Published online Apr 15, 1998. doi: 10.3748/wjg.v4.i2.140
Revised: December 20, 1997
Accepted: January 18, 1998
Published online: April 15, 1998
AIM: To elucidate the effect of various solutions for small bowel graft preservation in pigs under hypothermic storage.
METHODS: The swine segmental small bowel graft was autotransplanted after it was preserved with lactated Ringer¡äs (LR), Euro-Collins (EC), hyperosmolarity citrate adenine (HC-A) and WMO-1 solu-tions for 10, 18 and 24 h, respectively. The recipient survival rate, morphological structure, graft mucosal energy substances and Na+ K+ ATPase activity were studied, and graft absorption was estimated with D-xylose absorption test.
RESULTS: The morphological study of the grafts preserved with LR or HC-A solution for 10 hours or with EC and WMO-1 solution for 18 h was nor-mal 6 d after operation. Mucosal ATP, total adenine nucleotides (TAN) contents and Na+-K+AT-Pase activity of the graft preserved with EC or WMO solution were higher than that of the graft preserved with LR or HC-A solution. Serum level of D-xylose was higher in EC and WMO-1 groups than in LR and HC-A groups when the graft was preserved for 24 h.
CONCLUSIONS: EC and WMO-1 solutions can preserve the swine small bowel up to 18 h, which are superior to LR and HC-A solutions.
- Citation: Li YS, Li JS, Li N, Jiang ZW, Zhao YZ, Li NY, Liu FN. Evaluation of various solutions for small bowel graft preservation. World J Gastroenterol 1998; 4(2): 140-143
- URL: https://www.wjgnet.com/1007-9327/full/v4/i2/140.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i2.140
One of the problems accompanying successful clinical small bowel transplantation is the graft preservation, for the intestinal mucosa is probably the most sensitive tissue to ischemia in the body. The effective time of cold storage for small bowel graft (< 8 h) is much shorter than that of other organs, and optimal preservation solution is not yet clearly defined for small bowel grafts. In this study, the swine small bowel was preserved with lactated Ringer’s (LR), hyperosmolarity citrate adenine (HC-A), Euro-Collins (EC) and WMO-1 solutions to elucidate which is appropriate for swine small bowel preservation.
Outbred pigs weighing 18.5 kg-22.5 kg were used. The pigs were fasted for 24 h before operation, and general anesthesia was achieved with intravenous sodium pentobarbital.
Heterotopic segmental small bowel autotransplantation was performed as described previously[1]. The small bowel was sectioned before the ileocecal valve and mid-small intestine. The superior mesenteric artery of the graft was perfused with 400 mL-500 mL LR, EC, HC-A or WMO-1 solutions, and stored in the same solution at 4 °C for 10, 18 and 24 h, respectively. The perfusion pressure was 10.8 kPa-11.8 kPa, and perfusion time was 20 min. The lumen was perfused with metronidz-zole solution at 4¡æ after perservation, and the small bowel graft was autotransplanted. Both ends of the grafts were brought out as stomas (Thiry-Vella loops). Intestinal continuity was restored with two end-to-end anastomoses. The experimental groups and preservation time are shown in Table 1.
| Groups | Preservation time | ||
| 10 h | 18 h | 24 h | |
| LR | 5 | 5 | 6 |
| EC | 5 | 7 | 6 |
| HC-A | 6 | 5 | 5 |
| WMO-1 | 5 | 6 | 5 |
The recipient survival rateon the 21st postoperative day (POD) was observed.
Biopsy specimens obtained from the graft stomas, fixed in 10% neutral formation and embedded in paraffin, and sections were cut at 4 μm thickness, and stained with haemotoxylin and eosin. All specimens were observed under light microscopy.
