Published online Jul 14, 2025. doi: 10.3748/wjg.v31.i26.106509
Revised: April 29, 2025
Accepted: June 18, 2025
Published online: July 14, 2025
Processing time: 128 Days and 0.7 Hours
Esophageal cancer is a serious global health concern with poor prognosis in advanced stages. Immune checkpoint inhibitors (ICIs) have shown promise in enhancing survival, but they are associated with immune-related adverse events, including potentially life-threatening myocarditis. Moreover, ICI-induced myocarditis can be asymptomatic, necessitating early diagnosis. Specific risk factors and biomarkers for esophageal cancer remain poorly characterized.
To investigate the determinants of ICI-associated asymptomatic myocarditis in patients with esophageal cancer and explore potential early biomarkers.
A retrospective analysis was conducted on 202 cancer patients who received treatment at Shanxi Province Cancer Hospital from July 2019 to July 2024.
Older age, male gender, and elevated creatine kinase isoenzymes (CK-MB) and CK levels were found to be significant risk factors for asymptomatic myocarditis. The myocarditis occurrence group had higher CK-MB (3.05 ng/mL vs 5.02 ng/mL; P < 0.001) and CK levels (187.29 U/L vs 212.25 U/L; P = 0.005), and the predictive value of age, gender, CK, and CK-MB was low [are under the receiver operating characteristic curve (AUC) = 0.579-0.608]. However, their combination in a predictive model showed improved diagnostic capability, with an AUC of 0.808.
Age, gender, and cardiac biomarker levels considerably contribute to the risk of ICI-related myocarditis in patients with esophageal cancer. The integration of these factors into a predictive model enhances early diagnosis, facilitating personalized risk management.
Core Tip: This study investigates the determinants of immune checkpoint inhibitor (ICI)-associated asymptomatic myocarditis in patients with esophageal cancer. We identified age, gender, and elevated cardiac biomarker levels as signi
- Citation: Liu JY, Gao DL, Cao X. Risk factors and diagnostic biomarkers for asymptomatic immune checkpoint inhibitor-related myocarditis in patients with esophageal cancer after immunotherapy. World J Gastroenterol 2025; 31(26): 106509
- URL: https://www.wjgnet.com/1007-9327/full/v31/i26/106509.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i26.106509
Esophageal cancer remains a formidable global health challenge, with an estimated 604000 new cases and 544000 deaths annually[1-3]. Despite improvements in multidisciplinary approaches, prognosis for advanced-stage esophageal cancer has remained poor, necessitating novel therapeutic strategies[4,5]. In recent years, immune checkpoint inhibitors (ICIs) have emerged as a transformative modality in cancer immunotherapy, revolutionizing treatment paradigms across several malignancies, including esophageal cancer[6,7]. ICIs, such as programmed cell death protein 1 inhibitors, function by blocking regulatory pathways that suppress immune responses, thereby enhancing antitumor immunity[8]. ICIs have significantly improved survival outcomes for patients with various cancer types, but their use is not without challenges.
ICIs are associated with a spectrum of immune-related adverse events, which reflect their ability to reactivate immune responses[9,10]. Myocarditis, though relatively rare, is a serious and potentially life-threatening complication with an incidence rate ranging from 0.27% to 3.1% in patients with cancer on ICI therapy. Notably, ICI-related myocarditis can often be asymptomatic, and early diagnosis is vital for optimizing clinical outcomes and initiating timely interventions. The pathophysiology underlying ICI-related myocarditis involves excessive immune activation and autoimmunity against cardiac antigens, rendering the myocardium susceptible to inflammatory damage[11]. Despite the critical need to identify at-risk patients, current diagnostic approaches are limited by nonspecific clinical manifestations and standard laboratory assays.
Given the asymptomatic nature and potential severity of ICI-related myocarditis, reliable risk stratification methodologies and early diagnostic biomarkers are urgently needed. However, specific risk factors associated with ICI therapy in patients with esophageal cancer remain poorly defined. Additionally, although cardiac biomarkers such as troponins are established indicators of clinical myocarditis, their sensitivity in detecting subclinical or asymptomatic ICI-induced myocardial injury requires further investigation[12].
