Published online Apr 15, 1998. doi: 10.3748/wjg.v4.i2.162
Revised: December 30, 1997
Accepted: January 10, 1998
Published online: April 15, 1998
AIM: To investigate the effects of TRH in DVC on motility of the gallbladder in rabbits.
METHODS: fter fasted for 15h-18h, rabbits were anesthetized with urethane (1.0 g/kg). Gall-bladder pressure (GP) was measured by a frog bladder perfused with normal saline.
RESULTS: After microinjection of TRH (8.8 nmol, 1 μL) into DVC, GP was raised and the frequency of phasic contraction of gallbladder (FPCGB) increased. All the doses of TRH (0.13, 0.25, 0.50, 0.80, 1.30 nmol, 1 μL) injected into DVC could excite the motility of gallblader. As the dose of TRH was enlarged, the amplitude and duration of the reaction increased. Effects of TRH in DVC on motility of the gallbladder could be completely abolished by atropine (0.2 mg/g, i.v.) or vagotomy, but could not be inhibited by phentolamine iv (1.5 mg/g) or propranolol iv (1.5 mg/g)or by transecting the spinal cord.
CONCLUSION: Thyrotropin-releasing hormone in DVC can excite motility of gallbladder. This effect was mediated by vagus nerves and peripheral M receptor. Its physiological significance may be related to maintaining the phasic contraction of gallbladder in interdigestive period.
- Citation: Liu CY, Liu JZ, Zhou JH, Wang HR, Li ZY, Li AJ, Liu KJ. TRH microinjection into DVC enhances motility of rabbits gallbladder via vagus nerve. World J Gastroenterol 1998; 4(2): 162-164
- URL: https://www.wjgnet.com/1007-9327/full/v4/i2/162.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i2.162
Our previous studies indicated that the dorsal vagal complex (DVC) might play an important role in regulating motility of extrahepatic biliary tree[1], but we do not know which neurotransmitter mediates this effect.
Thyrotropin-releasing hormone (TRH) is one of important brain gut peptides[2]. There is a high density of TRH-immunoreactive nerve terminals in DVC[3]. Injecting TRH into DVC could excite the motility and secretion of stomach[2]. However, there is no report about TRH in DVC on the motility of extrahepatic biliary tree. In this study, TRH was injected into DVC of anesthetized rabbits, and then the effect on mean gallbladder pressure (GP) and frequency of phasic contraction of gallbladder (FPCGB) were observed, and the peripheral route of the effect of TRH was also investigated.
The experiments were conducted in 38 rabbits weighing 2.0 kg-2.5 kg. Fasted for 15 h-18 h, but allowed to drink water, the rabbits were anesthetized with urethane (1.0 g/k, iv.). A cannula was inserted into the trachea. Unilateral femoral artery was catheterized for measurement of blood pressure. The rectal temperature was maintained at 37 °C-38.5 °C.
A frog bladder filled with saline was placed in gallbladder and connected to a transducer (TP-200T) by a catheter. The blood pressure and GP were record-ed simultaneously by a polygraph (RM-6000, NI-HON KOHDEN).
The animal’s head was fixed in a stereotaxis (I-C model, JIANG-WANG, China). According to the Messen’s methods, a stainless steel guide tube (0.5 mm in outer diameter) containing a needle was inserted into DVC (rostral from obex 0.5 mm-1.0 mm, left or right from midline 0.7 mm-1.2 mm, depth from the surface 0.5 mm-1.2 mm). Normal saline or TRH solution(1 μL) was injected into DVC by a microsyringe connected to a cannula (0.3 mm in outer diameter).
Before the following tests were made, the basal level of GP of each animal was taken as control (i.e., basal GP as 0 kPa in place of real level). GP increase during the test, was considered as positive (+) value, while GP decrease was taken as negative (-). Student’s t test was used for the statistical analysis. All values were x-± sx-. P < 0.05 was considered to be statistically significant.
