Published online Apr 15, 1998. doi: 10.3748/wjg.v4.i2.121
Revised: December 20, 1997
Accepted: January 20, 1998
Published online: April 15, 1998
AIM: To determine the relationship between serum deprivation or serum levels and cell proliferation of human hepatoma SMMC-7721 cells.
METHODS: Human hepatoma SMMC-7721 cells were grown in RPMI 1640 supplemented with 10% fetal calf serum (FCS) in 5% CO2 incubator at 37 °C for 24 h, and culture media were replaced to serum-free or different serum FCS levels (2.5%, 5%, 10%, 20% and 25%). Six h, 12 h, 18 h and 24 h after the culture, the cells were incorporated [3H]-TdR for 4 h. At last [3H]-TdR incorporation was detected with liquid scintillation counting.
RESULTS: DNA synthesis of SMMC-7721 cells could be sharply stimulated by short-time (6 h) serum deprivation (the cpm value of 3H-TdR incorporation of cells in serum-free was 39.32-fold higher than cells in 25% serum), and the incorporation of 3H-TdR was negatively related to the serum levels. Longer-time serum starvation (12 h, 18 h and 24 h) also greatly stimulated DNA synthesis, although the cpm value of 3H-TdR incroporation was less than that in 6 h serum deprivation. Morphology of cells cultured in different serum levels also showed significant difference.
CONCLUSIONS: Compared with other cell lines such as BEL7404 and Swiss 3T3, human hepatoma SMMC-7721 cells had different response to the serum deprivation. Short-time serum deprivation could greatly stimulate DNA synthesis of human hepatoma SMMC-7721 cells. Precautions must be given to the changes of serum levels for the detection of growth factors and drugs using SMMC-7721 cells as a model.
- Citation: Jiang SM, Xu ZH. Serum deprivation enhances DNA synthesis of human hepatoma SMMC-7721 cells. World J Gastroenterol 1998; 4(2): 121-124
- URL: https://www.wjgnet.com/1007-9327/full/v4/i2/121.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i2.121
Mammalian cells in culture required serum in order to proliferation[1]. Serum was a mixture of protein and other substances, among which essential hormones and growth factors can support cell proliferation[2]. In order to reduce the effects of growth factors in serum on cells, the procedure of serum deprivation was often involved to study growth regulation on cultured mammalian cells[3-5]. Prolonged serum deprivation induced fibroblastic cells such as Swiss 3T3 to enter a quiescent state (G0)[6,7]. On the contrary, mouse embryo cells[8] and Hela S-3 cells[9] showed reversal response to serum deprivation and lower serum levels. Human hepatoma SMMC-7721 cells were established in our country[10] and were widely used for detecting the activity of growth factors and drugs. Therefore, it was very important to clarify the effects of serum deprivation and serum levels on the growth and metabolism of SMMC-7721 cells.
Human hepatoma SMMC-7721 cells were obtained from the Shanghai Cell Bank of Chinese Academy of Sciences and maintained in our laboratory. The cells were grown as monolayers in RPMI 1640 medium supplemented with 10%-20% fetal celf serum (FCS) and incubated at 37 °C in the humidified incu-bator with 5% CO2+ 95% air.
Exponent growing cells in flasks were harvested by trypsinization with 0.25% trypsin and suspended in RPMI 1640 medium plus 10% FCS. Cells were plat-ed at 1 × 105 cells/ml in 4 pieces of 24-well plates and incubated at 37 °C in 5% CO2+ 95% air for 24 h. After that, the medium was aspirated and the cells were washed with RPMI 1640 medium. The medium was replaced with RPMI 1640 plus different levels of serum (0%, 2.5%, 5%, 10%, 20% and 25%) in different treatment groups (each group having 4 wells of cells) respectively. The rates of DNA synthesis of cells cultured in different serum levels were detected at interval of 6 h (6, 12, 18 and 24 h).
