Published online Oct 15, 1998. doi: 10.3748/wjg.v4.iSuppl2.41
Revised: July 4, 1998
Accepted: August 1, 1998
Published online: October 15, 1998
AIM: To demonstrate whether bcl-2 gene can affect or alter biological behavior of a stomach carcinoma cell line MGC-803.
METHODS: To transduct a retrovirus containing bcl-2 antise nse RNA to MGC-803 cells and then to analyse the Bcl-2 protein expression in t he cells by Western blotting. To observe the morphology alteration, detect the G1 phase arrest by FCM, inhibition of proliferation by MTT method and tumorigenicity in nude mice.
RESULTS: The MGC-anti-bcl-2 cells shows contact inhibition, morphological alteration, from round or near round to shuttle like, decelerated growth rate, G1 phase arrest and weakened tumorigenicity in nude mice unlike the control (MGC-neo cells).
CONCLUSION: Our data show that antisense RNA to bcl-2, not only can induce apoptosis, but also reverse the biological behavior of MGC-803 cells. This would be a potential application to the gene therapy for stomach cancers.
- Citation: Cao GD, Wang SW, Wu SS, Li HF, Zhang WG. Retrovirus-ediated antisense RNA to bcl-2 alter the biological behavior of stomach carcinoma MGC-03 cell lines. World J Gastroenterol 1998; 4(Suppl2): 41-44
- URL: https://www.wjgnet.com/1007-9327/full/v4/iSuppl2/41.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.iSuppl2.41
bcl-2 gene, first detected as a putative oncogene located near to the breakpoint of t (14,18)(q32, q21) translocations in human follicular lymphoma[10], was the first one shown to be involved in the regulation of apoptosis[13]. Bcl-2 is expressed widely in tissues derived from all three germ layers, but its expression become more restricted during development[6]. The bcl-2 gene has been implicated in the oncogenicity of a wide variety of hematological malignancies and cancers including melanoma, breast, lung, prostate, gastric and bowel carcinoma. Overexpression of BCL-2 protein could promote cell survival or prevent apoptosis induced a variety of stimuli, including grow th factor deprivation, γ-irradiation, glucocorticoids, heat shock, and multiple chemotherapeutic agents[5,9,11]. There is strong in-vitro evidence that full phosphorothioated antisense oligonucleotides complementary to the open reading frame of the bcl-2 mRNA can downregulate BCL-2 protein expression, which results in reduced cell viability and induces apoptosis[1]. A phase I trial shows that in patients with relapsing non-Hodgkin lympho ma, bcl-2 antisense therapy led to an improvement in symptom, objective bio chemical evidence of tumor response, and down-regulation of the BCL-2 protein[12]. All those reports were only concerned about the relationship between bcl-2 to inhibition of apoptosis, and antisense bcl-2 to apoptosis. Whether bcl-2 antisense RNA or oligonucleotide can alter biological behavior of cells have not been reported. Here we report that retrovirus-mediated antisense RNA to bcl-2 can alter the biological behavior of MGC-803 cells (a bcl-2 positive stomach carcinoma), including alteration of morphology and cell cycle, and weanened tumorigenicity in nude mice.
Human bcl-2, a 1.9 kb long EcoR I cDNA, were cloned into the retroviru svector pLXSN. The reverse inserted recombinants were indentified by BamH I digestion and PCR methods using downstream primer of bcl-2 and upstream primer of pLXSN vector near polycloning sites. Purified DNA of pLXSN vector or vector containing the antisense bcl-2 cDNA were transfected into packaging cell lines PA317 using lipofectin according to the manufacturers instructions and G4 18 resistant colonies were obtained. To select the colonies with the highest titer, collect supernanants and used to transduce stomach carcinoma MGC-803 cells. G4 18 resistant colonies were picked and propagated in the selective medium (400 μg/mL of G418).
Exponentially growing cells were harvested into lysis buffer containing 50 mmol/L Tris, 2% SDS, 10% glycerol and 100 μg/mL PMSF, boiled for 10 min and centrifuged at 10000 g. After determining protein concentration, 50 μg of protein were electrophoresed on 12% SDS-PAGE and transferred to nitrocellulo se membrane by electoblotting. Membrane were blocked with 3% defatted milk power and then incubated with an anti-bcl-2 polyclonal antibody. Following incubati on with a biotin-rabbit secondary antibody and SA-AP, antigen-antibody complexes were detected by adding BCIP and NBT.
Human stomach carcinoma MGC-803 transduced with virus expressing bcl-2 antisen se RNA and neo were plated in six-well plates at a density of 5 × 104 cells/well. Cells number were measured by direct cell counting after being washed, trypsinized and stained with trypan blue every 24 h up to 4 d after plating. The experiment were performed in triplicates and reproduced three times.