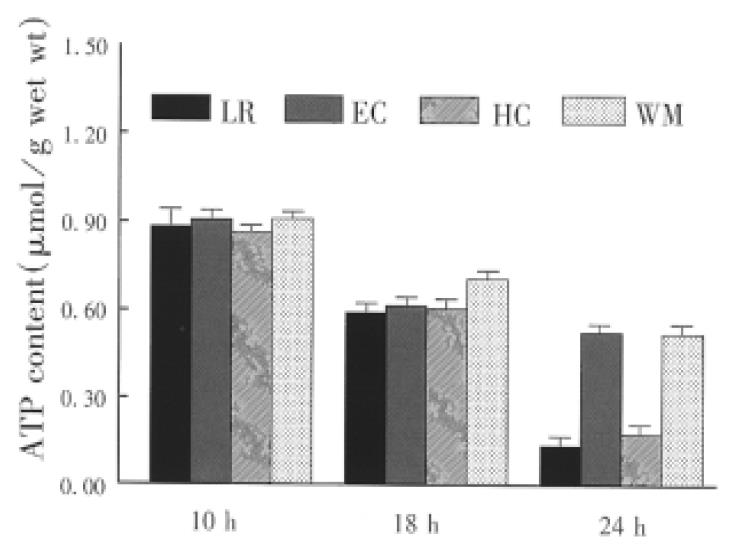
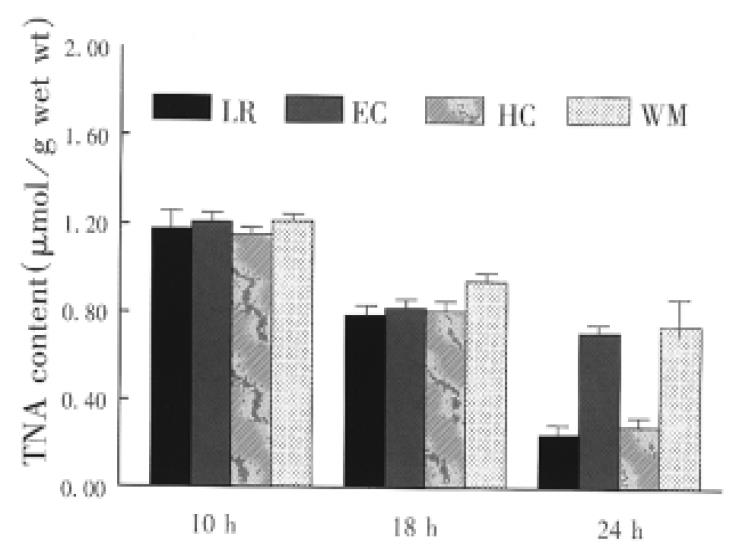
Small bowel graft energy phosphates (ATD, ADP and AMP) were determined by high performance liquid chromatography (HPLC) as described previously[2]. The mucosa (weighing about 0.5 g) was separated from the seromuscular wall and stored in liquid nitrogen. Atkinson energy charge (AEC) was calculated, AEC = (ATP + 0.5ADP)/TAN, total adenine nucleotides (TAN) ATP + ADP + AMP.
Small intestinal mucosal Na+-K+ ATPase activity was determined using Zemelman’s method[3]. The mucosa was separated and stored by the above-men-tioned method.
Absorptive function of the graft was estimated with D-xylose absorption test. Five g D-xylose (dissolved in 100 mL normal salt solution) was irrigated into the graft from the proximal stoma, and serum D-xylose level was determined by Eberts’ method[4].
All results were expressed as x-± s, statistical variances were made by Student’s t test or χ2 analysis, differences were considered significant if P < 0.05.
Six animals died because of failure in vascular anastomosis. Five animals were observed in each group. The survival rate on the 21st POD was similar in the graft preserved with each of the four solutions either for 10 or 18 h. When the graft was preserved for 24 h, the 21st POD survival rate was lower in LR group than in EC and WMO-1 groups (P < 0.05, Table 2).