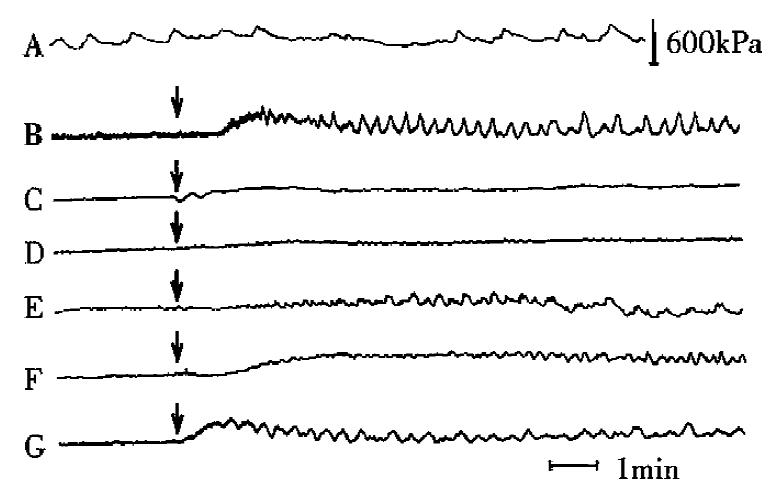
In interdigestive period, two kinds of motion of gallbladder were found, the tonic and phasic contractions. Tonic contraction, which means continuous and weak contraction, could maintain GP at a stable level (2.67 × 103± 190 Pa, n = 8). Phasic contraction, with a duration of 5-20 s and frequency of 1-3 times per minute, raised GP by 60 kPa-200 kPa rhythmically (Figure 1 a).
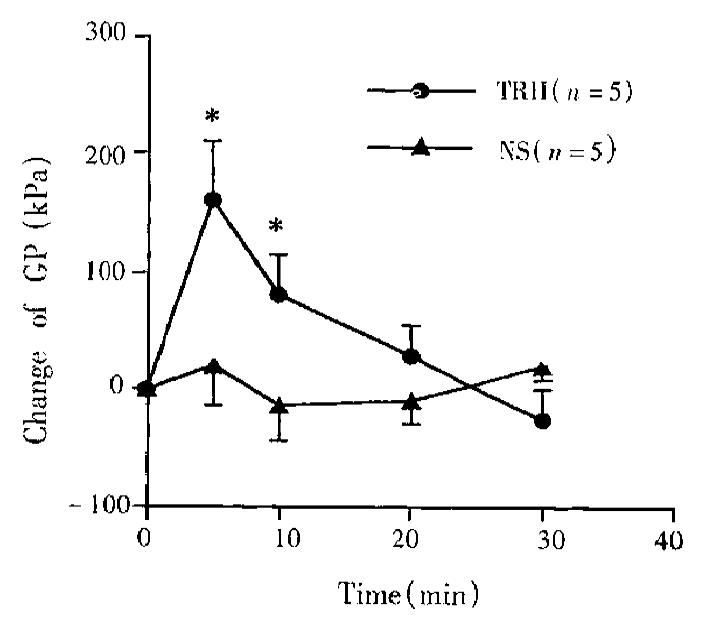
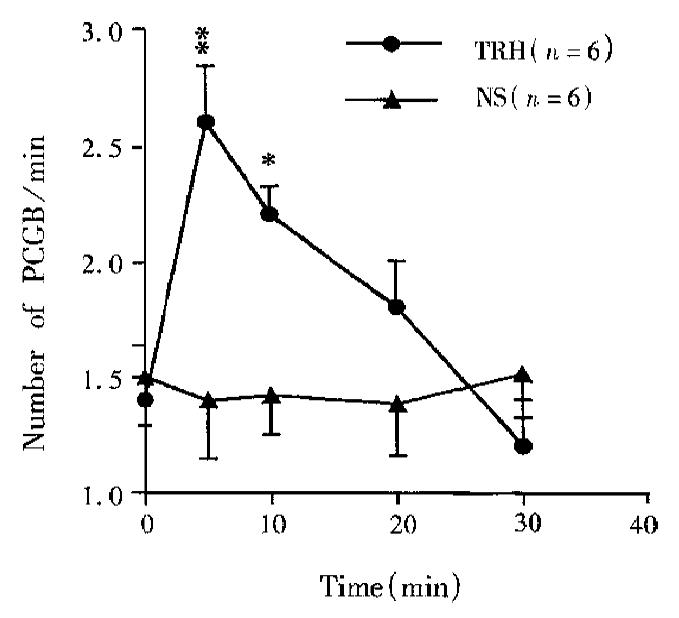
After TRH (0.8 nmol,1 μL) was microinjected into DVC, the motility of gallbladder was enhanced. The effect occurred in 1 min after the injection, reached its peak in 10 min and returned to normal within 20 min-30 min. At 5 min after injection, GP increased by 167 kPa ± 29 kPa (n = 5, P < 0.02); FPCGB increased from 1.14 ± 0.26 to 2.28 ± 0.26 per min (n = 5, P < 0.034) (Figure 1b, Figure 2, Figure 3). However, after normal saline (1 μL) injected into DVC or TRH (0.8 nmol, 1 μL) into the control position in medulla (lateral 0.5 mm-1.0 mm to the DVC), the GP and FPCGB did not change significantly.
