Published online Apr 15, 1999. doi: 10.3748/wjg.v5.i2.132
Revised: February 22, 1999
Accepted: February 26, 1999
Published online: April 15, 1999
AIM To detect hepatitis A virus-specific immunoglobulin M (IgM) antibody rapidly.
METHODS Colloidal gold with an average dia-meter of 15 nm was prepared by controlled reduction of a boiling solution of 0.2 g/L- chloroauric acid with 10 g/L-sodium citrate and labeled with anti-HAVIgG as gold probe. Dot immunogold filtration assay (DIGFA) has been developed by coating anti-human μ chain on nitrocellulose membrane (NCM) for capturing the anti-HAV IgM in serum, then using cultured hepatitis A antige n as a “bridge”, connecting anti-HAV IgM in sample and anti-HAV IgG labeled colloidal gold. If there was anti-HAV IgM in sample, gold probes would concentrate on NCM, which will appear a pink dot.
RESULTS A total of 264 serum samples were comparatively detected with both DIGFA and ELISA by “blind” method. Among them, 88 were positive and 146 were negative with the two methods. The sensitivity and the specificity of DIGFA were 86.27% and 90.12%, respectively. Fifteen negative serum samples and 15 positive serum samples were detected 3 times repeatedly, the results were the same.
CONCLUSION DIGFA is a simple, rapid, sensitive, specific and reliable method without expensive equipment and is not interfered with rheumatoid factor (RF) in serum. It is suitable for basic medical laboratories. The test could be applied for diagnosis and epidemiological survey of hepatitis A. It has a broad prospect in applica-tion
- Citation: Wu W, Xu DZ, Yan YP, Zhang JX, Liu Y, Li RL. Evaluation of dot immunogold filtration assay for anti-HAV IgM antibody. World J Gastroenterol 1999; 5(2): 132-134
- URL: https://www.wjgnet.com/1007-9327/full/v5/i2/132.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i2.132
Hepatitis A virus-specific immunoglobulin M antibody (anti-HAV IgM) is the specific serological marker for the early diagnosis of acute hepatitis A. It can be detected by radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), solid phase hemagglutination inhibition test (SPHIA) and other methods. At present, double sandwich ELISA is in widespread use[1]. However, it takes more time to finish the test and the procedure is complicated. The need of a simple, rapid, and noninstrumented test is evident in many basic units, where laboratory facilities and trained personnel are limited. In 1989, Chun developed a rapid test, DIGFA[2]. It has been used to detect HCG, C-reac tive protein, immunoglobulin G antibody and others[3,4]. We developed DI GFA for detection of anti-HAV IgM. The evaluation of this test is presented below.
Agents Gold chloride (HAuCl4) was produced by Shanghai No.1 Chemical Reagent Factory, and nitrocellulose membrane by Amersham International Co. (England). Anti-human μ chain was obtained from DAKO Co. (Denmark). Anti-HAV IgG and cell cultured hepatitis A virus were prepared by Nanjing Military Medical Institute. ELISA kits for detection of anti-HAV IgM and anti-HBcIgM were produced by Shanghai Kehua Biotech. Co., Ltd, and ELISA kits for detection of anti-HCV by Sino-American Biotechnology Co.
Samples From 1994 to 1997, 137 serum samples of acute viral hepatitis were collected from 5 hospitals in Xi’an city. During the outbreak of hepatitis A in a college in 1997, sera of 14 patients, 40 contacts and 33 classmates with no contact history were collected. Ten RF positive sera were obtained from Xijing Hospital. Sera of 30 normal blood donors were taken from blood bank of Xijing Hospital. All samples were stored at -20 °C.
Preparation of gold probe Colloidal gold with an average diameter of 15 nm was prepared by controlled reduction of a boiling solution of 0.2 g/L-chloroauric acid with 10 g/L-sodium citrate according to the method of Frens[5]. The quality of colloidal gold was controlled by transmission electron microscope (TEM) and spectrophotometers. Freshly prepared 10 g/L-K2CO3 solution was added into colloidal gold to adjust its pH. The minimal protecting amount was determined by constructing a concentration variable isotherm (CVAI)[6]. Anti-HAVIgG was added while stirring. It was incubated for 5-10 min at room temperature and followed by addition of bovine serum albumin (BSA) to final concentration of 10 g/L. After this was centrifuged, supernatant was discarded and the pellet was suspended. Gold probe obtained by the method described above was stored at 4 °C.
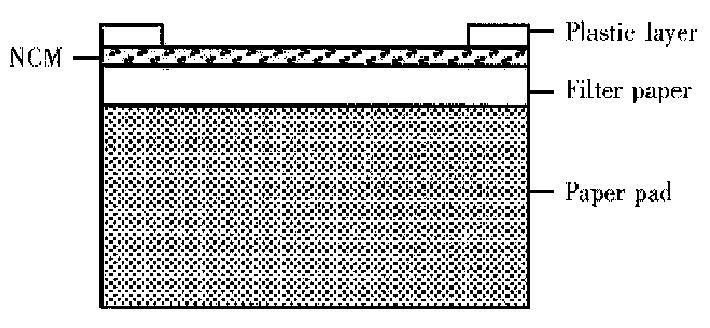
DIGFA An immunofiltration system containing a NCM was coated wi th anti-human μ chain, filter paper and an adsorbent pad . The NCM was positioned over an absorbent pad covered by a plastic layer with a hole exposing the NCM (Figure 1). A drop of liquid positioned over the hole was rapidly sucked through the NCM. Thus, anti-HAVIgM in serum was captured by anti-human μ chain on the NCM. Sequentially, hepatitis A antigen and gold probe were added. Cultured hepatitis A antigen as a “bridge” connected anti-HAV IgM in sample and anti-HAV IgG labeled colloidal gold. If there was anti-HAV IgM in sample, gold probes would concentrate on NCM, which would present a pink dot. The assay was routinely performed as follows. The membrane was activated by adding one drop of washing solution. Then 15 μL-serum was added. After it was soaked in, one drop of washing liquid was added and followed by 15 μL-cultured hepatitis A antigen. After it was soaked in, one drop of washing liquid were added, and gold probe and after this was soaked in, one drop of water was added. The process was completed within 10 min at room temperature.
