Published online Oct 15, 1998. doi: 10.3748/wjg.v4.i5.434
Revised: June 22, 1998
Accepted: July 14, 1998
Published online: October 15, 1998
AIM: To study the specific inhibition of HBV gene expression by liver-targeting antisense oligonucleotide (ASON) directed against pre-c and cregious in a sequence specific manner.
METHODS: According to the result of direct sequencing of PCR amplified products, a 16-mer phosphorothioate analogue of the antisense oligonucleotide (PS-ASOn) directed against the HBV U-5-like region was synthesized and then linked with one live-targeting ligand, the galactosylated poly-L-lysine.Their effect on the expression of HBV gene was observed using the 2.2.15 cells.
RESULTS: HBV DNA in the 2.2.15 cells was from HBV with surface antigen subtype ayw 1 by sequencing so that antisense oligonucleotides could bind specifically to the target sequence through base piring. Under the same experimental conditions, the inhibitory rates of PS-ASON to HBsAg and HBeAg were 70% and 58% at a concentration of 10 μmol/L, while by ligand-PS-ASON they were 96% and 82%, the amount of HBV DNA in cultured supernatant and cells was reduced significantly. An unrelated sequence oligonucleotide showed no effectiveness. All the oligonucleotides had no cytotoxicity.
CONCLUSION: Antisense oligonucleotides complexed by the liver-targeting ligand can be targeted to cells via asialoglycoprotein receptors, resulting in supecific inhibition of HBV gene expression and replication.
- Citation: Zhong S, Wen SM, Zhang DF, Wang QL, Wang SQ, Ren H. Sequencing of PCR amplified HBV DNA pre-c and c regions in the 2.2.15 cells and antiviral action by targeted antisense oligonucleotide directed against sequence. World J Gastroenterol 1998; 4(5): 434-436
- URL: https://www.wjgnet.com/1007-9327/full/v4/i5/434.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i5.434
Many studies have shown that antisense oligonucleotides (ASONs) can effitiently inhibit HBV DNA replication and expression in vitro and may become a new generation of anti-HBV drugs[1]. However, as a potential therapeutic agent, synthetic ASONs must fulfil three main requirements (i.e., the three S rule): (1) solubility, they must be water soluble and yet cross the lipopholic cell menbrane; (2) stability, they must resist enzymatic degradation to reach the target at an effective concentration; and (3) selectivity, they must bind specifically tothe target sequence through base pairing.
Basing on the three S rule, we first synthesized a 16mer phosphorothioate analogue of ASON (PS-ASON) directed against the HBV U5-ulike region according to the sequencing result, and then linked PS-ASOn with one Liver-targeting ligand, the galactosylated poly-L-lysine (Gal-PLL). The effect of PS-ASON and ligand PS-ASON on the expression of HBV gene was observed and compared by using the 2.2.15 cells. The results of this experiment and reported below.
HBV DNA in the 2.2.15 cells was tested by PCR. Primers were designed according to the pre c and c regions of HBV genome. The sequences of primers are P1 (5’-TTCCCGATACAGAGCTGAGGCC) and P2 (5’-AAGGTCTTTGTAGGAGGC). PCR amplified products were purified with Magic TMPCR Preps DNA pure system (Promega). Direct sequencing of pure products was carried out according to the manual of Pharmacia T7 kit.
The 2.2.15 cell was the hepatoblastoma cell line HepG2 transfected with cloned HBV DNA. Various parameters of the replicative cycle can be quantitated in the transfected HepG2 cell e.g., the secretion of HBsAg or HBeAg and the amount of episonal HBV DNA. Cells were grown in RPMI1640 (Sigma) medium supplemented with 15% fetal bovine serum, 2 mmol/L L-glutamine, 105 U/L penicilin, 105 U/L streptomycin and the neomycin analogue G418 (380 mg/L, Sigma). Cell cultures were mainitained at 37 °C in 5% CO2 atmosphere.
Conjugate Gal-PLL was prepared according to the reductive amination[2]. Laotose and poly-L-lysine were reacted by using borohydride sodium (the molar ratio of lactose/ poly-L-lysine/ borohydride sodium were 50:1:300). Sugar and amino group was determined at a molar ratio of 10:1 in the reaction products (Gal-PLL) according to the Lee YC’s method[3].
A 16-mer oligodeoxynucleotide, complementary to U5-like region, corresponding to nucleotides 1980-1905 of the sequenced viral genome, was synthesized on automated nucleotide synthesizer (Applied Biosystems) using phosphorothioate linkages. As a control, a random 16-mer sequence was prepared in an identical fashion. Antisense DNA was titrated with conjugte to form soluble complex using an agarose gel retardation system as described previously[4].
