Published online Dec 15, 1998. doi: 10.3748/wjg.v4.i6.550
Revised: August 20, 1998
Accepted: November 12, 1998
Published online: December 15, 1998
- Citation: Zhong SQ, Xu YD, Zhang YF, Zhang YF, Hai LS, Tang FC. Three-dimensional structure of lymphatics in rabbit stomach. World J Gastroenterol 1998; 4(6): 550-552
- URL: https://www.wjgnet.com/1007-9327/full/v4/i6/550.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i6.550
Recently, the stomach lymphatics have been studied, but there are different opinions on the lymphatic distribution of the stomach layers[1-6]. There has been no reported in China. Discribing the three-dimensional organization of the stomach lymphatics and revealing the correlation of the three-dimensional and the two-dimensional and organization.
In our study in the rabbit with the lymphatic corrosion cast with Mercox and semithin section methods we investigated the relationship of the three-dimensional organization with the drainage of the stomach lymphatics, which may provide the evidences of lymphology, pathology and the clinical medicine.
Twelve rabits of both sexes were used, two of them, undergone the procession of the seemitin section of electron microscopy, were observed under light microscopy. The other ten were used for the lymphatic corrosion casts.
The Mercox (CL-2B-5, Japan Velene Hospital, Tokyo,) diluted to 25%-30% (V/V) with methyl methacrylate monomer was injected in and around the mucosal submucosal, layers of the stomach. Shortly before the injection, a curing agent (MA. Japan Vilene Hospital Tokyo) was added to the injection medium to give a concentration of 1% (W/V). The injected parts of the stomach were removed and placed in a hot water bath (60 °C) for 3 h. They were put in concentrated NaOH (15%-20%) at about 60 °C until tissue elements were completely corroded away. The lymphatic corrosion casts were cut into blocks and observed under a SEM (S-520) (with an accelerating voltage of 10-15 kv).
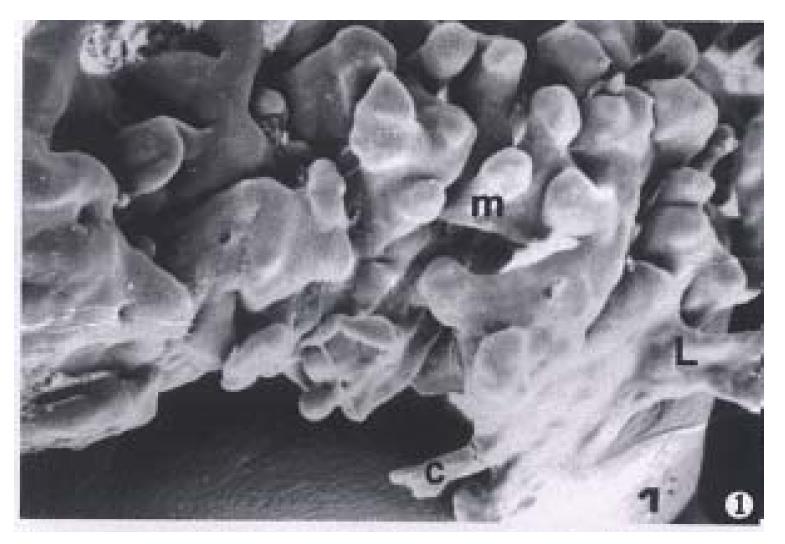
The samples filled with resin which were in the mucosal and the submucosal layers clearly showed the three-dimensional organization of the lymphatic capillaries and the lymphatics. There was a layer of the lymphatic capillary network in the deep layer of the tunica mucosa between the bottoms of gastric gland and the muscularis mucosa. The networks extended short tube with blind ends into gastric glands. The tubers were called intergland circular cones. which were 20 μm-30 μm in diameters. The cones were round, hook, V and finger in shap. In the cardia and the fundus of the stomach the cones were sparse and connected to the lymphatic capillary networks of the tunica mucosa. In the body of the stomach 2 or 3 circular cones were connected in one group. The roots of the circular cones were connected to the sinus (50 μm-60 μm in diameters), then drained to the lymphatic capillary networks of the tunica mucosa (Figure 1).