When cells were cultured in different serum levels for 6, 12, 18 and 24 h respectively, rates of DNA synthesis were determined by pulse labeling for 4 h in 74 KBq ml-1 3H-TdR. The media were aspirated and the cells were gently rinsed with phosphate buffer saline (PBS), trypsinized properly with 0.25% trypsin, collected on 49 model filter mem-brane and rinsed with 5% TCA (trichloroacetic acid) and 100% ethanol (three times). The mem-branes were dried at 80 °C for 30 min. The incorporation of 3H-TdR was determined by liquid scintillation counting.
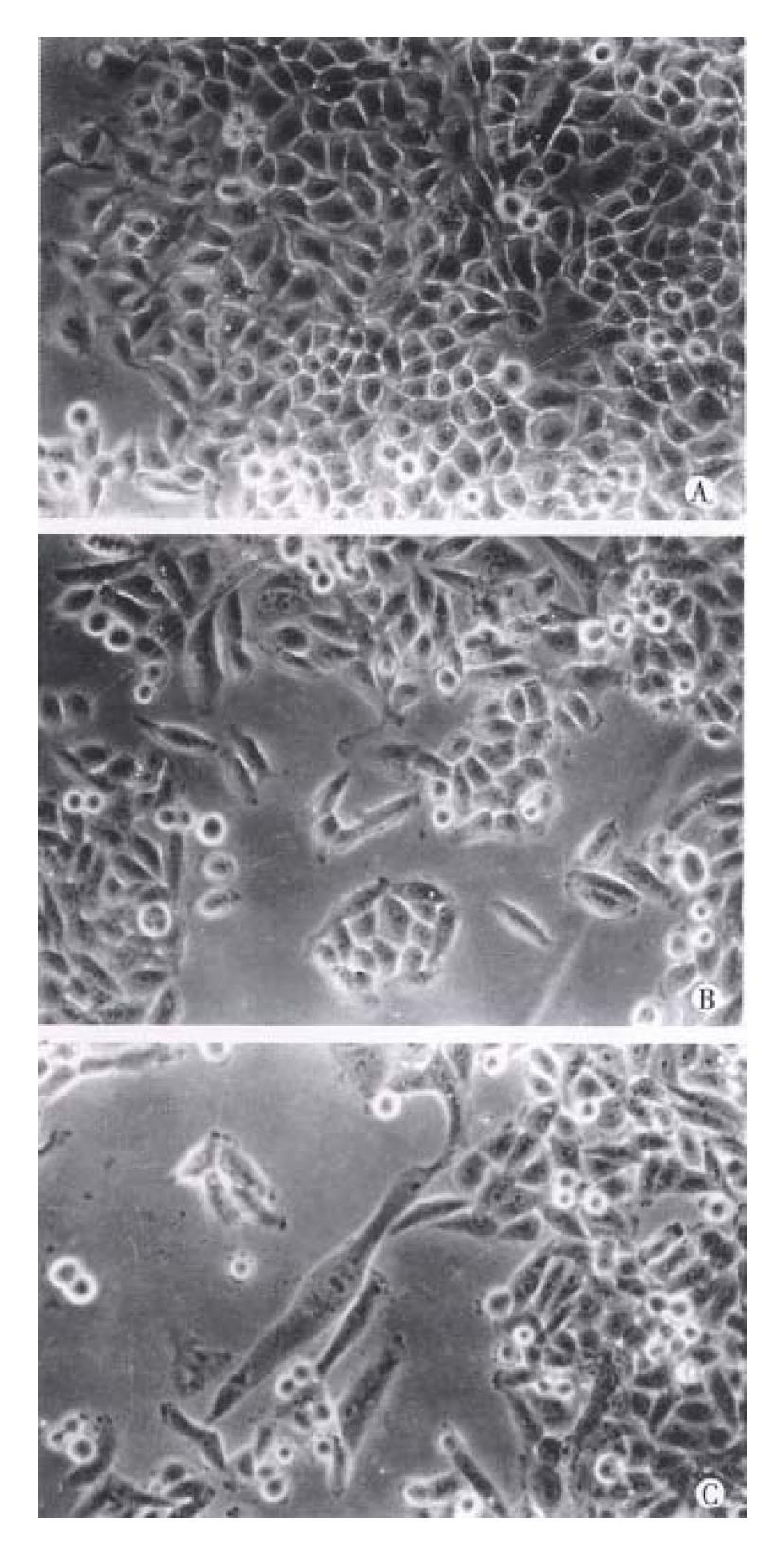
Before collected for liquid scintillation counting, the cells in different treatment groups were ob-served and photographed under inverted phase contrast microscope.
