Published online Aug 15, 1998. doi: 10.3748/wjg.v4.i4.307
Revised: April 22, 1998
Accepted: May 14, 1998
Published online: August 15, 1998
AIM: To observe the effects of ricin (RT) with and without chemicalmodification on both hepatotoxicity of mice and activity against hepatocellular cancer (HCC), and evaluate the possibility to improve RT anticancer activity via chemical modification.
METHODS: RT was modified with N-succinimidyl3 (2-pyridyldithio) propionate (SPDP), a heterobifunctional cross-linker, and SPDP derivative of RT (PDP-R) was obtained. The serum glutathione-s-transferase (SGST) activity, as an index of liver damage, was determined in mice intoxicated with RT and PDP-R, at various doses and time.The tissue damage of HCC in the nude mice ip injected with PDP-R was compared with that with RT at the same dose by immunohistochemical method, the relative content of both RT and PDP-R in the HCC tissues was measured by computerized image-analysis.
RESULTS: The SGST activities increased with doses or/and time intoxicated with both RT and PDP-R, and the increase in the value of RT group was more significant than that in the PDP-R group; the SGST activity of RT group was 2.8-fold (P < 0.01) of PDP-R group at a dose of 12.5 μg/kg for 42 h, showing the much lower toxicity of R-PDP than that of RT. Under an optical microscope, hemolysis and necrosis of massive cells in the HCC tissues of PDP-R group were observed and the ratio of necrosis mounted to 90.5% while the corresponding value of RT group only to 62.5%. With computerized image-analysis, the average relative content of RT and PDP-R in the HCC tissues, represented as greyness, was 140.06 ± 3.43 and 169.10 ± 2.74, respectively. There was significant difference between the two (P < 0.05), indicating the higher content of PDP-R in the HCC tissue than that of RT.
CONCLUSION: The hepatotoxicity of PDP-R to mice may be reduced by chemical modification with SPDP, but both the affinity of PDP-R to the HCC tissues and ability to kill it may be stronger than that of RT. So this might be a valuable attempt to improve the anticancer activity of RT.
- Citation: Wang WX, Dong JY, Zhou SY, Li WL, Zhao Y. Modification of ricin and its hepatotoxicity and activity against hepatocellular cancer in mice. World J Gastroenterol 1998; 4(4): 307-310
- URL: https://www.wjgnet.com/1007-9327/full/v4/i4/307.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i4.307
Ricin toxin (RT), a glycoprotein with high cytotoxicity, isolated from caster beans (Ricinus communisbeans), has received extensive interest in anticancer research. The natural RT, however, has been restrained in treatment of cancer due to its extreme toxicity. In order to overcome its side effect, various attempts have been made by investigators[1,2], but no report so far has been presented in direct use as anticancer agent. We modified RT with N-succinimidyl3-(2-pyridyldithio) propionate (SPDP), aheterobifunctional cross-linker, and toxicity of the resultant (PDP-R) to normal tissues reduced but its inhibition to some tumor cells increased[2,3]. In the present paper, we reported the preparation, determination of PDP-R and comparison between PDP-R and RT in hepatotoxicity to normal mice and activity against hepatocellular cancer (HCC) in nude mice.
RT was prepared as described previously[5] and shown to be a single band by SDS-PAGE, Mr 65000. SPDP was synthesized by a modified method[6], mp 80 °C-81 °C. Glutathione reduced form (GSH, BRITISH) was freshly made into 10.0 mmol/L (pH 6.5) solution in 1.0 mol/L of PB prior to use. 1-chloro-2, 4-dinitrobenzene (CDNB, Xi’an Chemical Reagent Factory) was made into 1.11 mmol/L solution in 1.0 mol/L of PB containing 40 mL/L ethanol after second recrystallization in absolute ethanol, m.p 53 °C-54 °C. Kunming mice, weighing 17.5 g-22.5 g and nude mice weighing 18.0 g, with human HCC were supplied by The Research Center of Experimental Animals in our university.
Preparation and determination of PDP-R RT of a 4.2 mg in 1.0 mL of PBS (0.1 mol/L, pH 7.5, containing 1.5 mol/L of NaCl) was added under stirring, 20 µL solution of N, N-dimethylformamide containing 0.25 mg of SPDP and reacted for 30 min at 23 °C. The solution of reacted mixture was thoroughly dialyzed against saline at 4 °C. PDP-R in the solution and the number (n) of PDP groups bonding to molecules of RT were determined by ultraviolet spectrophotometry[7].