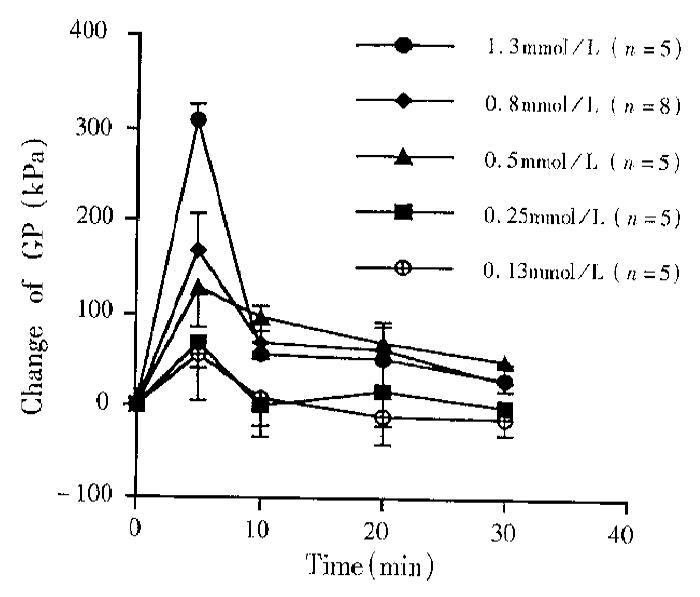
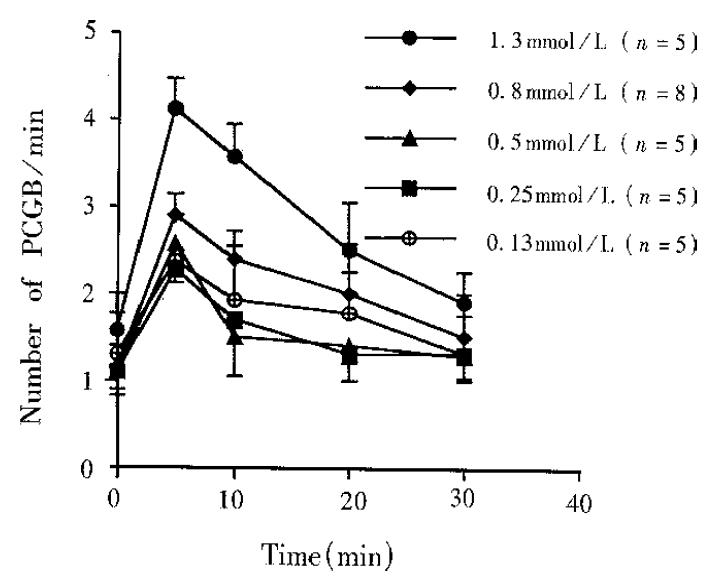
All the doses of TRH (0.13, 0.25, 0.50, 0.80, 1.30 nmol, 1 μL) microinjected into DVC could excite the motility of gallbladder. As the dose increased, the strength and duration of the effect of TRH on GP and PCGB went up, with a significant dose-response (Figure 4 and Figure 5).
Ten minutes after the cervical vagotomy (n = 5) or i.v. injection of atropine (0.2 g/kg, n = 5), mi-croinjection of TRH (0.8 nmol, 1 μL) could no longer excite the motility of gallbladder. Within 30 minutes after the injection of TRH, the GP and FPCGB had no significant changes (Figure 1b, c). However, ten minutes after transection of the spinal cord from T1 or i.v. injection of phentolamine (1.5 mg/kg) or propranolol (1.5 mg/kg), injection of TRH into DVC could also enhance the motility of gallbladder (Figure 1d, e, f).
In this study, we found that TRH microinjection into DVC raised GP and FPCGB. As an important brain-gut peptide, TRH in DVC plays an important role in regulating the function of gastrointestinal tract[2]. We found for the first time that TRH microinjected into DVC excited the motility of gallbladder in rabbits.
