Published online Oct 15, 1998. doi: 10.3748/wjg.v4.i5.421
Revised: September 22, 1998
Accepted: September 30, 1998
Published online: October 15, 1998
AIM: To compare the expression level of Fas gene and Bcl-2 gene in gastric cancer cells SGC7901 and gastric cancer multidrug resistant cells (MDR) SGC7901/VCR, to transduce Fas cDNA and Bcl-2 antisense nucleic acid into SGC7901/VCR cells respectively, and to observe the expression of two genes in transfectants and non-transfectants as well as their drug sensitivity.
METHODS: Eukaryotic expression vector pBK-Fas cDNA and pDOR-anti Bcl-2 were constructed and transfected into SGC7901/VCR cells by lipofectamine,respectively. Northern blot and Western blot were used to detect the expression of mRNA and protein in SGC7901/VCR and SGC7901 cells and transfectants, and drug sensitivity of transfectants for VCR, CDDP and 5-FU was analyzed with MTT assay.
RESULTS: After gene transfection, 80 for Fas and 120 for antisense Bcl-2 drug-resistant clones were selected from 2 × 105 cells, transfection rate being 0.04% and 0.06%. Two clones of SGC7901 Fas/VCR cells and SGC7901 anti Bcl-2/VCR cells were randomly selected for further incubation. Hybridization results showed that the expression level of Fas mRNA and protein in SGC7901/VCR cells was much lower, but that of Bcl-2 mRNA and protein was higher than that in SGC7901 cells. The expression of Fas mRNA and protein in SGC7901 Fas/VCR cells was higher, and of Bcl-2 mRNA and protein was lower in SGC7901 anti Bcl-2/VCR cells than that in non-transfectants. MTT assay showed that transfectants were more sensitive to VCR, CDDP, 5-FU than non-transfectants.
CONCLUSION: Bcl-2 gene displayed high expression while Fas gene had low expression in drug resistant gastric cancer cells. Expression of Bcl-2 protein was effectively blocked in SGC7901 anti Bcl-2/VCR cells by gene transfection. In contrast, the expression of Fas mRNA and protein in SGC7901 Fas/VCR cells increased. Fas gene and Bcl-2 antisense nucleic acid transfection sensitized drug resistant gastric cancer cells to chemotherapeutic drugs. These results suggest cell apoptosis plays an important role in the mechanism of MDR, and enhancing apoptosis might reverse MDR.
- Citation: Xiao B, Shi YQ, Zhao YQ, You H, Wang ZY, Liu XL, Yin F, Qiao TD, Fan DM. Transduction of Fas gene or Bcl-2 antisense RNA sensitizes cultured drug resistant gastric cancer cells to chemotherapeutic drugs. World J Gastroenterol 1998; 4(5): 421-425
- URL: https://www.wjgnet.com/1007-9327/full/v4/i5/421.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i5.421
Chemotherapy is one of the major methods in tumor treatment, but it often does not work due to multidrug resistance (MDR). Recent studies indicated that inhibition of cancer cell apoptosis and longer cell life may be one of MDR mechanisms[1]. So it is assumed that inducing apoptosis might reverse MDR. We transfected-Fas-gene and Bcl-2 antisense nucleic acid into drug resistant gastric cancer cells, and observed the expression of target genes in transfectants and the sensitivity of transfectants to chemotherapeutic agents in order to find the ground for reversing gastric cancer MDR.
