Published online Jun 15, 1998. doi: 10.3748/wjg.v4.i3.202
Revised: April 20, 1998
Accepted: May 24, 1998
Published online: June 15, 1998
AIM: To study the relationship between intercellular adhesive molecule-1 (ICAM-1) and liver cancer metastasis and to search for factors to predict metastasis of liver cancer.
METHODS: ICAM-1 expression in fresh tissues of normal liver and hepatocellular cancer (HCC) was examined by immunoperoxidase staining. The expression of ICAM-1 in human hepatoma, tumor surrounding tissues and normal livers were semiquantitatively analyzed by Dot immuno blot. Tissue ICAM-1 expression at mRNA level was detected by Northern blot.
RESULTS: All 6 cases of normal liver samples were negative in anti-ICAM-1 immunohistochemical staining, 80.0% (36/45) of HCC presented various ICAM-1 expression. The number of positive cells was a little higher in large tumors, tumors with intact capsule and metastasis, but there was no significant difference. Two cases with cancer embolus also had high ICAM-1 expression. ICAM-1 concentration in HCC (13.43 ± 0.09) was higher than that in tumor surrounding tissues (5.89 ± 0.17, P < 0.01) and normal livers (4.27 ± 0.21, P < 0.01). It was also higher in metastasis group (20.24 ± 0.30) than in nonmetastasis group (10.23 ± 0.12, P < 0.05). Northern blot analysis revealed that ICAM-1 expression at mRNA level was also higher in HCC and cancer embolus than that in tumor surrounding tissues and normal livers.
CONCLUSION: Tissue ICAM-1 could indicate the growth and metastasis of HCC, and may be an index that can predict liver cancer metastasis.
- Citation: Sun JJ, Zhou XD, Zhou G, Liu YK. Expression of intercellular adhesive molecule-1 in liver cancer tissues andliver cancer metastasis. World J Gastroenterol 1998; 4(3): 202-205
- URL: https://www.wjgnet.com/1007-9327/full/v4/i3/202.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i3.202
Tumor metastasis is one of the main causes of poor prognosis of hepatocellular carcinoma (HCC)[1]. The processe of metastasis includes dissociation of tumor cells from primary location; arrest, adhesion and extravasation of metastatic tumor cells. Adhesive molecules on endotheliocyte suchas selectin cause circulating tumor cells to roll along the surface. Other molecules on tumor cells including intercellular adhesive molecule-1 (ICAM-1), vascular cellular adhesive molecule-1 (VCAM-1) and so on, subsequently stop the cells completely, then extravasated through the blood vessels into selective target tissues. During the metastatic cascade, tumor cells interact with various host cells (endothelial cells, platelets or lymphocyte), and/or extracellular matrix and basement membrane components, the homotypic and heterotypic cell clumps form a multicellular embolus, which can enhance the survival, arrest and invasiveness of tumor cells[2]. The metastatic potential of cancer cells is related to the activity of their surface adhesive molecule[3]. Recently, it was noticed that ICAM-1 plays an important role in this processe. In this study, we investigated the relationship between ICAM-1 expression and the metastasis of HCC.
The tissues of HCC used for immunohistochemical study were collected from 45 patients (39 males and 6 females, aged 10-82 years with a median of 51.1) who had undergone hepatectomy for HCC. All the cases were diagnosed pathologically. Eighteen of the patients were in early stage (without symptoms and signs of HCC), and 27 in moderate stage (having symptoms and signs of HCC, but no ascites, jaundice and long distance metastasis). Thirty patients had positive AFP (≥ 30 μg/L) and 15 had negative AFP. The median diameter of the resected tumor was 6.7cm (1.5-18), 30 had large tumors (> 5 cm) and 15 were had small tumors (≤ 5 cm). Patients with embolus in portal vein and its main branch or with intrahepatic and ultrahepatic metastasis nodules belonged to the metastasis group, the other patients were to nonmetastasis group. Six normal livers were from the surrounding noncirrhotic liver tissues of pathologically proved benign tumors (4 hemangioma, 1 adenoma and 1 cyst).