The effect of dosage Fifty Kunming mice were randomly divided into PDP-R group and RT group. Twenty-five mice in each group were equally subdivided into 5 groups, of which each mouse was injected ip with 2.5, 5.0, 7.5, 10.0 and 12.25 µg/kg body weight respectively. Ten mice served as control group, of which each mouse was ip injected with the same volume of saline. The mice intoxicated were continuously observed for 42 h, blood was collected from fundus veniplex, and serum was separated and SGST activity of each specimen measured by dynamics method[8].
Effect of intoxication time Eighty Kunming mice were randomly divided into PDP-R group and RT group. Forty mice in each group were equally subdivided into 8 groups, of which each mouse was ip injected with 12.5 µg/kg body weight and the intoxication time was 6, 12, 18, 24, 30, 36, 42 and 48 h, respectively. Then the procedure was followed as mentioned above.
Comparison between PDP-R and RT in hepatotoxicity to normal mice and activity against HCC in nude mice Eighteen nude mice with human-HCC were randomly divided into PDP-R group, RT group and control group. Six mice of each group were ip injected with the same dose of PDP-R, RT and saline, respectively. The HCC tissue of mouse was taken out 4 h later and was treated by routine procedure to obtain the pathological slices with thickness of 4 µm-6 µm, which were stained immunohistochemically (ABC method). The slices were observed under the optical microscope and the relative content of both RT and PDP-R in the HCC tissues was measured by computerized image-analysis.
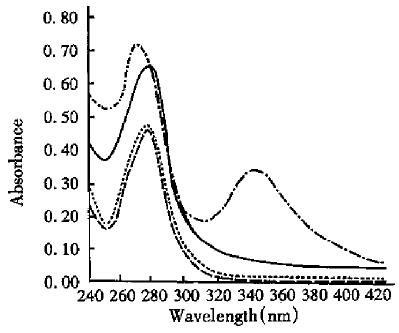
A small amount of PDP-R solution was added to dithiotheol (DTT) to a final concentration of 40 mmol/ L and treated for 30 min at room temperature. The ultraviolet absorption spectrum of the reaction solution was determined in spectrophotometer. After thorough dialysis of the reaction solution against PB, the ultraviolet absorption spectrum was determined again and compared with those of both solution of PDP-R and RT. The results are shown in Figure 1 and the absorbence (A) of the four solutions at 280 nm and 343 nm are shown in Table 1. The A value of PDP-R solution at 280 nm is 0.19 more than that of RT (Table 1). The number (n) of PDP bonding to each molecule of RT was 6.02 according to both molecule absorption coefficient of SPDP at 280 nm and the concentration of RT. On the other hand, based on both molecular absorption coefficient of α-mercaptopyridine (α-MP) and the concentration of RT, the value was calculated to be 5.8 because when the solution of PDP-R was treated with excess of DTT, it results in equimolecular α-MP to PDP and the α-MP in the solution had a strong absorption at 343 nm. Then value obtained from the two ways is close.
| λ/nm | Absorbence | |||
| RT | PDP-R | PDP-R+DTT | (PDP-R+DTT) dialyzed | |
| 280 | 0.476 | 0.665 | 0.700 | 0.484 |
| 343 | 0.007 | 0.065 | 0.345 | 0.066 |
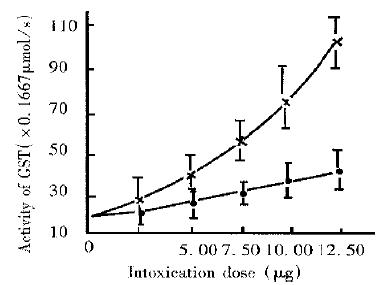
Effect of dose Under the same conditions, the SGST activities increased with doses intoxicated with both RT and PDP-R, but the increase in the value of RT group was more significant than that in the PDP-R group (Figure 2). When the dose increased to 12.5 µg/kg death in the mice of RT group occurred, the survivor’s blood volume decreased and the blood viscosity increased with apparent symptoms of intoxication. The average SGST activity of RT group was as high as 1.797 µm/s ± 0.087 µmol/s, being 5.3-fold that of the control group (0.340 µm/s ± 0.025 µm/s). However, it seems that the symptoms of PDP-R group resulting from intoxication with the same dose were not at all serious, no death for 42 h, no difference between PDP-R group and control group in the blood volume and viscosity in these mice, their average SGST activity being only 0.642 µm/s ± 0.054 µm/s, 36% (P < 0.01) of that of RT group at a dose of 12.5 µg/kg for 42 h, which showed much lower toxicity of R-PDP than that of RT.
