Published online Dec 15, 1998. doi: 10.3748/wjg.v4.i6.461
Revised: November 20, 1998
Accepted: December 2, 1998
Published online: December 15, 1998
- Citation: Baldwin GS, Shulkes A. Gastrin as an autocrine growth factor in colorectal carcinoma: implications for therapy. World J Gastroenterol 1998; 4(6): 461-463
- URL: https://www.wjgnet.com/1007-9327/full/v4/i6/461.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i6.461
There is now considerable experimental support for the hypothesis that progastrinderived peptides stimulate proliferation of the normal colonic mucosa[1], and act as autocrine growth factors in colorectal carcinoma (CRC). In a previous review[2], we summarized the evidence for the presence of progastrin-derived peptides and their receptors in CRC, and presented a model in which amidated and non-amidated progastrin-derived peptides stimulate proliferation of CRC cells via distinct receptor classes. In this editorial we will consider the various strategies available to interfere with the individual components of the autocrine loop, and the potential of the strategies to yield novel diagnostic and therapeutic agents.
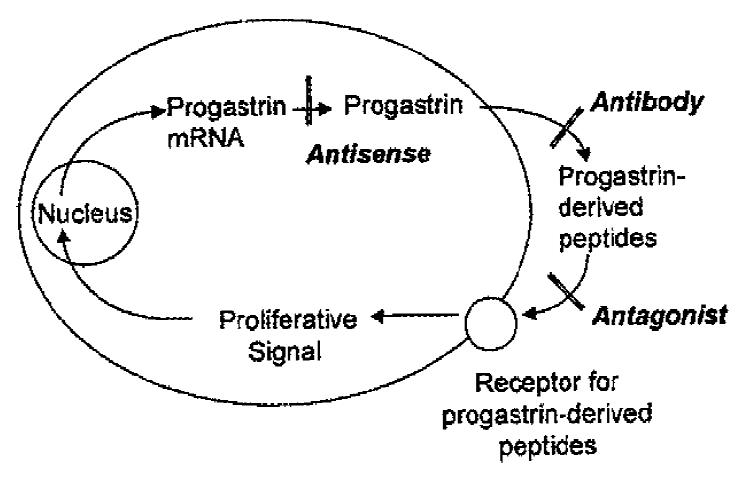
In the autocrine model (Figure 1) a cell synthesises its own growth factor, which is released into the surrounding medium. Binding of the growth factor to cell surface receptors then results in the transmission of a mitogenic signal to the cell nucleus, with a consequent increase in cell proliferation. In principle, autocrine loops could be disrupted in at least 3 ways:
1. By reduction of growth factor mRNA with introduction of antisense RNA or oligonucleotides,
2. By reduction of the concentration of extracellular growth factor by treatment with specific antibodies, or
3. By blockade of growth factor receptors with selective antagonists.
In addition the presence of elevated concentrations of tumour-derived growth factors in the sera of patients with CRC may offer a sensitive method of tumour detection, via the development of
Antisense experiments have provided clear evidence for the involvement of progastrinderived peptides in an autocrine loop in some cell lines of colonic origin. Expression of antisense gastrin mRNA reduced in vitro growth of the CRC cell lines Colo 320 and HCT 116[3]. and of the conditionally immortalized mouse colon cell line YAMC[4]. The ability of HCT 116 cells to grow as tumours in nude mice was also reduced by antisense gastrin mRNA expression[3]. In control experiments in vitro and in vivo growth of the CRC cell line Colo 205A, which expressed negligible amounts of gastrin mRNA prior to transfection, was unaffected by expression of antisense gastrin mRNA[3]. However the inherent difficulty of selectively targeting antisense constructs to tumour cells may delay development of related clinical therapies.
The question of whether or not CRCs produce progastrin-derived peptides has been controversial, at least partly because of the large number of potential products of the gastrin gene[2]. Progastrin is processed to amidated gastrin via a number of intermediates which include glycine-extended gastrins. Some early reports were confined to measurement of amidated forms of gastrin only, and the variable extent of postranslational processing of progastrin in peptide-producing tumours may explain some of the negative findings reported in the literature. Progastrin or progastrin-derived peptides are now detected in 80%-100% of CRCs[2]. The concentrations of progastrin-derived peptides in the serum of patients with CRC are also elevated between 2.3-fold (H. pylori negative) and 5.2-fold (H. pylori positive)[5]. The availability of a panel of antibodies recognizing different regions of intact progastrin, and of antibodies selective for individual progastrin-derived peptides, may permit the early diagnosis of CRC by radioimmunoassay of serum samples. In this context a large prospective study has recently indicated that hypergastrinaemia was associated with a 3.9-fold increase in the risk of later development of CRC[6].
Antibodies against progastrin-derived peptides may also be useful for treatment of CRC. The proliferation of some, but not all, CRC cell lines was inhibited by antibodies recognizing the C-terminal amidated tetrapeptide of gastrin[2]. On the other hand, proliferation of the mouse colon cell line YAMC was inhibited by antibodies recognizing glycine-extended, but not amidated, gastrins[4]. A promising approach to future therapy has been provided by the observation that preimmunization of rats with Gastrimmune (a conjugate of amino acids 1-9 of gastrin17 and diphtheria toxoid which recognises both gastrin17 and gastrin17-gly) reduced the in vivo growth of the rat CRC cell line DHDK12, either alone or in conjuction with 5-fluorouracil and leucovorin[7].