The tissues used for detection of ICAM-1 were collected from 40 patients with HCC, 20 of them had large tumors and 22 encapsulated: 9 patients were in early stage and 31 in moderate stage. Positive AFP was found in 30 cases. There were 16 patients in the metastasis group, and 24 in nonmetastasis group. The 8 normal liver tissues were collected form thesurrounding noncirrhotic tissues of pathologically proved benign liver tumors (5 hemangioma, 1 adenoma and 2 cyst).
Monoclonal antibody of ICAM-1(1 g/L) was purchased from R-G Company, Britain; AKP goat anti mouse IgG andbiotinylated rabbit anti-mouse IgG were from Sino-Amercain Company, China; TRIzolTM total isolation reagent from Gibro Company, USA; and megaprimeTM DNA labeling systems from Amersham Company, UK.
Indirect immunoperoxidase staining The fresh specimens embedded by O.C.T were frozen in liquid nitrogen, and then stored at -70 °C. Serial frozen sections (6 μm) of liver specimens were fixed with acetone. After preincubated with human serum to reduce background, the sides were incubated with the primary antibody (1:50) at 4 °C overnight, biotinylated rabbit anti mouse immunoglobulin (1:200) was used at 37 °C for 1 h, added with avidin biotin HRP mixture (1:100 v/v), and counterstained with hematoxylin.
Dot immuno blot Three hundred mg tissue was homogenized in 1.5 mL suspended buffer solution (0.1 mol/L NaCl, 0.01 mol/L Tris. Cl, pH 7.6, 0.001 mol/L EDTA pH 8.9, 1% Triton-X-100), the protein concentration was determined by Hatree method[4]. The supernatant (30 μL) was spotted onto nitrocellulose membrane in a dot blot format. After non-specific blocking with 5% lipid free milk, the blots were incubated with ICAM-1 antibody (1:500) at room temperature for 2 h, and then incubated with AKP conjugated goat anti-mouse IgG (1:200) for 2 h at room temperature, then stained with NBT/BCIP (2:1 v/v). The integrated optical density (IOD) of the blot was measured by MIAS 300 automatic image analyzer. Tissue ICAM 1 = (sample IOD background IOD) × sample area/(μg protein concentration × 1000).
Northern blot Total RNA was extractedby TRIzolTM reagent according to the manufacturer’s instruction (Gibco Life Technologies Cat No.15596.026). Total RN A of 20 μg was denatured in formaldehyde, electrophoresed, and transferred to nitrocellulose membrane. The plasmid containing ICAM-1 probe sequence was kindly provided by Dr. Christian Stratowa (Ernst Boehringer Institute, Vienna, Austria). Northern transfer membranes were prehybridized for 4 h at 42 °C with 5 × SSPE/5 × SSPE/5 × Denhardt’s solution/1% SDS/100 mg/L Samon sperm DNA. The 1.8 kb cDNA was labeled with 32 Pisotope by random primer method (MegaprimeTM DNA Labeling Systems, Amersham). Nothern blot analysis was performed under stringent washing conditions, 1 × SSC 0.1% SDS, 30 min at room temperture and 1 × SSC 0.1% SDS, 1 h at 50 °C. Autoradiography was carried out at -70 °C for 7 d.
Data analysis Significance was assessed by Student’s t test. The results were expressed as x-±s.