Materials RPMI 1640 medium was from Gibco, USA; trypsin was from Sigma;3H-thymidine (3H-TdR) was from the Institute of Atomic Energy of China; 24-well plates were from NUNC; and Triton X100, POPOP, PPO were from Serva.
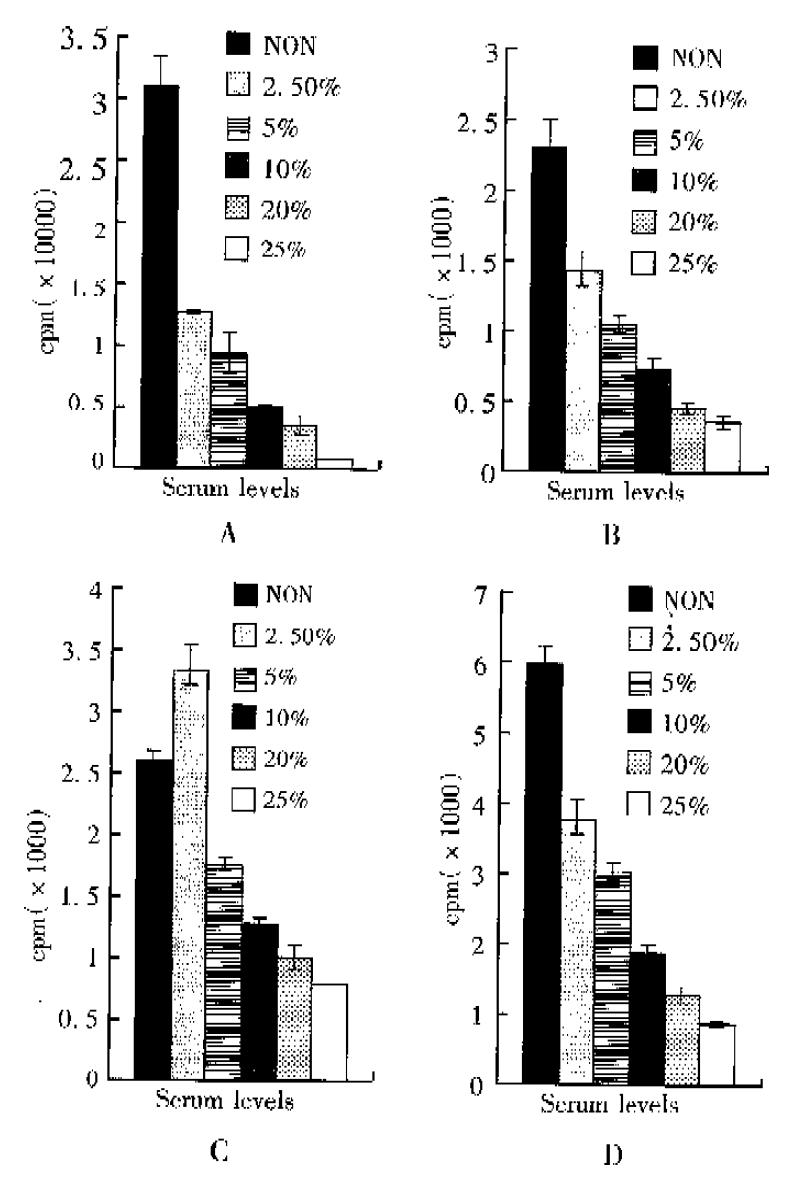
Serum contained significant amounts of thymidine, which would affect the incorporation of their appropriate exogenous labeled forms. This was beacuse the formulated media such as RPMI 1640 for cell culture did not usually contain unlabeled nucleotides, therefore serum provided the only source other than the labeled form. SMMC-7721 cells were pulse labeled with 3H-TdR for 4 h at 74 KBq ml-1 after serum deprivation and treatment of different serum concentrations for different times. The results showed decreased specific activity of the incorporation of 3H-TdR with increased serum concentrations, that was, the incorporation of 3H-TdR had negative relation with serum levels in medium. Cells cultured in serum-free medium for 6 hours after media replacement incorporated 3H-TdR 39.32-fold higher than cells in 25% serum medium (Figure 1A). On the other hand, within 18 h as the time of serum deprivation going down, the incorporation of 3H-TdR decreased (Figure 1B, C, D). The ratio of 3H-TdR incorporation between cells in serum free and in 25% serum decreased to 3.53-fold (Table 1).
| Treatments | 6 h | 12 h | 18 h | 24 h |
| SF/2.5% FCS | 2.47 | 1.63 | 0.778 | 1.67 |
| SF/5% FCS | 3.53 | 2.26 | 1.51 | 1.98 |
| SF/10% FCS | 7.34 | 3.35 | 2.19 | 3.27 |
| SF/20% FCS | 11.61 | 5.30 | 2.72 | 4.88 |
| SF/25% FCS | 39.32 | 7.21 | 3.53 | 7.35 |
Cells cultured in different serum levels showed significant difference in morphology under inverted phase contrast microscope. Cells cultured in serum-free or lower serum level (2.5%) (Figure 2A) were less well spread and smaller than that in higher serum levels. Cells cultured in 5% serum and more (Figure 2B, C) were epithelial-like and well spread.
Most mammalian cells were serum dependent and usually passaged in medium containing serum, and they would die if they were cultured in serum-free medium for long time. Therefore, short-time serum-free (serum deprivation) cell culture was one of the main protocols for the study of cell growth and regulation. How cell responses to the serum deprivation has called extensive attention. The results varied greatly even reversal because of the different cells used. Brooks, Larsson[3,4] and Zetterberg[7] indicated that the proliferation of non-transformed fibroblastic cells usually depend on serum or purified growth factors in the tissue culture medium. If the serum concentration was drastically reduced, the cells ceased proliferation and entered a reversible state of quiescence (G0). Larsson et al[4] reported serum-dependent proliferating 