Epithelial necrosis and edema occurred by all the solutions. On the 3rd or the 4th POD, the grafts be-came normal in all groups histologically when the graft was preserved with each of four solutions for 10 h 6 or 7 d after operation. When the graft was preserved for 18 or 24 h, more severe tissue reperfusion injury was seen after revasculation in LR and HC-A groups than in EC and WMO-1 groups. On the 21st POD mucosa histological structure of the grafts preserved with EC and WMO-1 solutions for 18 h were normal. Necrosis was present in the graft preserved with LR and HC-A solutions. When the graft was preserved with each of the four solutions for 24 h, the mucosal histological structure can not recover to normal.
The concentration of phosphorylated adenine nucleotides was similar in the graft preserved with the four solutions for 10 h. Cold storage significantly reduced the contents of ATP and TAN (Figure 1 and Figure 2). Tissue contents of ATP and TAN were significantly higher in the graft perserved with EC and WHO-1 solutions after 24 h of cold storage. The energy change as a parameter for the freely available energy in the cell was significantly higher in EC and WMO-1 groups (0.66 nmol/g ± 0.03 nmol/g wet weight, 0.63 nmol/g ± 0.03 nmol/g wet weight) than in LR and HC-A groups (0.55 nmol/g ± 0.04 nmol/g wet weight, 0.58 mol/g ± 0.03 nmol/g wet weight).
Mucosal Na+-K+ ATPase activity remained unchanged during cold storage despite storage time and preservation solution. Na+-K+ ATPase activity was decreased after 30 min of reperfusion (Table 3) in all the grafts. Mucosal Na+-K+ ATPase activity was significantly lower in LR and HC groups than in EC and WMO-1 groups after 24 h of cold storage.
After 10 and 18 h of cold storage, serum level of D-xylose was similar in all groups while after 24 h it was higher in EC and WMO-1 groups than in LR and HC-A groups (Figure 3).
Simple cold storage is the most practical method in organ preservation. Several solutions have been used in organ preservation, however, the optimal one is not clearly defined for small bowel grafts. A recently developed organ preservation solution, UW (University of Wisconsin) has provided 72 h preservation for pancreas and kidney, and 30 h or longer for the liver, but provided only 8-10 h preservation for small bowel in human[5].
LR solution, an extracellular fluid, is widely used in experimental small bowel preservation. Luther et al[6] and Zhang et al[7] reported that LR solution is superior to UW and EC solutions for small bowel storage in the rat model. In our studies, no animal receiving the graft preserved for 24 h survived more than 21 d. The recipient survival rate in EC and WMO-1 groups was 60%, respectively. Mucosal contents of ATP and TAN, and Na+-K+ ATPase activity in EC and WMO-1 groups were higher than in LR and HC-A groups after 24 h of cold storage. Our results indicated that EC and WMO-1 solutions are superior to LR solution for small bowel preservation in the pig model.
HC-A solution, a modified Ross solution, contains adenine, precursor for ATP production, and its osmolarity was reduced from 400 to 380 mOsm/L. The solution has provided 48 h or longer preservation for the cadaveric kidneys. In our study, mucosal contents of ATP and TAN and Na+-K+ ATPase activity in HC-A preserved small bowel was not higher than in EC and WMO-1 preserved one, so adenine added into the HC-A solution could neither improve the ATP production nor inhibit the tissue ATP degradation.
WMO-1 solution, containing ATP and Ca2+ could provide 48 h preservation for the liver in the rat model. It is believed that ATP in WMO-1 solution could increase the energy state of tissue and prevent ATP degradation and the calcium could stabilize the membranes. Our study indicated that mucosal contents of ATP and TAN were not higher in WMO-1 preserved small bowel than in EC- preserved one. Mucosal Na+-K+ ATPase activity was similar in the two groups, therefore, ATP added into WMO-1 solution could not prevent ATP degradation in tissues. It was not introduced in latest-developed organ preservation solution, such as UW. In the healthy cells, cytosolic calcium is at very low level (10-7 mmol/L), and hypothermic preservation may disrupt the cell membrane, resulting in influx of calcium into the cell. Elevated intracellular calcium was also shown to potentiate cell damage[8], therefore the effects of ATP and Ca2+ in the WMO-1 solution should be studied.