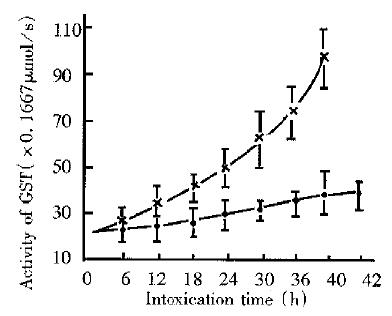
Effect of intoxication time The SGST activities of mice of both PDP-R and RT group increased with intoxication time extended, although within 10 h difference between the two were insignificant (Figure 3). After 10 h, however, increase of RT group was gradually getting more quick and difference between the two at 42 h was up to a maximum measurable value. After 42 h this value was unable to measure due to increased number of death of mice in RT group, while the SGST activity of PDP-R group smoothly decreased.
Comparison between PDP-R and RT in activity against hepatocellular cancer Under an optical microscope, hemolysis and necrosis of massive cells in the HCC tissue of PDP-R group was observed and the ratio of necrosis mounted to 90.5%, while it was only to 62.5% in RT group. With computerized image-analysis, the average relative content of RT and PDP-R in the HCC tissue, represented as greyness, was 140.06 ± 3.43 and 169.10 ± 2.74, respectively. There was significant difference between the two (P < 0.05), indicating the higher content of PDP-R in the HCC tissue than that of RT and PDP-R has stronger affinity and activity against HCC than that RT.
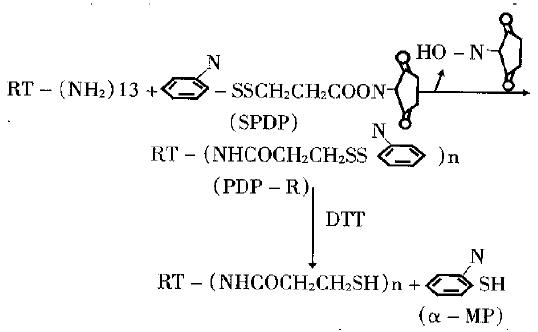
Reaction of RT with SPDP and determination of PDP-R The molecular structure of SPDP and its reaction with RT is shown in Figure 4.There were 13 amino groups (ª²NH2) in each molecule of RT which may be acylated by SPDP. The experiment showed that acylation ratio of -NH2 in RT molecule is not high under general conditions, possibly due to that some - NH2 was located at hydrophobic domain of RT molecule and being not easy to get access to SPDP. In this experiment 26 equimolecular SPDP was used to react with RT to increase the acylation ratio. The resultant, PDP-R, was determined by two ways: first, based on the number (n) of PDP bonding to each molecule of RT to be positively proportional to increased value in absorbence of PDP-R at 280nm, so then value can be calculated from absorbence of solution, the molecular absorption coefficient of SPDP at 280 nm and the concentration of RT. Second, when the solution of PDP-R was treated with excessive DTT, it resulted in equimolecular α-MP to PDP and the α-MP in the solution had a strong absorption at 343 nm. So the n can be calculated from the absorbence of reacted solution at 343 nm, molecular absorption coefficient of α-MP and the concentration of RT. The n value is close by the two ways and it can be used as the evidence for each other. After dialysis of the reaction solution, its absorption spectrum was the same as that of RT, which showed that the absorption at 343 nm was caused by α-MP.