In this context, our study examined the determinants of asymptomatic myocarditis associated with ICI therapy in patients with esophageal cancer and investigated potential early biomarkers for its detection.
This retrospective cohort study included 202 patients diagnosed with esophageal cancer and treated at Shanxi Province Cancer Hospital from July 2019 to July 2024. Demographic data were collected from medical records composed of general patient information, cancer biomarker levels, cardiac injury indicators, and electrocardiogram and echocardiogram data.
Given that this retrospective study utilized de-identified patient data, it presented no potential risk or harm to patient care. Consequently, informed consent was waived. The consent form and the study itself were approved by the Shanxi Province Cancer Hospital’s institutional review board and ethics committee, adhering to regulatory and ethical guidelines for retrospective studies.
The inclusion criteria were as follows: (1) Diagnosed esophageal cancer; (2) Treatment with ICIs; (3) Mental stability and ability to accurately describe own’s physical condition; (4) Complete pathological data available; and (5) over 18 years old.
The following individuals were excluded: (1) Individuals with severe immunodeficiency disorders; (2) Patients who had died due to non-cancer-related causes; (3) Individuals with significant organ dysfunction, such as cerebrovascular or renal failure; and (4) Patients with interstitial lung disease, adrenal insufficiency, or other related conditions.
Grouping criteria: Patients with esophageal cancer and on immunotherapy were divided into two groups according to the occurrence of ICI-associated asymptomatic myocarditis: The occurrence (68 patients) and non-occurrence groups (134 patients).
Immunotherapy methods: Patients received ICI combination therapy or monotherapy with a single ICI agent. The primary ICIs used in this study included sintilimab, nivolumab, camrelizumab, pembrolizumab and tislelizumab. All inhibitors were administered intravenously.
Diagnostic criteria for ICI-associated asymptomatic myocarditis: Diagnosis was based on considerable changes in cardiac biomarkers compared to pretreatment levels. No notable changes in electrocardiogram and echocardiography were observed, and patients did not present with myocarditis-related symptoms.
Blood samples were collected from the antecubital vein. Each patient positioned his or her forearm on the examination table with the palm facing upward, and a pillow supported the elbow. The medical staff drew blood with a disposable syringe. A tourniquet was applied 6 cm above the collection site, and the ends were facing upward. The skin at the collection site was disinfected with iodine. Approximately 2-5 mL of blood was collected. After blood withdrawal, a disinfectant cotton swab was used to stop bleeding. Routine blood testing was conducted with a fully automated blood cell analyzer (D2-CRP, DYMIND BIOTECH, China). Cardiac injury markers and tumor biomarkers were measured using ELISA according to the reagent kit’s instructions (Supplementary Table 1). The following biomarkers were tested: Cancer antigen 125 (CA125), carcinoembryonic antigen (CEA)[13], squamous cell carcinoma antigen (SCC), cardiac troponin I, creatine kinase isoenzymes-MB (CK-MB), creatine kinase (CK), lactate dehydrogenase[14], myoglobin and N-terminal pro-B-type natriuretic peptide (NT-proBNP).
Each patient was positioned comfortably on the examination table in a relaxed state. Clothing was loosened, and the wrists and upper parts of both ankles were exposed, which were subsequently cleaned with alcohol gauze. The patients assisted the clinician in cleaning the skin and applying conductive gel to ensure optimal contact between the skin and electrodes. The patient remained still for 8-15 minutes during detection.