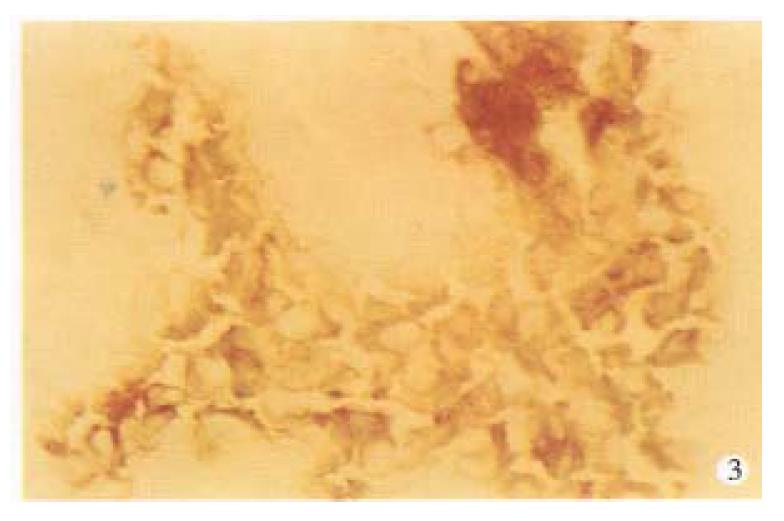
By using anti ICAM-1 monoclone antibody, no ICAM-1 was foundin normal liver tissues (Figure 1). ICAM-1 was positive in fresh HCC (Figure 2), mainly on cell membrane, with a rate of 80% (36/45). In this group, 42.6% showed strong positive (+ +, 30%-70% positive cells), others were weak positive (+, 0%-30% positive cells). ICAM 1 was also positive in 2 cases with tumor embolus in portal vein (Figure 3).
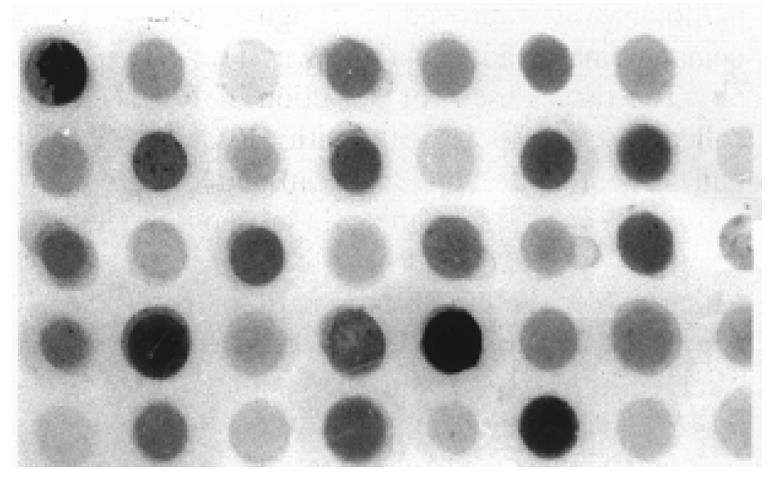
Dot immuno-blot was employed in semi-quantive analysis of tissue ICAM-1 (Figure 4). Table 1 demonstrats that ICAM-1 content in HCC was about two-fold higher than that in the tumor surrounding liver tissues and normal controls (P < 0.01), no significant difference was found between the latter two.
| Groups | Cases | ICAM-1 |
| HCC | 40 | 13.43 ± 0.09 |
| Tumor surrounding liver tissues | 40 | 5.89 ± 0.17b |
| bNormal liver | 8 | 4.27 ± 0.21b |
The expression of tissue ICAM 1 was also compared with tumor biological characters (Table 2), ICAM-1 in cancer tissues was only related to tumor metastasis, and there was no relationship with tumor size and capsule formation.