Drug resistant gastric cancer SGc7901/VCR cells and JM109 bacterial strain were kept in our department. Retrovirus vector pDOR-SV40 and expression vector pBK-CMV were generous gifts from Dr. Cui Da-Xiang, Department of Biochemistry, Fourth Military Medical University. The pBluescript Bcl-2 cDNA plasmid was from our department. The pBluescript Fas-cDNA was presented by professor Itoh N, Japan. Eco R I, Bam H I, Sal I, Xho I, CIP, RNaseA, T4 DNA ligase, PMSF, Aprotinin, SDS, ABC kit, probe labeled kit and guanidinium isothiocyanate were purchased from SABC and Promega, and Lipofectamine from Gibco BRL. MTT, Protease k, DEPC, MOPS, G418, FCM and nitrocellulose filter were products of Sigma. [α32P]dATP was derived from Yahui Ltd, Beijing, primer and oligonucleotides from Sangon, Shanghai, and rat anti-human Bcl-2 monoclonal antibody and rabbit anti human Fas polyclonal antibody from Orient Ltd, Beijing.
Construction and identification of recombinant vectors 1.9 kb Bcl-2 cDNA was inserted into pBluescript KS at Eco R I site and 2.5 kb Fas cDNA into pBluescript KS at Xho I site. According to the construction protocol, primary plasmids were digested by enzyme. The results of agarose gel electrophoresis showed that primary plasmids contained complete Bcl-2 cDNA and Fas-cDNA. We used Eco RI and Bam HI to cut pBluescript Bcl-2 cDNA, Sal I and Eco R I to cut pBluescript Fas-cDNA, and obtained 0.622 kb Bcl-2 cDNA and 1.83 kb Fas-cDNA fragments by frozen thaw methods. Under T4 DNA ligase, cDNA fragments were connected with the corresponding pDOR and pBK-CMV vectors digested by enzyme, the products were transformed into competent cells for routine culture. Ampresistant colonies and mini-prepared plasmids were selected randomly, and recombinant plasmids were identified through enzyme digestion and agarose gel electrophoresis. Three pairs of primers designed according to Fas-cDNA sequence were used for PCR amplification of pBK-Fas-cDNA, and sequence of PCR product was analyzed to confirm the correctness of open reading frame.
Purified pDOR-anti-Bcl-2 or pBK-Fas cDNA (0.5 µg) diluted in 100 µL RPMI 1640 was mixed with 5 µL lipofectamine, and placed at room temperature for 10 min. The mixture was then transfected into 2 × 105 SGC7901/VCR cells. The cells were selected by 500 mg/L G418 24 h later. At the same time, pDOR or pBK-CMV vector which lacked target genes transfected cells and non-transfectants served as negative controls.
Genomic DNA and total RNA were obtained from the well growing 1 × 107 cells. Genomic DNA digested with Eco R I ran agarose gel electrophoresis. The denatured total RNA underwent formaldehyde denaturation and gel electrophoresis. DNA and RNA were transferred to nitrocellulose filter by vacuum aspiration. [α-32P] dATP labeled probe was made for prehybridization, hybridization and autoradiography.
Half cell lytic protein was used for SDS-PAGE, and comassie brilliant blue stained, the other half was electrotransfered 100 v overnight, nitrocellulose filter denatured by SDS and visualized by ABC method.
Cells (103-104) diluted with 200 µL 10% RPMI 1640 were seeded into 96-well plates, respectively, added cisplatin (1 µg, 10 µg, 100 µg), 5-FU (7 µg, 70µg, 700 µg), and VCR (0.1 µg, 1.0 µg, 10 µg) according to the clinically established plasma peak concentration. Three days later, 20 µL MTT solution (5 g/L) was dropped into plates, the supernant was discorded after 4 h, and 150 µL DMSO was added to melt crystal. OD value was read at 590 nm wavelength.