Cardiac ultrasound was used in assessing cardiac function indicators. Examination was conducted using a cardiac ultrasound diagnostic device (EPIQ, Philips Ultrasound, Inc., Netherlands). During the procedure, a patient was positioned on the examination table in a left lateral position, and undergarments were unbuttoned to expose the anterior chest area, specifically from the second to fifth intercostal spaces. The physician applied a coupling agent to the ultrasound probe or directly to the examination site and then performed sectional scanning with the probe. The orientation of the probe was adjusted as necessary to measure the left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), left ventricular ejection fraction (LVEF), and stroke volume (SV). Once the examination was complete, the coupling agent was wiped off, and the patients were allowed to readjust their clothing.
Data analysis was performed using SPSS 29.0 statistical software (SPSS Inc., Chicago, IL, United States). Categorical variables were expressed as [n (%)]. A χ2 test was applied using the basic formula when the sample size was ≥ 40 and the expected frequency (T) was ≥ 5. When the sample size was ≥ 40 but the expected frequency was 1 ≤ T < 5, a corrected χ2 test was used. For a sample size of < 40 or T value of < 1, Fisher’s exact test was employed. The Shapiro-Wilk test was used to assess the normal distribution of continuous variables. Normally distributed continuous variables were expressed as mean ± SD and analyzed using a t-test with corrected variance. A two-tailed P value less than 0.05 was considered statistically significant. Variables showing significant differences in univariate and multivariate analyses, including age, CK-MB and CK levels, were incorporated as covariates in a logistic regression analysis. The diagnostic efficacy of age, CK-MB, and CK levels for ICI-associated asymptomatic myocarditis was evaluated using the area under the receiver operating characteristic[15] curve (AUC).
This study examined patients with esophageal cancer who were on immunotherapy. Participants who developed myocarditis were older, with a mean age of 65.49 years vs 62.36 years in the non-occurrence group (P = 0.009; Table 1). Gender distribution differed significantly, males had a higher proportion in the occurrence group (61.76%) than in the non-occurrence group (41.79%; P = 0.007). The incidence of myocarditis was significantly higher in patients receiving ICI combination therapy (48.53%) than in patients receiving ICI monotherapy (32.09%; P = 0.023). Other measured factors, such as body mass index, education level, marital status, lesion segmentation and length, tumor staging system, histological classification, and coexisting conditions, including hypertension, diabetes, and coronary heart disease, were not statistically significantly different between the groups (P > 0.05). Chemotherapy use was similar between the groups
