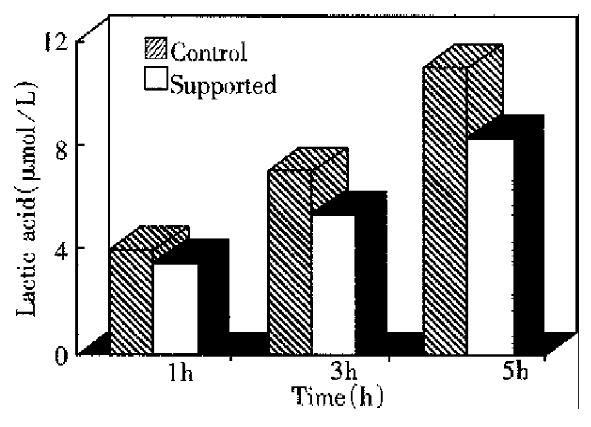
Dogs supported with EBLSS showed significant improvement in their survival time as compared with the control group (10.7 h × 1.1 h and 5.8 h × 0.5 h, respectively) (P < 0.01, Figure 2). The changes in blood pressure, heart rate and ECG of the support dogs were slow. After 3 h and 5 h of treatment, the blood ammonia and serum lactate levels were signi ficantly lower in the support group as compared with the control animals (P < 0.01, Figures 3 and 4). Liver cell viability at the end of the 5 h experimental period was 52.3% to 70.8%, and the aggregate adherence rate was about 50%.
Up to now, considerable progress has been made in the bioartificial liver system. There are two extracorporeal systems, which have reached the stage of preliminary clinical assessment. One based on a human hepatoblastoma cell line was developed by Sussman and Kelly (extracorporeal liver assist devices)[5] and the other on isolated pig hepatocytes (bioartificial liver) was developed by Demetriou’s group[6]. Although primarily isolated porcine hepatocytes proliferate poorly in vitro, they possess normal liver function and the source is almost unlimited. Unfortunately, animal hepatocytes can not replace the synthesis of human plasma proteins. On the other hand, it should be considered that besides the immunological rejection of xenogenic biological tissue, xenogenic proteins and enzymes in the blood will produce side effects. Human cell line is readily available in large quantities, but its differential function is rather poor. Sufficient replacement of liver function by the lines cannot be expected. Besides, they are all genetically transformed, potentially tumorigenic, and characterized by tremendous alterations in genetic, metabolic and synthetic parameters. The side effects and risks of these cells cannot be ruled out com pletely.
Recently, a workshop sponsored by the National Digestive Diseases Advisory Board on “Current Issues in the Management of Fulminant Hepatic Failure” discussed the development of EBLSS[7]. The ideal hepatocyte for such devices was described as being human in origin, of normal (nonmalignant) phenotype, readily available, rapidly and easily grown in cell culture to a high density, stable and remaining in a well-differentiated state for days or weeks, and capable of the full range of synthetic and detoxifying features of mature hepatocytes.
In this study, we found that the human hepatocytes and nonperenchymal cells could be easily isolated by an alternative extracorporeal two-step perfusion method. The viability of cells was 92% to 96% and the yield of cells reached 10.8/100 g of liver. Using a synthetic technique, we successfully performed the aggregate culture of hepatocytes and nonperenchymal cells. Hepatocytes grown in suspension as self-induced multicellular spheroids with three-dimensional structures resembled in vivo. It has become clear that three dimensional rather than monolayer gr owth is particularly important for maintaining differentiated hepatocyte function in culture[8] and the co-culture with nonparenchymal cells also significantly enhances hepatocyte viability and function. Perhaps for this reason, our EBLSS consisting of hepatocyte aggregate spheroids and hollowfiber reactor not only possesses metabolic function (metabolism of ammonia and lactate) but also prolongs the survival in dizhepatic dogs.
These results have important implications concerning use of human hepatocytes in bioartificial liver design. Such EBLSS has provided effective liver support for dizhepatic animal, at least in a short time. It can be expected to maintain the patients in the best condition until an adequate liver graft becomes available or provide liver support for a longer period of time to await liver regeneration in some patients.
Edited by Xian-Lin Wang
| 1. | Nyberg SL, Peshwa MV, Payne WD, Hu WS, Cerra FB. Evolution of the bioartificial liver: the need for randomized clinical trials. Am J Surg. 1993;166:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Hughes RD, Williams R. Use of bioartificial and artificial liver support devices. Semin Liver Dis. 1996;16:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Rozga J, Holzman MD, Ro MS, Griffin DW, Neuzil DF, Giorgio T, Moscioni AD, Demetriou AA. Development of a hybrid bioartificial liver. Ann Surg. 1993;217:502-509; discussion 502-509;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 167] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Bismuth H, Figueiro J, Samuel D. What should we expect from a bioartificial liver in fulminant hepatic failure. Artif Organs. 1998;22:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Sussman NL, Kelly JH. Extracorporeal liver support: cell-based therapy for the failing liver. Am J Kidney Dis. 1997;30:S66-S71. [PubMed] |
| 6. | Demetriou AA, Rozga J, Podesta L, Lepage E, Morsiani E, Moscioni AD, Hoffman A, McGrath M, Kong L, Rosen H. Early clinical experience with a hybrid bioartificial liver. Scand J Gastroenterol Suppl. 1995;208:111-117. [PubMed] |
| 7. | Hoofnagle JH, Carithers RL, Shapiro C, Ascher N. Fulminant hepatic failure: summary of a workshop. Hepatology. 1995;21:240-252. [PubMed] |
| 8. | Koide N, Sakaguchi K, Koide Y, Asano K, Kawaguchi M, Matsushima H, Takenami T, Shinji T, Mori M, Tsuji T. Formation of multicellular spheroids composed of adult rat hepatocytes in dishes with positively charged surfaces and under other nonadherent environments. Exp Cell Res. 1990;186:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 332] [Article Influence: 9.5] [Reference Citation Analysis (0)] |