To determine the effect of antisense DNA on viral gene expression, after the 2.2.15 cells were seeded for 60 h, culture fluid was taken out. Then the cells were incubated for 72 h in medium containing antisense DNA alone, complexed antisense DNA, complexed random DNA and medium alone. All media containing DNA, the DNA concentration was 10 μM. The medium was changed to RPMI1640 without oligodeoxynucleotides and the cells culture was continued for another 72 h. Supernatant (200 μL) was collected and assayed for HBsAg and HBeAg by ELISA (ABC) method as described by the manufacturer. HBV DNA was tested by dot hybridization.
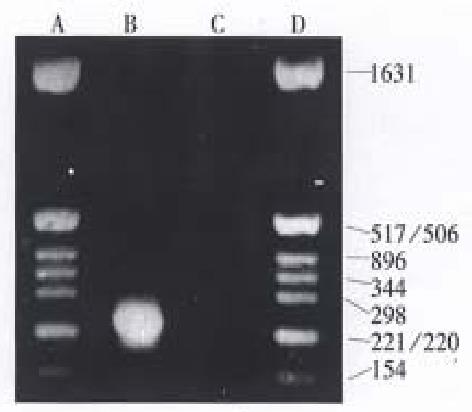
Total DNA was isolated from the 2.2.15 cells. PCR amplification of pre-c and c regions was done with P2 and P2 primers. The length of amplified sequence was limited at 260 base pairs by the primers. Agarose gel electrophoresis showed that amplified products located between nucleotides 221 and 298, which corresponded well with our needs (Figure 1). The purified PCR products were directly sequenced with T7 DNA sequencing system. One hundred and thirty-two base pairs could be read on the film of autoradiograph as follows:
CCAGCACCATGCAACTTTTTCACCTCTGCCTAATCATCTCTTGTTCATGTCCTACTGTTCAAGCCTCCAAGCTGTGCCTTGGGTGGCTTTGGGGCATGGACATCGACCCTTATAAAGAATTTGGAGCTACTG
The sequence was the same as what HBV (ayw1 subtype) had, including prc-c sequence (1816-1902), U5-like region (1857-1918) and part of poly-A addition singal sequence (1919-1962).
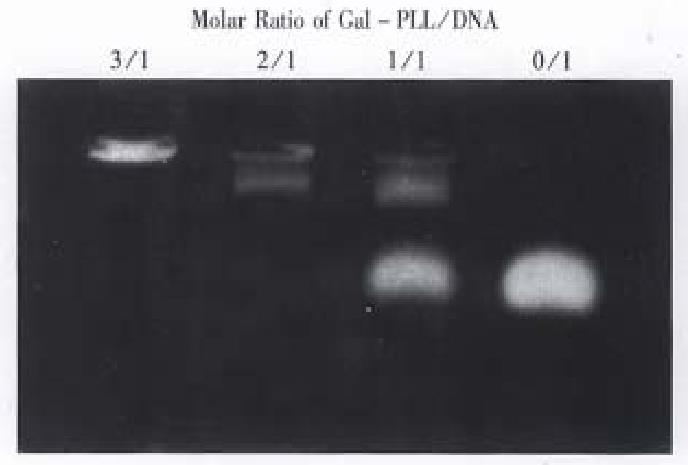
Purified Gal-PLL was incubated with DNA by means of increasing concentrations. The extent of Gal-PLL: DNA complex formation was measured by agarose gel electrophoresis. Judged on the basis of charge neutralization, as seen by the reduction of electrophoretic mobility of the DNA, interaction between the Gal-PLL and DNA started at a molar ratio of 1:1 (Figure 2). The DNA migration is completely retarded at molar ratios of 2:1 and greater.
To determine whether the targeted antisense DNA was functional, effects on HBV gene expression were evaluated. Table 1 shows that the influence of oligodeoxynucleofides on the sceretion of HBsAg and HBeAg was determined 8.5 days after seeding. HBsAg and HBeAg secretions from cells were inhibited by 70% and 50% respectively at antisense DNA alone concentration of 10μM. The inhibition rates gradually increased to 96% and 82% when the complexed antisense DNA (Gal-PLL:ASON) was used. HBsAg and HBeAg secretions were not markedly inhibited by the complexed random DNA (19% and 10%). The amount of HBV DNA in the culture supernatant and cells was reduced significantly with complexed antisense DNA compared with the complexed random DNA and antisense DNA alone (data not shown).