| Parameters | Non-occurrence group (n = 134) | Occurrence group (n = 68) | t/χ2 value | P value |
| Age (years) | 62.36 ± 7.34 | 65.49 ± 8.96 | 2.654 | 0.009 |
| Gender (male/female) | 56 (41.79)/78 (58.21) | 42 (61.76)/26 (38.24) | 7.205 | 0.007 |
| Body mass index (kg/m²) | 21.36 ± 3.25 | 20.56 ± 3.44 | 1.622 | 0.106 |
| Education level | 0.782 | 0.676 | ||
| Primary and below | 24 (17.91) | 9 (13.24) | ||
| Middle school | 45 (33.58) | 23 (33.82) | ||
| College and above | 65 (48.51) | 36 (52.94) | ||
| Marital status | 0.640 | 0.726 | ||
| Married | 119 (88.81) | 60 (88.24) | ||
| Divorced | 11 (8.21) | 7 (10.29) | ||
| Unmarried | 4 (2.99) | 1 (1.47) | ||
| Segmentation of lesions | 3.179 | 0.365 | ||
| Neck | 42 (31.34) | 16 (23.53) | ||
| Upper thoracic segment | 35 (26.12) | 25 (36.76) | ||
| Mid thoracic segment | 36 (26.87) | 19 (27.94) | ||
| Lower thoracic segment | 21 (15.67) | 8 (11.76) | ||
| Length of lesion | 0.509 | 0.476 | ||
| > 5 cm | 78 (58.21) | 36 (52.94) | ||
| ≤ 5 cm | 56 (41.79) | 32 (47.06) | ||
| Tumor staging system staging | 0.552 | 0.907 | ||
| Phase I | 12 (8.96) | 6 (8.82) | ||
| Phase II | 35 (26.12) | 21 (30.88) | ||
| Phase III | 41 (30.6) | 20 (29.41) | ||
| Phase IV | 46 (34.33) | 21 (30.88) | ||
| Organizational classification | 0.031 | 0.861 | ||
| Squamous cell carcinoma | 109 (81.34) | 56 (82.35) | ||
| Adenocarcinoma | 25 (18.66) | 12 (17.65) | ||
| Hypertension | 16 (11.94) | 4 (5.88) | 1.856 | 0.173 |
| Diabetes | 11 (8.21) | 3 (4.41) | 0.506 | 0.477 |
| Coronary heart disease | 14 (10.45) | 4 (5.88) | 1.158 | 0.282 |
| ICI | 5.195 | 0.023 | ||
| ICI combination therapy | 43 (32.09) | 33 (48.53) | ||
| ICI monotherapy | 91 (67.91) | 35 (51.47) | ||
| Chemotherapy | 52 (38.81) | 26 (38.24) | 0.006 | 0.937 |
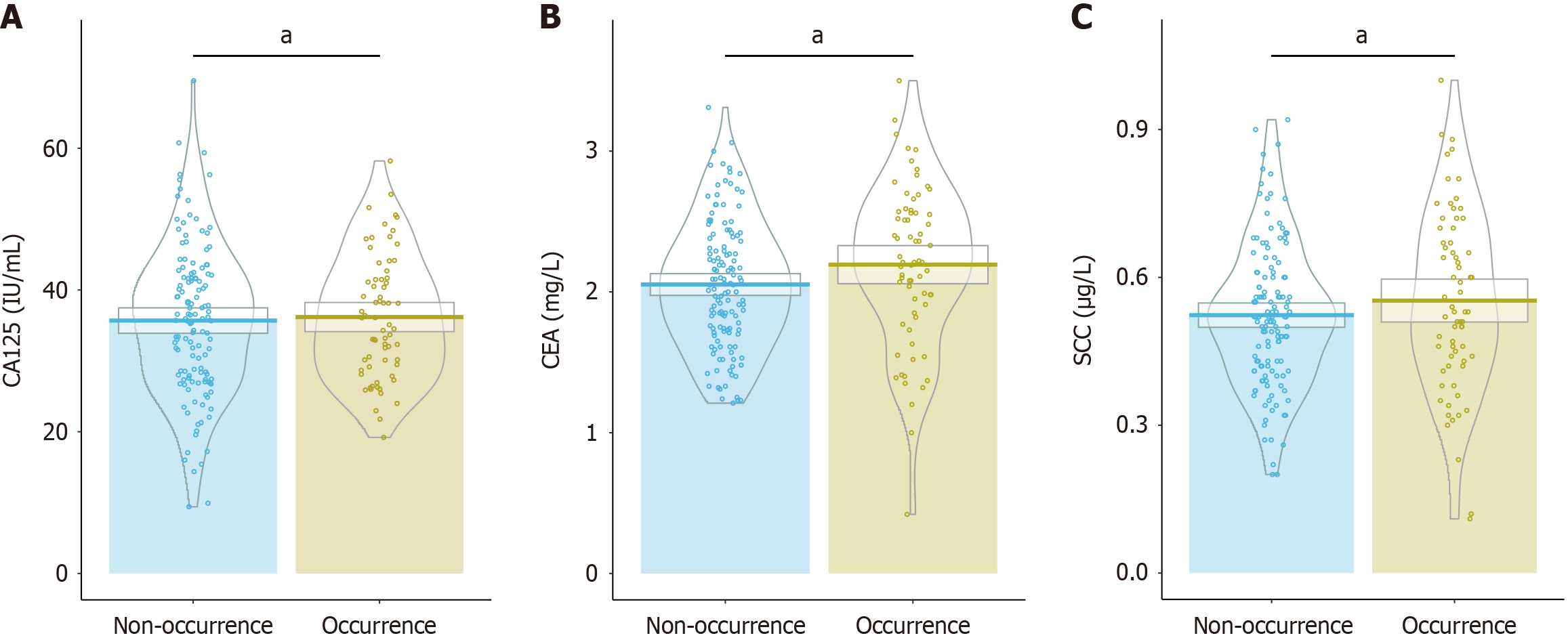
Analysis of tumor biomarker levels revealed that the mean level of CA125 was 36.18 IU/mL in the occurrence group and 35.69 IU/mL in the non-occurrence group (P = 0.738; Figure 1). Similarly, CEA levels were 2.19 mg/L in the occurrence group and 2.05 mg/L in the non-occurrence group, showing no statistically significant difference (P = 0.078). Additionally, SCC levels did not significantly vary, with 0.55 μg/L in the occurrence group and 0.52 μg/L in the non-occurrence group (P = 0.249). These results indicate that CA125, CEA, and SCC levels are not associated with the development of myocarditis in this cohort.