ELISA All commercial kits were used according to their manufac turers’ instruction.
Statistical analysis The following definitions and formulae wer e used in the analysis of the data generated in this study. A true-positive (a) sample was reactive by DIGFA and ELISA. A true-negative (d) sample was nonreactive by DIGFA and ELISA. A false-positive (b) sample was reactive by DIGFA but negative by ELISA and a false-negative (c) sample was negative by DIGFA but positive by ELISA (Table 1). The sensitivity of DIGFA was defined as the probability that a sample containing anti-HAV IgM would be positive in DIGFA. The specificity of DIGFA was defined as the probability that a sample without anti-HAV IgM would be negative in DIGFA. The formulae are shown in Table 2.
| ELISA | Total | |||
| + | - | |||
| DIGFA | + | a | b | a+b |
| - | c | d | c+d | |
| Total | a+c | b+d | N | |
| Index | Formulae | Index | Formulae |
| Sensitivity | a/(a+c) | Specificity | d/(b+d) |
| Positive predictive value | a/(a+b) | Negative predictive value | d/(c+d) |
| Accuracy | (a+d)/N | Youden index | [a/(a+c)]/[d/(b+d-1)] |
| Positive likelihood ratio | [a/(a+c)]/[b/(b+d)] | Negative Likelihood ratio | [c/(a+c)]/[d/(b+d)] |
A total of 264 serum samples were comparatively detected with both DIGFA and ELISA by “blind” method, 88 were positive and 146 were negative (Table 3). The sensitivity, the specificity, the accuracy, the positive and the negative predi ctive value, the positive likelihood ratio, the negative likelihood ratio and Youden index of DIGFA were 86.27%, 90.12%, 88.64%, 84.62% 91.25%, 8.735, 0.1523, 0.7639, respectively. Sensitivity, specificity and accuracy (95%CI) were 75.59%, 92.95%, 85.53%, 94.71% and 84.81%, 92.45%, respectively. Serum samples of 34 hepatitis B, 15 hepatitis C and 10 RF positive patients were deter-mined with DIGFA, none of them was positive.
| ELISA | Total | |||
| + | - | |||
| DIGFA | + | 88 | 16 | 104 |
| - | 14 | 146 | 160 | |
| Total | 102 | 162 | 264 | |
NCM coating anti-human μ chain and gold probe were kept at 4 °C. In order to establish the stability of the reagents, 15 negative and 15 positive serum samples were selected at random and detected 3 times once a month. All results were the same. It indicates that the reagents are stable at least for 3 months at 4 °C and repeatable.
Incidence of hepatitis A is very high in developing countries, especially in poor hygiene area. In order to control epidemic of hepatitis A, it is very important to detect anti-HAV IgM as early as possible. DIGFA is a rapid method, requiring 10 min to perform. Additionally, it is simple enough to be used by paramed ical personnel without special training. It allows reliable measurement of anti-HAV IgM from a small volume of serum (15 μL) without using special equipment. DIGFA showed very good performance. Its sensitivity and specificity are similar to that of ELISA. Rheumatoid factor, anti-HB-cIgM and anti-HCV do not markedly interfere with the assay. It attributes to double sandwich method used in DIGFA and cell cultured hepatitis A antigen which is purer. The sample is drawn quickly through the porous membrane, thereby reducing low-affinity nonspecific binding and minimizing chemical interference. However, if there is fibrin clot or much blood lipid in serum, they will interfere with reaction between antigen and antibody, and the drawing rate of NCM, which will result in false positive or false negative. So, before detection, such sera must be centrifuged with fibrin clot and blood lipid removed.
In brief, rapid and simple procedure, with the visual interpretation of results and reagent stability make DIGFA particularly suitable for field testing and epi demiological surveys.
Edited by Xian-Lin Wang
| 1. | Guo KS. Detection of virus-specific immunoglobulin antibody. Chin J Exp Clin Virol. 1988;2:79-81. |
| 2. | Urdal P, Borch SM, Landaas S, Krutnes MB, Gogstad GO, Hjortdahl P. Rapid immunometric measurement of C-reactive protein in whole blood. Clin Chem. 1992;38:580-584. [PubMed] |
| 3. | Beristain CN, Rojkin LF, Lorenzo LE. Evaluation of a dipstick method for the detection of human immunodeficiency virus infection. J Clin Lab Anal. 1995;9:347-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Spielberg F, Kabeya CM, Ryder RW, Kifuani NK, Harris J, Bender TR, Heyward WL, Quinn TC. Field testing and comparative evaluation of rapid, visually read screening assays for antibody to human immunodeficiency virus. Lancet. 1989;1:580-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature Phys Sci. 1977;241:20-22. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5277] [Cited by in RCA: 4327] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 6. | Horisberger M, Rosset J. Colloidal gold, a useful marker for transmission and scanning electron microscopy. J Histochem Cytochem. 1977;25:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 407] [Article Influence: 8.5] [Reference Citation Analysis (0)] |