The effects of TRH microinjected into DVC on the motility and secretion of gut may be physiological. In DVC, there is a high density of TRH-immunoreactive nerve terminals which come from the nucleus raphe pallidus and raphe obscurus. Some of these terminals make synaptic contacts on the dendrities of gastric vagal motoneurons[5]. Multicolon TRH antibody injected into DVC can inhibit the gastric acid secretion[6].
Through vagus nerve and peripheral cholinergic M receptors, TRH could excite the gallbladder motility, but its physiological significance is unknown. Bile evacuation occurrs during sham feeding, and is abolished by vagotomy[7]. After vagotomy or gastrectomy, the incidence of gall-stones increases[8,9]. In this experiment, TRH in DVC raised GP and then facilitated the bile evacuation. Thus TRH may be a possible candidate for the transmitter in DVC mediating the cephalic bile output.
The present experiment showed that there was rhythmic phasic contraction of gallbladder in rabbits in the interdigestive period, which was similar to that found in dogs[10]. It was believed that this phasic contraction stirred the bile in gallbladder and prevented gallstones forming in the interdigestive period[11]. Our experimental results also showed that TRH microinjected into DVC enhanced the phasic contraction via vagus nerve. Thus TRH in DVC may be regarded as an important neurotransmitter in mediating the phasic contraction of gallbladder. Similar to Ura’s data in dogs[11], vagotomy almost inhibited the phasic contraction completely in our study. It was suggested that the tonic excitation of vagus nerve center may be important in maintaining this type of motion in the interdigestive period. TRH may participate in this effect.
In conclusion, we believed that TRH in DVC may play an important role in regulating the motility of gallbladder via vagal nerves and peripheral cholinergic M receptors, and may participate in cephalic gallbladder emptying during ingestion and maintain the phasic contraction of gallbladder in the interdigestive period.
We are grateful to Professor Zhu-Li He, Director of the Department of English, Shandong Medical University, for polishing the manuscript.
Project supported by the Natural Science Foundation of Shangdong Province (Y95c1032).
| 1. | Liu CY, Liu JZ, Zhou JH, Wang HR, Li ZY. The effects of electric and chemical stimulation of DVC on the motility of biliary system. Acta Acad Med Shandong. 1997;35:207-210. |
| 2. | Taché Y, Stephens RL, Ishikawa T. Central nervous system action of TRH to influence gastrointestinal function and ulceration. Ann N Y Acad Sci. 1989;553:269-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Lynn RB, Kreider MS, Miselis RR. Thyrotropin-releasing hormone-immunoreactive projections to the dorsal motor nucleus and the nucleus of the solitary tract of the rat. J Comp Neurol. 1991;311:271-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Palkovits M, Mezey E, Eskay R, Brwnstein MJ. Innervation of the nucleus of the solitary tract and the dorsal vagal nucleus by thyrotropin-releasing hormone-containing raphe neurons. Brain Res. 1996;373:246-251. |
| 5. | Rinaman L, Miselis RR. Thyrotropin-releasing hormone-immunoreactive nerve terminals synapse on the dendrites of gastric vagal motoneurons in the rat. J Comp Neurol. 1990;294:235-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Hernandez DE, Jennes L, Emerick SG. Inhibition of gastric acid secretion by immunoneutralization of endogenous brain thyrotropin-releasing hormone. Brain Res. 1987;401:381-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Fisher RS, Rock E, Malmud LS. Gallbladder emptying response to sham feeding in humans. Gastroenterology. 1986;90:1854-1857. [PubMed] |
| 8. | Hauters P, de Neve de Roden A, Pourbaix A, Aupaix F, Coumans P, Therasse G. Cholelithiasis: a serious complication after total gastrectomy. Br J Surg. 1988;75:899-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Ihasz M, Griffith CA. Gallstones after vagotomy. Am J Surg. 1981;141:48-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Matsumoto T, Sarna SK, Condon RE, Dodds WJ, Mochinaga N. Canine gallbladder cyclic motor activity. Am J Physiol. 1988;255:G409-G416. [PubMed] |
| 11. | Ura K, Sarna SK, Condon RE. Antral control of gallbladder cyclic motor activity in the fasting state. Gastroenterology. 1992;102:295-302. [PubMed] |