As shown previously, the EC solution is a standard organ preservation solution and has been widely used in liver, kidney, pancreas and small bowel transplantations[5]. In this study, EC solution is superior to HC-A and LR solutions, and mucosal contents of ATP and TAN and Na+-K+ ATPase activity were higher in EC group than in LR and HC-A groups and were similar to that of WMO-1 group.
Recently, a direct correlation between ATP content during cold preservation and outcome in animal and clinical organ transplantation has been reported[9]. Na+-K+ ATPase is an important membrane-bound enzyme that acts by active extrusion of Na+ from the intracellular compartment to counter-balance the osmotic effect of impermeable intracellular macromolecules. We investigated the effect of four solutions in recipient survival rate, mucosal energy substances, Na+-K+ ATPase activity and absorption of the graft. The results showed that Na+-K+ ATPase activity maintained unchanged during cold storage and reduced significantly after reperfusion but the contents of ATP and TAN decreased during cold storage. There was a direct correlationship between contents of ATP and TAN after cold storage and Na+-K+ ATPase activity after reperfusion and recipient outcome after transplantation. EC and WMO-1 solutions were superior to LR and HC-A solutions for small bowel graft.
In conclusion, the swine small bowel can be successfully preserved in good condition for 18 h using simple cold storage in EC and WMO-1 solutions as assessed by the recipient survival rate, mucosal energy substances, Na+-K+ ATPase activity, and D-xylose absorption test and the EC and WMO-1 solutions are superior to LR and HC-A solutions for preservation of small bowel in the pig model.
Project supported by the National Natural Science Foundation of China, No. 39070828.
| 1. | Li N, Li JS, Liao CX, Li YS, Wu XH. Segmental small bowel allotransplantation in pigs. Chin J Organ Transplant. 1993;14:2-4. [Cited in This Article: ] |
| 2. | Schweinsberg PD, Loo TL. Simultaneous analysis of ATP, ADP, AMP, and other purines in human erythrocytes by high-performance liquid chromatography. J Chromatogr. 1980;181:103-107. [PubMed] [Cited in This Article: ] |
| 3. | Zemelman BV, Walker WA, Chu SH. Expression and developmental regulation of Na+,K+ adenosine triphosphatase in the rat small intestine. J Clin Invest. 1992;90:1016-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Eberts TJ, Sample RH, Glick MR, Ellis GH. A simplified, colorimetric micromethod for xylose in serum or urine, with phloroglucinol. Clin Chem. 1979;25:1440-1443. [PubMed] [Cited in This Article: ] |
| 5. | Toledo-Pereyra LH. Small bowel preservation: evolution of methods and ideas, and current concepts. Transplant Proc. 1992;24:1083-1084. [PubMed] [Cited in This Article: ] |
| 6. | Luther B, Lehmann C, David H, Klinnert J. Preservation of isolated intestinal segments using the University of Wisconsin solution. Transplant Proc. 1991;23:2459. [PubMed] [Cited in This Article: ] |
| 7. | Zhang S, Koluda Y, Neato Em. Biochemical evidence on mucosal damage of in-testinal graft during cold preservation in University of Wisconsin, Euro-collins and lactated Ringer's solution. Transplant Proc. 1994;24:1087-1088. [Cited in This Article: ] |
| 8. | Clavien PA, Harvey PR, Strasberg SM. Preservation and reperfusion injuries in liver allografts. An overview and synthesis of current studies. Transplantation. 1992;53:957-978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 639] [Cited by in F6Publishing: 600] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 9. | Harvey PRC, In S, Mckeown CMB, Petranka CN, Ilson RG, Strasberg SM. Adenine nucleotide tissue concentrations and liver allograft viability after cold preservation and warm ischemia. Transplantation. 1988;45:1016-1020. [Cited in This Article: ] |