3T3 cells prolonged their intermitotic time by 9-10 h after exposure to serum-free medium for only 1 h and a short exposure to serum-free medium was sufficient for cells to leave the cell cycle. Zetterberg[7] showed that in 3T3 cells, in all stages of cell cycle, serum deprivation resulted in inhibition of protein synthesis, but only in postmitotic cells in the first 3-4 h of G1 did it produce cell-cycle arrest, a 1-h exposure to serum-free medium was sufficient to force most G1 cells into a state of quiescence (G0). Loo et al[8] demonstrated that mouse embryo cells established and maintained in the absence of serum depend on epidermal growth factor for survival and their proliferation was reversibly inhibited by serum or platelet-free plasma. Yin et al[9] showed that Hela cells cultured in 10% serum medium incorporated 3H-TdR to 25% of cells cultured in 0.2% serum medium. Xu et al[11,12] had demonstrated that human hepatoma BEL-7404 could grow in serum-free medium and there were only less than 2%-3% apoptotic cells after serum starvation for 24 h. Human hepatoma SMMC-7721 cell were serum-dependent proliferating cell line and widely used in the detection of growth factors and anti-cancer drugs, therefore it was very important to as-certain the effects of short-time serum deprivation and serum levels on growth and metabolism of SMMC-7721 cells, which would affect the correct assessment of the activity of growth factors and drugs. Our results suggested that short-time serum deprivation (6 h) could stimulate the synthesis of SMMC-7721 cells and the incorporation of 3H-TdR was negatively related to the serum levels in medium. For longer (12, 18, and 24 h) expo-sure to serumª² free medium, the incorporation of 3H-TdR was also negatively related to the serum level, although the ratio of 3H-TdR incorporation of cells in serumª²free medium and in 25% serum medium decreased from 39.32-fold (6 h) to 3.53-fold (18 h). These results were reversal to that with 3T3 cells, which could easily be overlooked, or even mistakenly attributed to the activity of growth factors and drugs. The results might be due to two main reasons, one was that DNA synthesis of SMMC-7721 cells cultured in serum-free medium was inhibited by the lack of thymidine in RPMI 1640 medium and the cells were accumulated to the G1/S. Once 3H-TdR was added to the medium, the cells began to enter S phase quasisynchronously and start DNA synthesis, the other reason was that most of malignant cells appeared less dependent on serum and could secrete some growth factors and stimulate themselves by feedback mechanism. The differences of cell morphology in serum-free and different serum levels were mainly induced by fibronectin and fetuin in serum[13], which promoted cell attachment and spread on the surfaces of culture plates. So necessary precautions must be given to the serum level changes in the medium in detecting growth factors and drugs with human hepatoma SMMC-7721 cells, otherwise false conclusion might be implied.