At least four receptors exist for the gastrin/CCK family of peptides 2. The CCK-A and gastrin/CCK-B receptors are specific for amidated peptides, while the glycine-extended gastrin receptor is selective for non-amidated forms of gastrin. The low affinity gastrin/CCK-C receptor binds amidated and non-amidated forms of gastrin with equal affinity. While the nonselective antagonists proglumide and benzotript inhibit the binding to gastrin/CCK-A, B and C receptors, antagonists selective for either-A or -B receptors have also been developed[2].
The non-selective antagonists proglumide and benzotript inhibit proliferation of many gastrointestinal carcinoma cell lines both in vitro and in vivo[8]. Comparison of the inhibitory potencies of proglumide, benzotript and other selective gastrin/CCK receptor antagonists with receptor affinities suggests that the gastrin/CCK-C receptor is the probable target[9]. However a clinical trial of proglumide in patients with gastric carcinoma did not reveal any benefits, perhaps because the concentrations achieved were not sufficient to saturate gastrin/CCK receptors. Gastrin/CCK-B receptor antagonists have also been shown to inhibit the growth of some CRC cell lines in vitro, and of primary human CRCs in vitro and in vivo, but have not yet been subjected to clinical trials. However the observation that most CRCs do not express gastrin/CCK-B receptors indicates that it will be unlikely that gastrin/CCK-B receptorselective antagonists will be a general treatment for CRC[2].
Epidemiological studies have revealed that non-steroidal anti-inflammatory drugs (NSAIDs), and in particular aspirin, reduce by approximately 50% the risk of CRC and other cancers of the gastrointestinal tract[10]. The NSAID sulindac also reduces the size and number of colorectal polyps in patients with familial adenomatous polyposis, and inhibits the development of chemically-induced CRC in rodents. Although selective antagonists have indicated that the inducible isozyme cyclooxygenase-2 is one of the targets for the inhibitory effects of NSAIDs on CRC growth in vivo, several lines of evidence suggest that other targets may contribute to the anti-proliferative effects in vitro[10].
The gastrin/CCK-C receptor may be one such alternative target. All of a panel of 17 NSAIDs tested inhibited the binding of gastrin to the gastrin/CCK-C receptor with affinities which correlated well with their potencies as inhibitors of the proliferation of CRC cell lines[11]. The most potent antagonist of gastrin binding to date is sulindac sulphide, which has an IC50 value of 40 μM, and more potent antagonists of the gastrin/CCK-C receptor may well be of use in the treatment of CRC.
This editorial has summarized several promising avenues for future research into the effects of progastrin-derived peptides as autocrine growth factors in CRC. In particular the development of antibodies against progastrin-derived peptides, and of antagonists selective for progastrin-derived peptide receptors, may provide new opportunities for diagnosis and therapy of CRC.
| 1. | Wang TC, Koh TJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Dockray GJ. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest. 1996;98:1918-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 231] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Baldwin GS, Shulkes A. Gastrin, gastrin receptors and colorectal carcinoma. Gut. 1998;42:581-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Singh P, Owlia A, Varro A, Dai B, Rajaraman S, Wood T. Gastrin gene expression is required for the proliferation and tumorigenicity of human colon cancer cells. Cancer Res. 1996;56:4111-4115. [PubMed] |
| 4. | Hollande F, Imdahl A, Mantamadiotis T, Ciccotosto GD, Shulkes A, Baldwin GS. Glycine-extended gastrin acts as an autocrine growth factor in a nontransformed colon cell line. Gastroenterology. 1997;113:1576-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Ciccotosto GD, McLeish A, Hardy KJ, Shulkes A. Expression, processing, and secretion of gastrin in patients with colorectal carcinoma. Gastroenterology. 1995;109:1142-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 6. | Thorburn CM, Friedman GD, Dickinson CJ, Vogelman JH, Orentreich N, Parsonnet J. Gastrin and colorectal cancer: a prospective study. Gastroenterology. 1998;115:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 207] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Watson SA, Michaeli D, Grimes S, Morris TM, Robinson G, Varro A, Justin TA, Hardcastle JD. Gastrimmune raises antibodies that neutralize amidated and glycine-extended gastrin-17 and inhibit the growth of colon cancer. Cancer Res. 1996;56:880-885. [PubMed] |
| 8. | Hoosein NM, Kiener PA, Curry RC, Rovati LC, McGilbra DK, Brattain MG. Antiproliferative effects of gastrin receptor antagonists and antibodies to gastrin on human colon carcinoma cell lines. Cancer Res. 1988;48:7179-7183. [PubMed] |
| 9. | Baldwin GS. Antiproliferative gastrin/cholecystokinin receptor antagonists target the 78-kDa gastrin-binding protein. Proc Natl Acad Sci USA. 1994;91:7593-7597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Gupta RA, DuBois RN. Aspirin, NSAIDS, and colon cancer prevention: mechanisms? Gastroenterology. 1998;114:1095-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Baldwin GS, Murphy VJ, Yang Z, Hashimoto T. Binding of nonsteroidal antiinflammatory drugs to the alpha-subunit of the trifunctional protein of long chain fatty acid oxidation. J Pharmacol Exp Ther. 1998;286:1110-1114. [PubMed] |