| Treatment | HBsAg | Inhibitory rate (%) | HBeAg | Inhibitory rate (%) |
| Untreated control | 9.40 ± 0.16 | 15.10 ± 0.15 | ||
| Antisense DNA alone | 4.30 ± 0.25 | 70 | 7.60 ± 1.10 | 58 |
| Complexed antisense DNA | 2.40 ± 0.26 | 96 | 4.50 ± 0.42 | 82 |
| Complexed random DNA | 8.10 ± 0.13 | 19 | 13.80 ± 0.76 | 10 |
Specificity of antisense oligonucleotides depends on the selectivity of Watson-Crick or other types of base pairing. The affinity associated with a mismatched base pair varies with the function of the specific mismatch, the position of the mismatch in a region of complementarity, and the sequence surrounding the mismatch. Gibbs free energy of binding induced by a single mismatch decreased from 0.2 to 4.9 kcal/mol at 100 mM NaCl. Thus, a single base mismatch may result in affinity change by approximately 500 fold[5]. We identified that HBV DNA in the 2.2.15 cells were from HBV with surface antigen subtype syw-2 by sequencing so that antisense DNA could bind specifically to the target sequence through base pairing without any mismatch.
Oligonucleotides may be degraded by exonucleases and endonucleases, which exist extensively in serum, cells and fluid of body. Work from many laboratories has demonstrated that a wide range of modification may be used to enhance the stability of oligonudeotides. Phosphorothioate oligonucleotides have been shown to be extremely stable in media, cells and cell extracts, serum, various tissues, urine and stable to most nucleases. In this experiment, we chose phosphorothioate analog to study. Phosphorothioates are negatively charged, but because of the sulfur atoms they may be slightly more lipophilic than phosphodiesters and tend to bind nonspecifically to serum proteins, those may effect on the action of oligonucleotides. Many techniques have been developed to introduce foreign DNA into cells in vitro. For example, methods such as electroporation, microinjection, liposomes. Some of these methods have been used successfully in viro. The approach on epecificity of DNA delivery has been to take advantage of cell surface receptors as natural internalization sites for targeting substances to specific cells. There have been particularly interests in liver cells because of the presence on these cells of umique receptors that are able to recognize galactose-terminal (asialo) glycoproteins. Wu GY et al[6] reports that asialoglycoprotein (asialoorosomucoid, ASOR)poly(L-lysine) conjugates can be used to target genes in a soluble form resulting in specific delivery to cells possessing surface asialoglycoprotein receptors.
In this study an artificial ligand, Gal-PLL conjugate, was used for targeting antisense DNA to the 2.2.15 cells. With increasing proportins of conjugate in the sample, more DNA was retained by the Gal-PLL conjugate in the wells. A 2:1 molar ration of the conjugate to DNA optimized the complex formation, and this molar ratio was the same as what Wu GY et al[7] reported using ASOR.
In the same experimental conditions, the inhibitarg effects of HBsAg and HBeAg by PS-ASON were 70% and 58%, while by ligand PS-ASON were 96% and 82%. HBsAg and HBeAg scretion were not markedly inhibited by the random DNA. The results indcate that antisense oligonucleotids complexed by a soluble DNA-carrier system can be targeted to cells via asialoglycoprotein receptors in specific inhibition of HBV gene expression and replication.
Gal-PLL, as a hepatotropic carrier of DNA, has some advantages: (1) It is obtain by a simple synthetic method; (2) It might not cause allergy in vivo. So it is very important to study synthetic low-molecular weight carriers.
Project supported by the National Natural Schience Foudation of China, No.39370648.
| 1. | Korbe B, Wells F, Jones K, Engle R, Buckler-white A, Gerin I. Inhibition of hepatitis B virus replication in vitro by antisense oligonudeotides. Antivi-ral Res. 1994;20:78. |
| 2. | Schwartz BA, Gray GR. Proteins containing reductively aminated disaccharides. Synthesis and chemical characterization. Arch Biochem Biophys. 1977;181:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 183] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Lee YC. Synthesis some cluster glycosides suitable for attachment to protein or solid matrices. Carbohydr Res. 1978;67:509-514. |
| 4. | Cristiano RJ, Smith LC, Woo SL. Hepatic gene therapy: adenovirus enhancement of receptor-mediated gene delivery and expression in primary hepatocytes. Proc Natl Acad Sci USA. 1993;90:2122-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 107] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Crooke ST. Therapeutic applications of oligonucleotides. Annu Rev Pharmacol Toxicol. 1992;32:329-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 301] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Wu GY, Wu CH. Evidence for targeted gene delivery to Hep G2 hepatoma cells in vitro. Biochemistry. 1988;27:887-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 141] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Wu GY, Wu CH. Specific inhibition of hepatitis B viral gene expression in vitro by targeted antisense oligonucleotides. J Biol Chem. 1992;267:12436-12439. [PubMed] |