Human stomach carcinoma MGC-803 transduced with virus expressing bcl-2 antisense RNA and control pLXSN virus were plated in six-well plates at a density of 1 × 105 cells/well. Cell were collected at 70% confluence, or at 100% confluence with additional culturing for 24 h respectively, and then fixed with 75% ethanol at 4 °C overnight. After centrifuged and washed with PBS, the cells were digested with RNase A (20 μg/mL) at room temperature for 1 h, and then resuspended in solution containg 50 μg/mL of propidium iodied, 0.3% sodium citrate, and 0.01% Triton X-100. Cell cycle were analyzed on the FACScan using lysis II software.
Four-week-old athymic nude mice were supplied by Chinese Institute of Cancer. The transduced MGC-neo and MGC-anti-bcl-2 cells were propagated, trypsized and resuspended in Hank’s balanced salt solution at a concentration of 2.5 × 1 07 cells/mL, 5 × 106 of MGC-anti-bcl-2 and MGC-neo cells were injected into the right and left flank of nude mice respectively. After sacrifice, the tumor mass were weighted. The significance of differences in tumor size between control and bcl-2 antisense RNA virus transduced group was evaluated using paired t test.
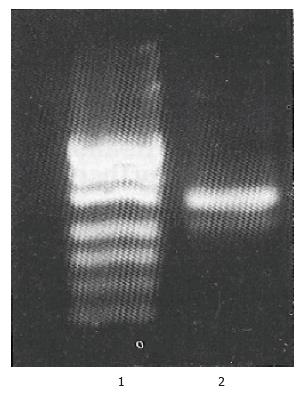
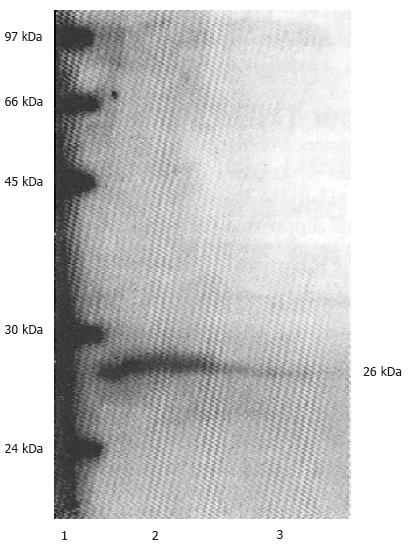
MGC-803 was a stomach carcinoma cell line which established by Shandong Institute of cancer, China in 1983. It has not been reported that whether MGC-803 cell express bcl-2 mRNA and protein or not. RT-PCR was performed using GAPDH as internal control (Figure 1), and the mRNA proportion of bcl-2 to GAPDH (BCL-2/GAPDH) analyzed by laser scanning is 0.25. A decreased level of BCL-2 protein was detected in MGC-803 cells after being transduced with retrovirus expressing bcl-2 antisense RNA by Western blot analysis (Figure 2). Our results showed that MGC-803 cells expresses both bcl-2 mRNA and protein in a high level, retrovirus-mediated bcl-2 antisense RNA was expressed and coul d down-regulate BCL-2 protein expression in MGC-803 cells.
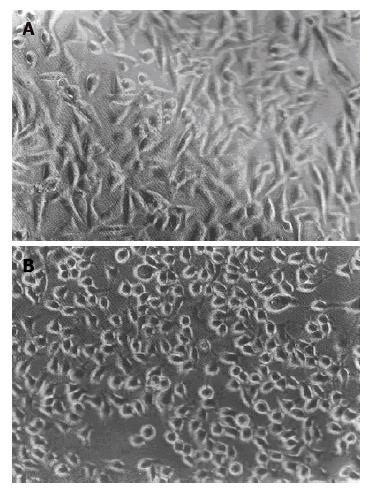
MGC-803 was an anchorage-dependent, round or a slightly flat cells. After being transduced with retrovirus expressing antisense bcl-2 RNA, morphology of MGC-803 cell altered, from round or slightly flat to shuttle-like, and it become larger than the parental MGC-803 or control MGC-neo cells (Figure 3). Contact inhibition was also observed in MGC-anti-bcl-2 cells. The result shows that MGC-803 cells transduced with retrovirus expressing antisense bcl-2 RNA was prone to differentiate. Vauk et al (1988) reported that bcl-2 did not morphological transform NIH3T3 fibroblasts, and our data showed that bcl-2 antisense RNA also did not alter T-cell leukemia Jurkat morphologically ( data not shown). The mechanism that bcl-2 antisense RNA alter morphologically MGC-803 cells remains for further investigation.