Project supported by a grant from Science and Technology Committee of Shandong Province, ª©No. J97K01.
| 1. | Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955;122:501-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1118] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 2. | Hayashi I, Sato GH. Replacement of serum by hormones permits growth of cells in a defined medium. Nature. 1976;259:132-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 267] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Brooks RF. Regulation of fibroblast cell cycle by serum. Nature. 1976;260:248-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 139] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Larsson O, Dafgård E, Engström W, Zetterberg A. Immediate effects of serum depletion on dissociation between growth in size and cell division in proliferating 3T3 cells. J Cell Physiol. 1986;127:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Zaitsu H, Kimura G. Serum-dependent regulation of proliferation of cultured rat fibroblasts in G1 and G2 phases. Exp Cell Res. 1988;174:146-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Campisi J, Morreo G, Pardee AB. Kinetics of G1 transit following brief starvation for serum factors. Exp Cell Res. 1984;152:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Zetterberg A, Larsson O. Kinetic analysis of regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. Proc Natl Acad Sci USA. 1985;82:5365-5369. [PubMed] |
| 8. | Loo DT, Fuquay JI, Rawson CL, Barnes DW. Extended culture of mouse embryo cells without senescence: inhibition by serum. Science. 1987;236:200-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 154] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Yin Z, Wheatley DN. Sensitivity of 3T3 cells to low serum concentration and the associated problems of serum withdrawal. Cell Biol Int. 1994;18:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Tong R, Zhou R, Lu F, Tao W. The establishment and primary biological char-acteristics of human hepatoma SMMC-7721 cell line. J 2nd Milit Med Univ. 1980;1:5-9. |
| 11. | Xu Y, Jiang W, Peng S, Chen Y. Antisense EGFR sequence reverses the growth properties of human liver carcinoma cell line BEL-7404 in vitro. Cell Res. 1993;3:75-83. |
| 12. | Fu T, Liu H, Liu F, Gu J, Jiang W, Xu Y. Antisense EGFR sequence enhances apoptosis in a human hepatoma cell line BEL-7404. Cell Res. 1996;6:145-153. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 13. | Freshney RI. Culture of animal cells. New York:. Alan R Liss Inc. 1983;74-77. |