The white blood cell count was 4.76 × 109/L in the occurrence group vs 4.65 × 109/L in the non-occurrence group (P = 0.759; Table 2). Hemoglobin levels were slightly higher in the occurrence group (127.06 g/L) than in the non-occurrence group (121.56 g/L), but the difference was not statistically significant (P = 0.110). Similarly, platelet counts were 149.46 × 109/L in the occurrence group and 157.64 × 109/L in the non-occurrence group (P = 0.317). The group had nearly identical neutrophil counts, which were 3.49 × 109/L in the occurrence group and 3.52 × 109/L in the non-occurrence group (P = 0.921). Lymphocyte counts were also similar, with 1.46 × 109/L in the occurrence group and 1.52 × 109/L in the non-occurrence group (P = 0.478). These findings suggest that routine blood test parameters did not significantly differ between patients with and without myocarditis.
| Parameters | Non-occurrence group (n = 134) | Occurrence group (n = 68) | t value | P value |
| White blood cell (× 109/L) | 4.65 ± 2.42 | 4.76 ± 2.35 | 0.307 | 0.759 |
| Hemoglobin (g/L) | 121.56 ± 22.34 | 127.06 ± 24.25 | 1.606 | 0.110 |
| Platelet (× 109/L) | 157.64 ± 55.27 | 149.46 ± 53.79 | 1.003 | 0.317 |
| Neutrophil (× 109/L) | 3.52 ± 1.96 | 3.49 ± 2.05 | 0.099 | 0.921 |
| Lymphocyte (× 109/L) | 1.52 ± 0.77 | 1.46 ± 0.48 | 0.711 | 0.478 |
CK-MB levels were significantly higher in the myocarditis occurrence group (5.02 ng/mL) than in the non-occurrence group (3.05 ng/mL; P < 0.001; Table 3). Similarly, CK levels were elevated in the occurrence group, averaging 212.25 U/L vs 187.29 U/L in the non-occurrence group (P = 0.005). However, the levels of cardiac troponin I, lactate dehydrogenase, myoglobin, and NT-proBNP were not significantly different between the groups (P > 0.05). These findings suggest that elevated CK-MB and CK levels are early biomarkers for the risk assessment of asymptomatic ICI-related myocarditis in this patient population.
| Parameters | Non-occurrence group (n = 134) | Occurrence group (n = 68) | t value | P value |
| Cardiac troponin I (ng/mL) | 0.07 ± 0.03 | 0.07 ± 0.04 | 0.112 | 0.911 |
| CK-MB (ng/mL) | 3.05 ± 0.95 | 5.02 ± 1.49 | 25.032 | < 0.001 |
| CK (U/L | 187.29 ± 58.25 | 212.25 ± 62.55 | 2.808 | 0.005 |
| LDH (U/L) | 210.35 ± 42.38 | 219.15 ± 41.94 | 1.400 | 0.163 |
| Myoglobin (ng/mL) | 60.58 ± 12.59 | 62.68 ± 13.96 | 1.082 | 0.280 |
| NT-proBNP (pg/mL) | 185.48 ± 36.89 | 194.13 ± 42.16 | 1.500 | 0.135 |
A larger proportion of patients in the occurrence group exhibited a QRS duration of ≥ 110 ms (30.88%) compared to the non-occurrence group (17.91%; P = 0.036; Table 4). Other electrocardiogram parameters, such as the incidence of premature atrial contractions (P = 0.894), premature ventricular contractions (P = 1.000), episodes of nonsustained supraventricular tachycardia (P = 0.090), and episodes of nonsustained ventricular tachycardia (P = 0.344), were not statistically significantly different between the groups. These findings suggest that QRS duration is a potential early diagnostic marker for myocarditis in this patient population.