Donini has observed the lymphatic capillary networks of the subepithelium were in the stomach pylorus. We observed the lymphatic capillaries between the bottoms of the tunica mucasa gland and the muscularies mucosa, but did not observe the lymphatic capillaries were in the subepithelium. Our observations were smilar to Han’s studies[5]. We found the lymphatic capillaries were in the tunica mucoa, but no thick lymphatics. A large number of lymphatic capillaries and lymphatics were found in the tela submucosa. The lymphatic capillaries formed a coarse network. The lympahtics (vessels) also formed a coarse plexus. The corrosion casts of the lymphatic clearly showed the three-dimensional organization of the lymphatics. The diameter of the lymphatic capillary was 10 μm-30 μm, but the lymphatic vessel’s diameter was 30 μm-100 μm. The size of the meshes of the lymphatic vessels were varied and interconnected, triangular, oval and polygon in shape. The semithin sections also showed rich lymphatic capillaries and lymphyatic vessels in the stomach tela submucosa. On the surface of the lymphatic casts, we found marked constrictions characteristric of bicuspid valves (Figure 2).
A rich lymphatic capillaries and lymphaties were found in the stomach tunic muscularis. Some the lymphatic capillaries of 7 μm-30 μm in diameter extended the short branches with blind ends. The diameter of the lymphatic was 30 μm-80 μm. Between three muscular layers there were lymphatic capillaries and the lymphatic vessels. The lymphatics were string of beads in shape and interconnected to plexus. There were break ends of the anastomotic channels to the superficial and the deep part from the lymphatics of the tunic muscularis. It suggested that the lymphatics of the tunica muscularis were interconnected with both the lymphatics of the tela submucosa and the lymphatics of the tunica serosa. The surface of the lymphatic casts in the tunica muscularis there were folds which run parelled to the lymphatic major axis. The imprints of endothelial nuclei were denser than other layers. On the surface, we could see the transverse imprints which were induced by the smooth muscle contraction. Between the lymphatic capillaries and lymphatic vessels of the tunica muscularis there were anstomotic channels which existed in each layer of perimysium. The lymphatics and the lymphatic capillaries of the tunica muscularis were seen in the histological sections.
Octrovekhov thought that there was not any lymphatic capillary. But Nariadchikova pointed out that the lymphatic vessels and the lymphatic capillaries of the tunica muscularis only existed among the three layer’s muscularis but not in each muscular layer. Our experiment proved that in the connective tissue there were both lymphatic capillaries and lymphatic vessels in each smooth muscularis layer. We also observed the lymphetic capillaries among perifascicular parts of each muscular layer.
There were both the lymphatic capillaries and the lymphatic vessels in a deep part of the tunica serosa (Figure 3). The meshes of the lymphatic capillary nextwork and the lymphatic plexus in the layer were larger than those of the ttela submucosa and the tunic musecularis. The meshes presented in willow leaf, oval or trianglar shape. The lymphatic capillaries and the lymphatic vessels were also observed in the semithin sections of the layers under the light microscopy. Donin reported that only in the curvatura veutriculi minor and major did the lymphatic capillaries of the tunica serosa exist. Rakhan tought the lymphatic capillaries of the tunica serosa only existed in the parts of pylorus. Ohtani[3] pointed out that only the lymphatic vessels existed in the longitudinal muscle layer. In our lymphatic casts and semithin setions we observed both the lymphatic capillaries and the lymphatic vessels existed in the tunica serosa (Figure 4).
Project supported by the National Natural Science Foundation of China, No. 39070462
| 1. | Wassilev W, Wedel T, Michailova K, Kühnel W. A scanning electron microscopy study of peritoneal stomata in different peritoneal regions. Ann Anat. 1998;180:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Ji RC, Kato S. Enzyme-histochemical study on postnatal development of rat stomach lymphatic vessels. Microvasc Res. 1997;54:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Ohtani O, Kikuta A, Ohtsuka A, Taguchi T, Murakami T. Microvasculature as studied by the microvascular corrosion casting/scanning electron microscope method. I. Endocrine and digestive system. Arch Histol Jpn. 1983;46:1-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 50] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Kato S. Histochemical localization of 5'-nucheotidase in the lymphatic endothilium. Acta Histochem Cytochem. 1990;23:613-617. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Han MD, Wang YX, Zhao LH. Ultrastructure of intramural lymph capillar-ies of the stomach in the rat. Acta Anatomy Sinica. 1990;23:129-133. |
| 6. | Zhao LH, Wang YX, Wang GX. Fine distribution of the lymph vessels in the stomach as studied by enzyme-histochemical method. Acta Anatomy Sinica. 1993;24:97-100. |