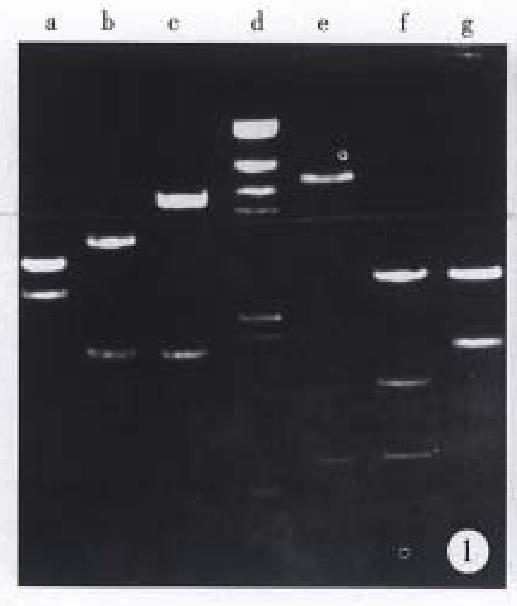
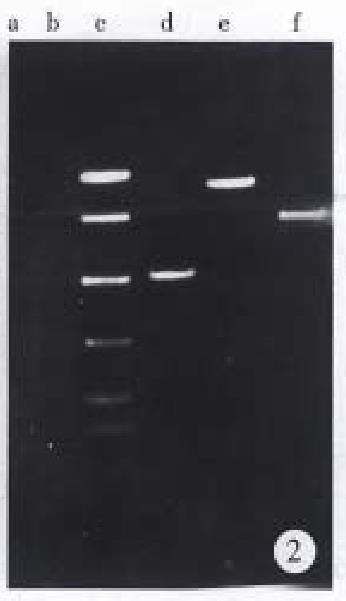
As shown in Figure 1, 1.9 kb Bcl-2 cDNA and 2.5 kb Fas-cDNA were separated from primary plasmids digested with Eco RI and Xho I, 0.622 kb anti-sense Bcl-2 fragment and 6.5 kb vector from recombinant vector pDOR- anti-Bcl-2 digested by Eco R I and Bam H I, 1.8 kb Fas cDNA and 4.5 kb vector from recombinant vector pBK-Fas-digested by Eco R I and Sal I. Electrophoresis of PCR product (Figure 2) indicated that three pairs of primers amplified 500 bp, 850 bp, 700 bp fragments respectively, which were consistent with our expectation.
When transfected cells were cultured selectively by G418 for 4-5 weeks, resistant clones formed gradually, the number of stable clones reached about 120, with a transfection rate of above 0.05%. In contrast, all non-transfectants died two weeks after G418 selection. Resistant clones were further incubated in the presence of low-dose G418 for 40-50 days. We got two resistant clones, SGC7901-Fas /VCR cells and SGC7901 anti-Bcl-2/VCR cells.
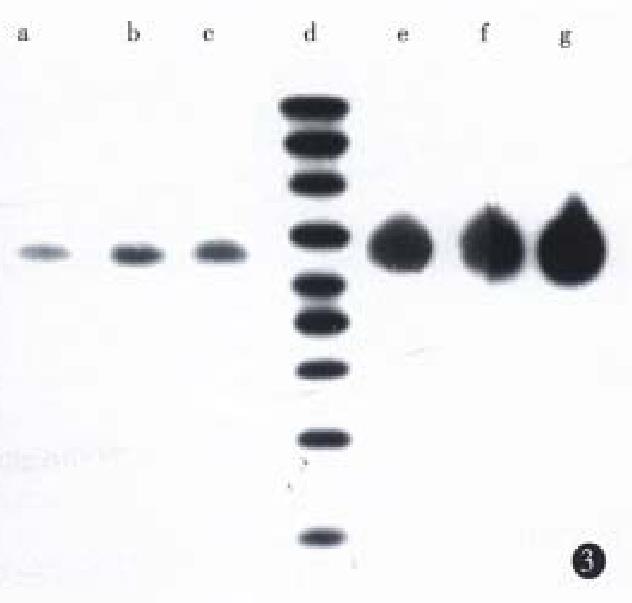
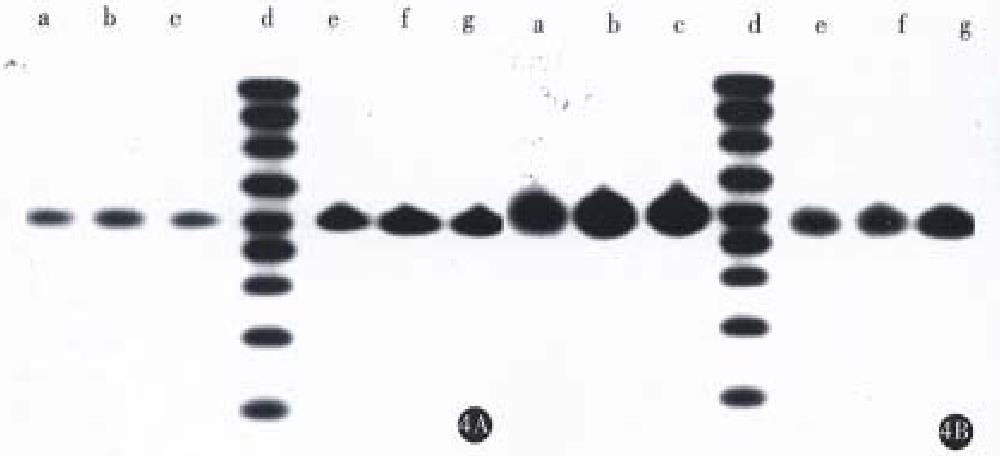
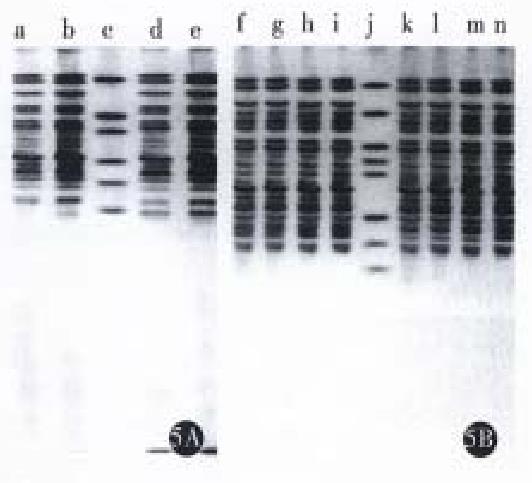
Northern blot was performed using a single strain cDNA fragment of Fas or Bcl-2 or antisense Bcl-2 as probes, respectively. The results (Figure 3 and Figure 4) showed that SGC7901 cells had weak signals of Fas and strong signal of Bcl-2, while SGC7901/VCR had weak signal of Fas and very strong signal of Bcl-2. The signal of Fas became stronger in SGC7901-Fas/VCR than that in SGC7901/VCR, which was also dependent on cell number. The signal of antisense Bcl-2 was stronger in SGC7901 anti -Bcl-2/VCR than in SGC7901/VCR and non-transfectants, but the signal of sense Bcl-2 was a little weaker than that in non-transfectants. SDS-PAGE and Western blot (Figure 5) showed that a band of Mr 36000-40000 was found in transfectants, but not in non-transfectants, the size of which was consistent with Mr of Fas- protein. A band of Mr 25000, which equaled to that of Bcl-2 was found in non-transfectants, but not in antisense Bel-2 transfected cells.
The survival rates of transfectants treated with cisplatin or 5-FU were obviously lower than non-transfectants, however, the survival rates of transfectants treated with VCR were only lowered lightly, compared with non-transfectants (Table 1).