| Parameters | Non-occurrence group (n = 134) | Occurrence group (n = 68) | t value | P value |
| Premature atrial contractions | 8 (5.97) | 3 (4.41) | 0.018 | 0.894 |
| Premature ventricular contractions | 5 (3.73) | 2 (2.94) | 0 | 1.000 |
| Episodes of non-sustained supraventricular tachycardia | 27 (17.91) | 21 (30.88) | 2.868 | 0.090 |
| Episodes of non-sustained ventricular tachycardia | 110 (82.09) | 52 (69.12) | 0.897 | 0.344 |
| QRS duration | 4.384 | 0.036 | ||
| ≥ 110 ms | 24 (17.91) | 21 (30.88) | ||
| < 110 ms | 110 (82.09) | 47 (69.12) |
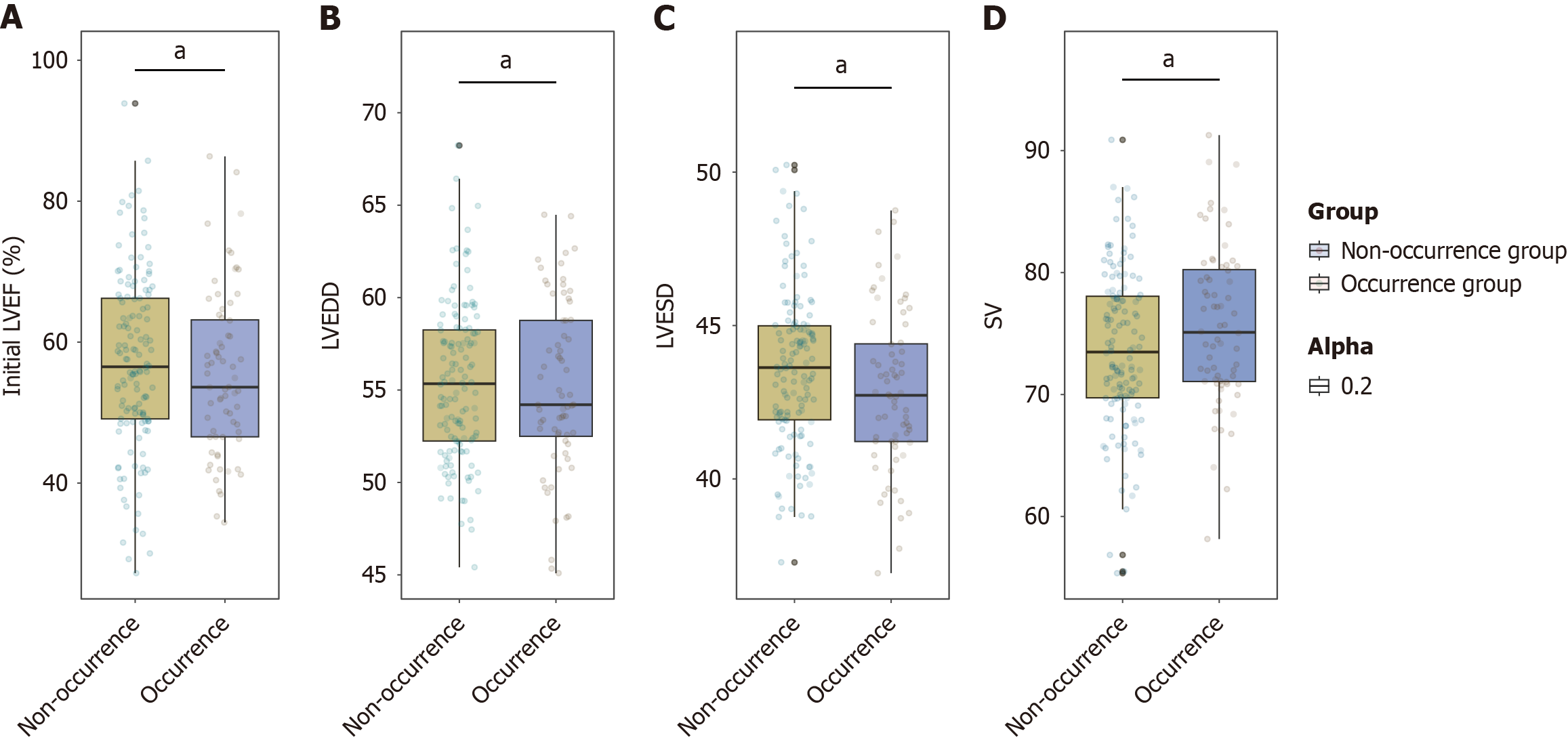
The initial LVEF of the occurrence group (55.34%) was comparable to that of the non-occurrence group (57.12%; P = 0.332; Figure 2). Similarly, the LVEDD showed no significant difference between the groups, with values of 55.16% in the occurrence group and 55.42% in the non-occurrence group (P = 0.693). Although the LVESD and SV appeared slightly lower and higher, respectively, in the occurrence group (42.87% for LVESD and 74.58% for SV) than in the non-occurrence group (43.58% for LVESD and 73.69% for SV), the differences were not statistically significant, with P values of 0.063 and 0.053, respectively. Overall, echocardiographic parameters were not significantly associated with myocarditis in this cohort.