Comparison between PDP-R and RT in effect on SGST activity of mice The liver injury caused from intoxication may lead to release of GST to blood flow, resulting in the significant increase of SGST activity and this has been a sensitive index to detect liver injury[9]. We compared the hepatotoxicity of PDP-R with that of RT by determination of SGST activity of intoxicated mice. The results were identical with that obtained by pathomorphological method[3]. The experiment showed, in view of effects of either dose or time, that the increase of SGST activity caused by PDP-R apparently lower than that by RT, indicating that the hepatotoxicity of PDP-R was significantly lower than that of RT, other symptoms intoxicated with PDP-R was less serious than that with RT and the mice had stronger tolerance to PDP-R than to RT. These differences may imply that the mechanism of intoxication of PDP-R differs from that of RT.
Some biological activities of PDP-R different from that of RT It is reported that after RT entered into a mouse body for 0.5h almost 50% of it was located in the liver, showing very strong hepatotoxicity[10]. Therefore, to determine the hepatotoxicity of PDP-R is of a typical significance to know its systemic toxicity. The experiment showed that the ability of RT to bind galactose (G) and the residue containing G will be decreased by reaction of RT with SPDP[11]. It may be inferred that the toxicity to normal cells including hepatocytes might be weakened because the interaction between RT and the receptor containing G on cellular surface can be decreased by modification of RT with SPDP. On the other hand, the PDP in PDP-R molecules were very sensitive to hydrosulfury (-SH) on the cellular surface of some tumors and easy to bind to these cellular surface. It is reported that the content of SH on cellular surface of some tumors was ten times more than that on normal cells[12]. PDP-R molecules may have a higher affinity to some cancers and stronger ability to kill them. These suggest that PDP-R molecules might have biological activities different from that of RT and might be a anticarcinogen worth investigating further. ModificationofRTwithheterobifuctionallinkercontaining group able to bind -SH groups, such as SPDP and so forth, might be a new way to improve activity of RT against cancer including hepatocellular cancer and related investigations are underway.
Project supported by the National Natural Science Foundation of China, No.3870638
| 1. | Lambert JM, McIntyre G, Gauthier MN, Zullo D, Rao V, Steeves RM, Goldmacher VS, Blättler WA. The galactose-binding sites of the cytotoxic lectin ricin can be chemically blocked in high yield with reactive ligands prepared by chemical modification of glycopeptides containing triantennary N-linked oligosaccharides. Biochemistry. 1991;30:3234-3247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Lambert JM, Goldmacher VS, Collinson AR, Nadler LM, Blättler WA. An immunotoxin prepared with blocked ricin: a natural plant toxin adapted for therapeutic use. Cancer Res. 1991;51:6236-6242. [PubMed] |
| 3. | Dong JY, Wang WX, Cao YX, Li YS, Zou BY. Study on comparison between ricin and modified it in localization at major viscera of mice. J 4th Military Medical University. 1995;16:17-20. |
| 4. | Li L, Wang WX, Zou BY. The preparation of modified ricin and its cytotoxicity. J 4th Military Medical University. 1996;17:178-180. |
| 5. | Wang WX, Guo ZR. Isolation of ricin using simple and economical method. J 4th Military Medical University. 1987;6:66-69. |
| 6. | Wang WX. Synthesis of heterobifunctional cross linking agent SPDP using modified method. J 4th Military Medical University. 1987;8:66-69. |
| 7. | Carlsson J, Drevin H, Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978;173:723-737. [PubMed] |
| 8. | Chen GZ, Li YH. Determination of activity of serum glutathione-s-trans-ferase and its clinical practices. Shaanxi J of Lab Med. 1994;9:8-10 (in Chinese). |
| 9. | Adachi Y, Horii K, Takahashi Y, Tanihata M, Ohba Y, Yamamoto T. Serum glutathione S-transferase activity in liver diseases. Clin Chim Acta. 1980;106:243-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Fodstad O, Olsnes S, Pihl A. Toxicity, distribution and elimination of the cancerostatic lectins abrin and ricin after parenteral injection into mice. Br J Cancer. 1976;34:418-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 86] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Wang WX, Dong JY, Li L, Yang HX, Zou BY. Change in bonding of modified ricin to galactose and hemoglutination. Med Info PLA. 1994;8:269. |
| 12. | Mehrishi JN, Grassetti DR. Sulphydryl groups on the surface of intact Ehrlich ascites tumour cells, human blood platelets and lymphocytes. Nature. 1969;224:563-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 1.1] [Reference Citation Analysis (0)] |