Apoptosis and mitosis both are important for keeping the balance of organism in vivo, which act in opposite way. Recent studies indicated that inhibition of cancer cell apoptosis and disturbance of apoptosis related genes can increase the anti-apoptosis protein or decrease pro-apoptosis protein, which is one of the mechanisms of tumorigenesis. Therefore, induction of apoptosis may treat tumors. Many chemotherapeutic agents are found to act through damaging DNA or regulating apoptosis related genes p53, c-myc, Bcl-2, c-H-ras to trigger apoptosis[2-4]. But MDR becomes an obstacle in tumor treatment. Recent researches have shown that MDR is the result of many mechanisms, in addition to the changes of Pgp, MRP, LRP, Topo II and GST/GSH, deregulation of apoptosis also produces cross drug resistance of cancer cells[1,5]. The a bility of drug resistant cells repairing damaged DNA and anti-apoptosis has strengthened greatly[6]. Introduction of Bcl xL gene may induce resistance of drug sensitive cells, and overexpression of Bcl-2 is linearly related to overexpression of Pgp, therefore, it is supposed that the expression level of apoptosis related genes determines the MDR phenomena of cancer cells[7]. Chinese hamster drug resistant ovarian fibroblastoma LR73/20E cells, which overexpress MDR and Pgp, are not sensitive at all to apoptosis inducing colchicine, but become sensitive to apoptosis after treated with drug resistant reversing agent verapamil[8]. Apoptosis can not be induced in drug resistant breast cancer MCF-7/ADR cells which overexpress Bcl-2 mRNA and protein by sugar starvation[9]. Drug resistant leukemia HL-60/AR, HL-60/VCR, HL-60/ TAX1000 cells, overexpressing MRP, Pgp, Bcl-2, and Bcl-xL, can inhibit the intercellular aggregation of chemotherapeutic agent paclitaxel and prohibit apoptosis. Bcl-2 and Bcl-xL transfected drug sensitive cells can antagonize apoptosis, but can not affect the intercellular aggregation of chemotherapeutic agent paclitaxel[10]. P53 protein and Fas antigen promote cell apoptosis, but they display low expression in drug resistant cells[1,11]. These results show that high expression of Bcl-2 and low expression of Fas are important for drug resistant cells to antagonize apoptosis and resist chemotherapeutic agents.
Although antiapoptosis gene Bcl-2 can not promote cell proliferation, it prolongs cell life. Its inhibition of apoptosis is caused by changing the permeability of Ca2+ channel and the expression of ICE[12], and is related with regulation of Bax, TNF and Fas[13]. Controlling the transcription of Bcl-2 mRNA by antisense nucleic acid technology can effectively suppress the expression of Bcl-2 and retard tumor cell growth[14].
Fas gene product, a type-I membrane protein, belongs to the TNF receptor family. Fas molecule on the surface of cell transmits death message into cell through combining Fas ligand or anti Fas monoclonal antibody, resulting in the death of cells in several hours[15]. Compared with normal tissues, malignant tumor cells, especially metastatic tumor cells present extremely lower expression of Fas, while most benign tumors are similar to their original tissues in the expression of Fas. It is hypothesized that malignant tumors might surppress Fas expression or lose Fas molecule in order to avoid the surveillance of Fas ligand. This explains the lack of Fas expression in drug resistant cells[1]. In one word, transduction of proapoptosis Fas gene or antisense Bcl-2 gene into drug resistant cells is obviously helpful in increasing the drug sensitivity. Chen XQ transiently transducted Bcl-2 antisense RNA into human leucemia cells (CEM), and found that intrinsic Bcl-2 protein was decreased, and transfected cells were more sensitive to etoposide and a lot of apoptotic bodies and small DNA fragments formed when cells died.
To study the significance of Fas and Bcl-2 protein in drug resistant gastric cancer SGC7901/ VCR cells, we constructed the eukaryotic expression vectors pBK Fas and pDOR-anti-Bcl-2 which were respectively transfected into SGC7901/VCR cells. After G418 selection, resistant clones overexpressing Fas protein or lacking Bcl-2 protein were obtained. Molecular hybridization showed drug resistant gastric cancer SGC7901/VCR cells expressed a tiny amount of Fas mRNA and protein, but highly expressed Bcl-2. Compared with control cells, Fas mRNA and protein expression of Fas transfected cells was obviously increased. In anti-Bcl-2 transfected cells, Bcl-2 mRNA expression was inhibited slightly, but Bcl-2 protein was blocked greatly. Drug sensitivity assay demonstrated that transfectants were more sensitive to VCR, CDDP and 5-FU than non-transfectants. It was concluded that suppression of apoptosis play an important role in inducing MDR of tumor cells, and promoting apoptosis could reverse MDR to some extent.