| Groups | Cases | ICAM-1 | P-value |
| Metastasis | 16 | 20.24 ± 0.30 | < 0.05 |
| Nonmetastasis | 24 | 10.23 ± 0.12 | |
| Metastasis tumor surrounding liver | 16 | 6.80 ± 0.08 | > 0.05 |
| Nonmetastasis tumor surrounding liver | 21 | 4.78 ± 0.04 | |
| Intact capsule | 22 | 13.55 ± 0.20 | > 0.05 |
| Nonintact capsule | 15 | 12.93 ± 0.32 | |
| Large HCC | 20 | 14.45 ± 0.26 | > 0.05 |
| Small HCC | 14 | 10.96 ± 0.47 |
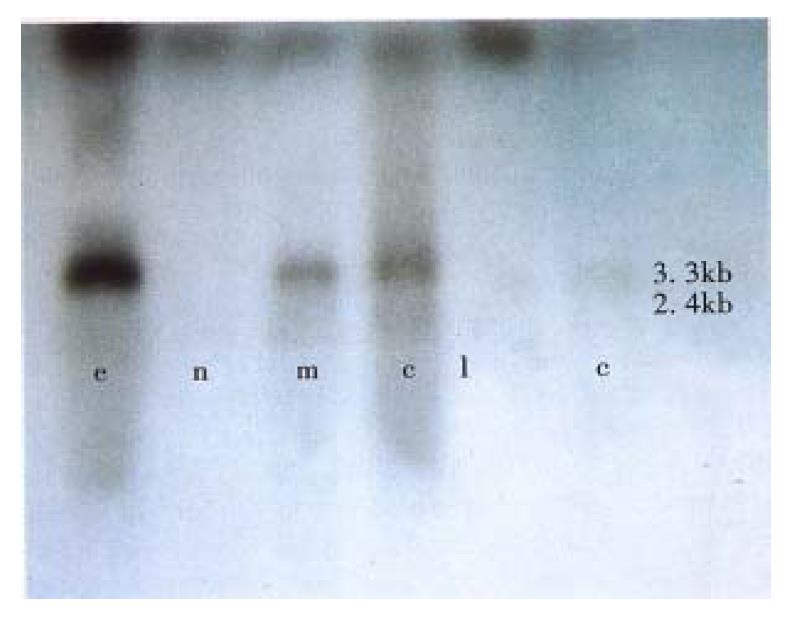
In this study, special DNA probe was used in Northern blot for detecting ICAM-1 mRNA, two transcripts with 3.3 kb and 2.4 kb in length was defined. β-actin was acted as internal standard. The data obtained from image analysis indicated that ICAM-1 mRNA expression in tumors was higher than that in tumor surrounding liver tissues and normal controls as well as cancer embolus (Figure 5).
Intercellular adhesive molecule-1 (ICAM-1, CD54), a transmembrane glucoprotein, is a member of the immunoglobulin superfamily and functions as counterreceptor for leukocyte function associated antigen-1 (LFA-1, CD11a/CD18) and complement receptor type 3 (MAC-1, CD11b/CD18)[5]. It mediates homotypic and heterotypic cell interaction, not only plays an important role in the tumor genesis, but also can reflect tumor curative effect[6], and may be a critical factor in the process of bloodborne metastasis of cancer[7]. Recently it wasfound that ICAM-1 plays an important role in tumor recurrence and metastasis. Hung et al[8] found that anti-ICAM-1Ab can prevent the growth of APH-77 tumors in SCID mice by altering the homing and growth of tumor cells in certain anatomical sites. Most of normal tissues do not express ICAM-1, but ICAM-1 expression has been observed in many tumors, such as renal cancer, uterine cancer, lung cancer, gastric cancer, bladder cancer, etc. Santarosa et al[9] demontrated that renal cancer recurrence after operation was related to ICAM-1 expression in cancer tissues and patients with < 50% of ICAM-1 positive cells in cancer tissues had prolonged disease-free survival after a median follow-up of 60 months.
In this study, all 6 cases of normal liver sample were negative in anti ICAM-1 immunohistochemical staining, 36/45 HCC presented various ICAM-1 expression. The rate of positive cell was a little higher in large tumors, tumors with intact capsule and tumors with metastasis, but there was no significant difference. It has been noticed that 2 cases with enbolus also had high ICAM-1 expression. As indicated in semiquantitative analysis of tissue ICAM-1, ICAM-1 concentration in liver cancer was higher than in tumor surrounding liver tissues and normal livers. Tissue ICAM-1 was higher in metastasis group than that in nonmetastasis group. Northern blot analysis revealed that ICAM-1 expression at mRNA level was also higher in HCC and cancer embolus than in tumor surrounding liver tissues and normal controls. So it was suggested that ICAM-1 might be one of the characters of liver cancer, and could be a diagnostic indicator for metastatic status of liver cancer.