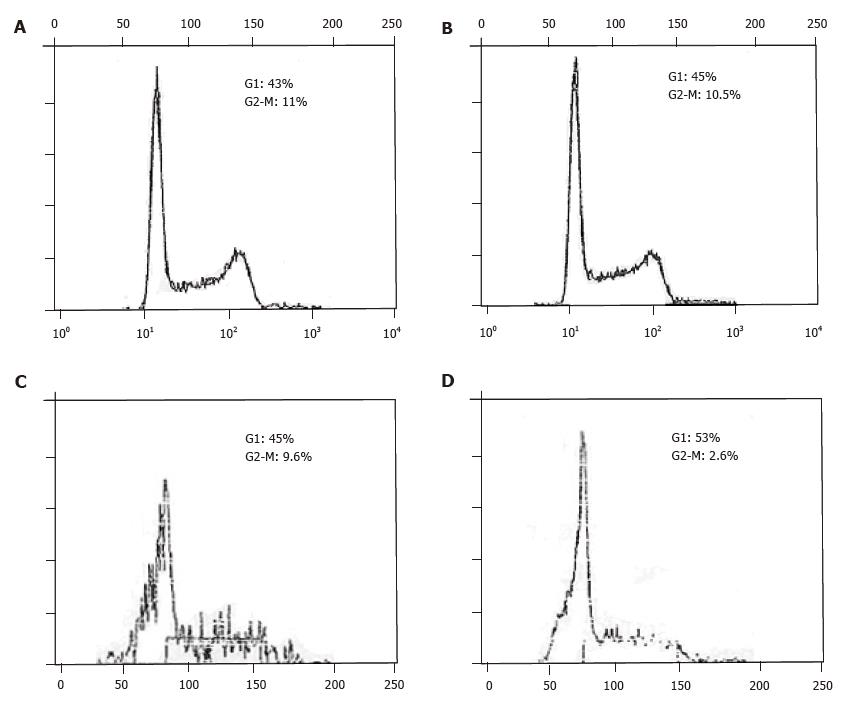
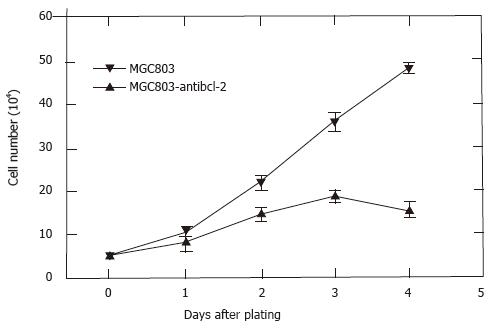
Cell cycle analysis of cells in logarithmic growth showed that antisense bcl-2 RNA had not altered the distribution of MGC-803 cells, the main proportion of MGC-anti-bcl-2 and MGC-neo cells in G1 and G2-M were as follows respectively: 45%, 10.5% and 43%, 11%. However, antisense bcl-2 RNA cause G1 phase arrest in MGC-anti-bcl-2 cells if cells were continued to culture for 24 h after reaching 100% confluent, the percentage of G1 and G2-M phase were 53%, 2.6%, and 45%, 9.6% for MGC-anti-bcl-2 and MGC-neo cells respectively (Figure 4). To study whether bcl-2 antisense RNA had effect on proliferation, the cell number was counted directly after being stained with trypan blue. The MGC-anti-bcl-2 cells grew slowly and ceased growing when it reached 100% confluent at the third day, while the control MGC-neo cells grew rapidly and continued growing even it was 100% confluent (Figure 5). All those data show that bcl-2 antisense RNA inhibit proliferation of MGC-803 cells, and cause G1 phase arrest.
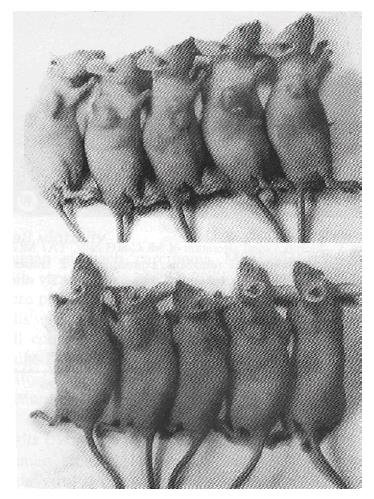
We tested the tumorigenicity in vivo of MGC-803 cells transduced with virus expressing antisense RNA to bcl-2 and with pLXSN virus as control in nude mice. Subcutaneous injection of MGC-anti-bcl-2 and MGC-neo cells into nude mice both resulted in the development of tumors, but the weight of tumors, 330.6 ± 69.4 mg and 496.5 ± 92.5 mg respectively, has significant difference (P < 0.05, P = 0.028). This indicated that the tumorigenicity of MGC-803 cells was weakened by retrovirus-mediated antisense RNA to bcl-2 (Figure 6).