| 1. | Friesen C, Fulda S, Debatin KM. Deficient activation of the CD95 (APO-1/Fas) system in drug-resistant cells. Leukemia. 1997;11:1833-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 137] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Arceci RJ. Tumor cell survival and resistance to therapy. Curr Opin Hematol. 1996;3:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Ketley NJ, Allen PD, Kelsey SM, Newland AC. Modulation of idarubicin-induced apoptosis in human acute myeloid leukemia blasts by all-trans retinoic acid, 1,25(OH)2 vitamin D3, and granulocyte-macrophage colony-stimulating factor. Blood. 1997;90:4578-4587. [PubMed] |
| 4. | Nooter K, Boersma AW, Oostrum RG, Burger H, Jochemsen AG, Stoter G. Constitutive expression of the c-H-ras oncogene inhibits doxorubicin-induced apoptosis and promotes cell survival in a rhabdomyosarcoma cell line. Br J Cancer. 1995;71:556-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Van Waardenburg RC, Meijer C, Burger H, Nooter K, De Vries EG, Mulder NH, De Jong S. Effects of an inducible anti-sense c-myc gene transfer in a drug-resistant human small-cell-lung-carcinoma cell line. Int J Cancer. 1997;73:544-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Colvin OM. Drug resistance in the treatment of sarcomas. Semin Oncol. 1997;24:580-591. [PubMed] |
| 7. | Minn AJ, Rudin CM, Boise LH, Thompson CB. Expression of bcl-xL can confer a multidrug resistance phenotype. Blood. 1995;86:1903-1910. [PubMed] |
| 8. | Robinson LJ, Roberts WK, Ling TT, Lamming D, Sternberg SS, Roepe PD. Human MDR 1 protein overexpression delays the apoptotic cascade in Chinese hamster ovary fibroblasts. Biochemistry. 1997;36:11169-11178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 110] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Lee-YJ , Galoforo SS, Berns CM, Tong WP, Kim HR, Corry PM. Glucose deprivationinduced cytotoxicity in drug resistant human breast carci-noma MCF-7/ADR cells: role of c-myc and Bcl-2 in apoptotic cell death. J Cell Sci. 1997;110:681-686. |
| 10. | Huang Y, Ibrado AM, Reed JC, Bullock G, Ray S, Tang C, Bhalla K. Co-expression of several molecular mechanisms of multidrug resistance and their significance for paclitaxel cytotoxicity in human AML HL-60 cells. Leukemia. 1997;11:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Reeve JG, Xiong J, Morgan J, Bleehen NM. Expression of apoptosis-regulatory genes in lung tumour cell lines: relationship to p53 expression and relevance to acquired drug resistance. Br J Cancer. 1996;73:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Shimizu S, Eguchi Y, Kamiike W, Matsuda H, Tsujimoto Y. Bcl-2 expression prevents activation of the ICE protease cascade. Oncogene. 1996;12:2251-2257. [PubMed] |
| 13. | Allouche M, Bettaieb A, Vindis C, Rousse A, Grignon C, Laurent G. Influence of Bcl-2 overexpression on the ceramide pathway in daunorubicin-induced apoptosis of leukemic cells. Oncogene. 1997;14:1837-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Webb A, Cunningham D, Cotter F, Clarke PA, di Stefano F, Ross P, Corbo M, Dziewanowska Z. BCL-2 antisense therapy in patients with non-Hodgkin lymphoma. Lancet. 1997;349:1137-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 372] [Article Influence: 13.3] [Reference Citation Analysis (0)] |