The deduced ICAM-1 amino acid sequence analysis revealed the presence of C3/C4 binding protein consensus sequence between residues 341 and 404, so ICAM-1 may be involved in C3b binding. Since C3b binding protein generally acts to accelerate the dissociation of active complement complexes, ICAM-1 may help the tumor escape immune destruction, providing an important property needed for successful metastasis. Functioning as a ligand of LFA-1, ICAM-1 can establish the heterotypic adhesion of tumor cells with migratory and invasive leukocytes present in the tumor cells, enabling individual cell to dissociate from primary tumor and go into circulation[10]. There is soluble ICAM-1 (sICAM-1) in serum, which comes from proteolytic cleavage of membrane associate ICAM-1. Since LFA-1 could also be blocked with soluble ICAM-1, sICAM-1 may also block the attachment of cytotoxic T cell and/or NK cells to cancer cells. This could be one of the mechanisms of cancer cells escaping from the immunosurveillance system of the host, and can inhibit lymphocyte mediated cytotoxicity, therefore a favorable environment for tumor cell proliferation would be created[11]. Spontaneous regression of human liver tumor was quite rare and the tumors were unusually resistant to various immumotherapy, probably due to the high content of sICAM-lin those patients.
In our experiment, we also found that there was no statistically significant difference in ICAM-1 expression between tumor surrounding liver tissues and normal livers, and between tumor metastasis group and nonmetastasis group. So we think that ICAM-1 reflects the metastatic ability of cancer cells but not the cancer environment.
In conclusion, tissue ICAM-1 could reflect the growth and metastatic status of HCC and may be a factor to predict liver cancer metastasis.
Supported with Grant for Leading Specialty by the Shanghai Health Bureau, National Natural Science Foundation No.396707 and China. Medical Board No.93-583
| 1. | Zhou XD, Tang ZY, Yu YQ, Yang BH, Lu JZ, Lin ZY, Ma ZC, Zhang BH. Recurrence after resection of alpha-fetoprotein-positive hepatocellular carcinoma. J Cancer Res Clin Oncol. 1994;120:369-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Yasoshima T, Denno R, Kawaguchi S, Sato N, Okada Y, Ura H, Kikuchi K, Hirata K. Establishment and characterization of human gastric carcinoma lines with high metastatic potential in the liver: changes in integrin expression associated with the ability to metastasize in the liver of nude mice. Jpn J Cancer Res. 1996;87:153-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Nasu K, Narahara H, Etoh Y, Kawano Y, Hirota Y, Miyakawa I. Serum levels of soluble intercellular adhesion molecule-1 (ICAM-1) and the expression of ICAM-1 mRNA in uterine cervical cancer. Gynecol Oncol. 1997;65:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Hartree EF. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972;48:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4025] [Cited by in RCA: 3697] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 5. | Budnik A, Grewe M, Gyufko K, Krutmann J. Analysis of the production of soluble ICAM-1 molecules by human cells. Exp Hematol. 1996;24:352-359. [PubMed] |
| 6. | Shimizu Y, Minemura M, Tsukishiro T, Kashii Y, Miyamoto M, Nishimori H, Higuchi K, Watanabe A. Serum concentration of intercellular adhesion molecule-1 in patients with hepatocellular carcinoma is a marker of the disease progression and prognosis. Hepatology. 1995;22:525-531. [PubMed] |
| 7. | Regimbald LH, Pilarski LM, Longenecker BM, Reddish MA, Zimmermann G, Hugh JC. The breast mucin MUCI as a novel adhesion ligand for endothelial intercellular adhesion molecule 1 in breast cancer. Cancer Res. 1996;56:4244-4249. [PubMed] |
| 8. | Huang YW, Richardson JA, Vitetta ES. Anti-CD54 (ICAM-1) has antitumor activity in SCID mice with human myeloma cells. Cancer Res. 1995;55:610-616. [PubMed] |
| 9. | Santarosa M, Favaro D, Quaia M, Spada A, Sacco C, Talamini R, Galligioni E. Expression and release of intercellular adhesion molecule-1 in renal-cancer patients. Int J Cancer. 1995;62:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Johnson JP, Stade BG, Holzmann B, Schwäble W, Riethmüller G. De novo expression of intercellular-adhesion molecule 1 in melanoma correlates with increased risk of metastasis. Proc Natl Acad Sci USA. 1989;86:641-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 242] [Article Influence: 6.7] [Reference Citation Analysis (0)] |