In this report, we have investigated the effect of retrovirus mediated antisense RNA to bcl-2 on biological behavior of stomach carcinoma MGC-803 cell lines. The results of these studies shows that bcl-2 antisense RNA can inhibit the expression of BCL-2 protein in MGC-803 cells, by which it cause morphological alteration, from round or a slightly round to shuttle-like, enlargement of cellular volume, G1 phase arrest, Contact inhibition and weakened tumorigenicity in nude mice. All these data indicate that bcl-2 antisense RNA can inhibit proliferation and alter the biological behavior of MGC-803 cells.
c-myc gene plays an important role in determinating the deovelpment of cells to proliferate, silent, differentiate or die depending on the stimuli given. c-myc can provide the first signal, leading to apoptosis or to progression, and bcl-2 may provide a second signal to inhibit apoptosis and allows c-myc to drive cells into the cell cycle[2]. Bcl-2 has synergy with c-myc to promote proliferation of pre-B cells[12]and to markedly enhance the tumorigenicity in athymic nude mice[7]. The transduced retro virus can express bcl-2 antisense RNA, and downregulate BCL-2 protein expression in MGC-803 cells, thus the synergy between bcl-2 and c-myc was aborted and cause reversion of bilogical behavior. In addition, bcl-2 can modulate p53 fuction by altering p53 subcellular trafficking during cell cycle[8] and block or delay the apoptosis induced by p53, ICE and other apoptosis-promoting genes[4]. All these data make us to hypothese that retrovirus mediated bcl-2 antisense RNA inhibit the expression of BCL -2 protein, and upregulation of genes which were associated with cell prolifera tion or cell cycles caused by supression of BCL-2 protein alter the biological behavior of MGC-803 cells. The actual mechanism remains for further investigation.
E- Editor: Li RF
| 1. | Cotter FE, Johnson P, Hall P, Pocock C, al Mahdi N, Cowell JK, Morgan G. Antisense oligonucleotides suppress B-cell lymphoma growth in a SCID-hu mouse model. Oncogene. 1994;9:3049-3055. [PubMed] |
| 2. | Bissonnette RP, Echeverri F, Mahboubi A, Green DR. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992;359:552-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 719] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 3. | Fisher TC, Milner AE, Gregory CD, Jackman AL, Aherne GW, Hartley JA, Dive C, Hickman JA. bcl-2 modulation of apoptosis induced by anticancer drugs: resistance to thymidylate stress is independent of classical resistance pathways. Cancer Res. 1993;53:3321-3326. [PubMed] |
| 4. | Hale AJ, Smith CA, Sutherland LC, Stoneman VE, Longthorne VL, Culhane AC, Williams GT. Apoptosis: molecular regulation of cell death. Eur J Biochem. 1996;236:1-26. [PubMed] |
| 5. | Miyashita T, Reed JC. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993;81:151-157. [PubMed] |
| 6. | Novack DV, Korsmeyer SJ. Bcl-2 protein expression during murine development. Am J Pathol. 1994;145:61-73. [PubMed] |
| 7. | Reed JC, Cuddy M, Haldar S, Croce C, Nowell P, Makover D, Bradley K. BCL2-mediated tumorigenicity of a human T-lymphoid cell line: synergy with MYC and inhibition by BCL2 antisense. Proc Natl Acad Sci USA. 1990;87:3660-3664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Ryan JJ, Prochownik E, Gottlieb CA, Apel IJ, Merino R, Nuñez G, Clarke MF. c-myc and bcl-2 modulate p53 function by altering p53 subcellular trafficking during the cell cycle. Proc Natl Acad Sci USA. 1994;91:5878-5882. [PubMed] |
| 9. | Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 881] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 10. | Tsujimoto Y, Croce CM. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci USA. 1986;83:5214-5218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 744] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 11. | Tsujimoto Y. Stress-resistance conferred by high level of bcl-2 alpha protein in human B lymphoblastoid cell. Oncogene. 1989;4:1331-1336. [PubMed] |
| 12. | Webb A, Cunningham D, Cotter F, Clarke PA, di Stefano F, Ross P, Corbo M, Dziewanowska Z. BCL-2 antisense therapy in patients with non-Hodgkin lymphoma. Lancet. 1997;349:1137-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 322] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2187] [Cited by in RCA: 2230] [Article Influence: 60.3] [Reference Citation Analysis (0)] |