Age was a significant risk factor, and each additional year correlated with a 5% increase in the odds of developing myocarditis [odds ratio (OR) = 1.050; 95% confidence interval (CI): 1.008-1.094; P = 0.020; Table 5]. Male gender was also associated with increased risk, with males being over twice as likely to develop myocarditis compared to females (OR = 2.196; 95%CI: 1.120-4.305; P = 0.022). Elevated CK-MB levels were significant, with each μg/L increase linked to a 14% higher risk (OR = 1.142; 95%CI: 1.029-1.267; P = 0.012), and increased CK levels were similarly noteworthy, showing a 2.5% increased risk per μg/L (OR = 1.025; 95%CI: 1.007-1.043; P = 0.007). ICI use and prolonged QRS duration had elevated OR = 1.624 (95%CI: 0.818-3.223) and 2.028 (95%CI: 0.947-4.342), respectively. However, the differences were not statistically significant (P = 0.166 and P = 0.069, respectively). These findings revel that age, sex, and specific cardiac biomarkers are independent risk factors for identifying patients at high risk for myocarditis.
| Parameters | Coefficient | SE | Wald | P value | OR | OR CI lower | OR CI upper |
| Age (years) | 0.049 | 0.021 | 2.325 | 0.020 | 1.050 | 1.008 | 1.094 |
| Gender (male/female) | 0.787 | 0.343 | 2.290 | 0.022 | 2.196 | 1.120 | 4.305 |
| ICI | 0.485 | 0.350 | 1.386 | 0.166 | 1.624 | 0.818 | 3.223 |
| CK-MB (μg/L) | 0.133 | 0.053 | 2.499 | 0.012 | 1.142 | 1.029 | 1.267 |
| CK (U/L) | 0.024 | 0.009 | 2.681 | 0.007 | 1.025 | 1.007 | 1.043 |
| QRS duration | 0.707 | 0.388 | 1.820 | 0.069 | 2.028 | 0.947 | 4.342 |
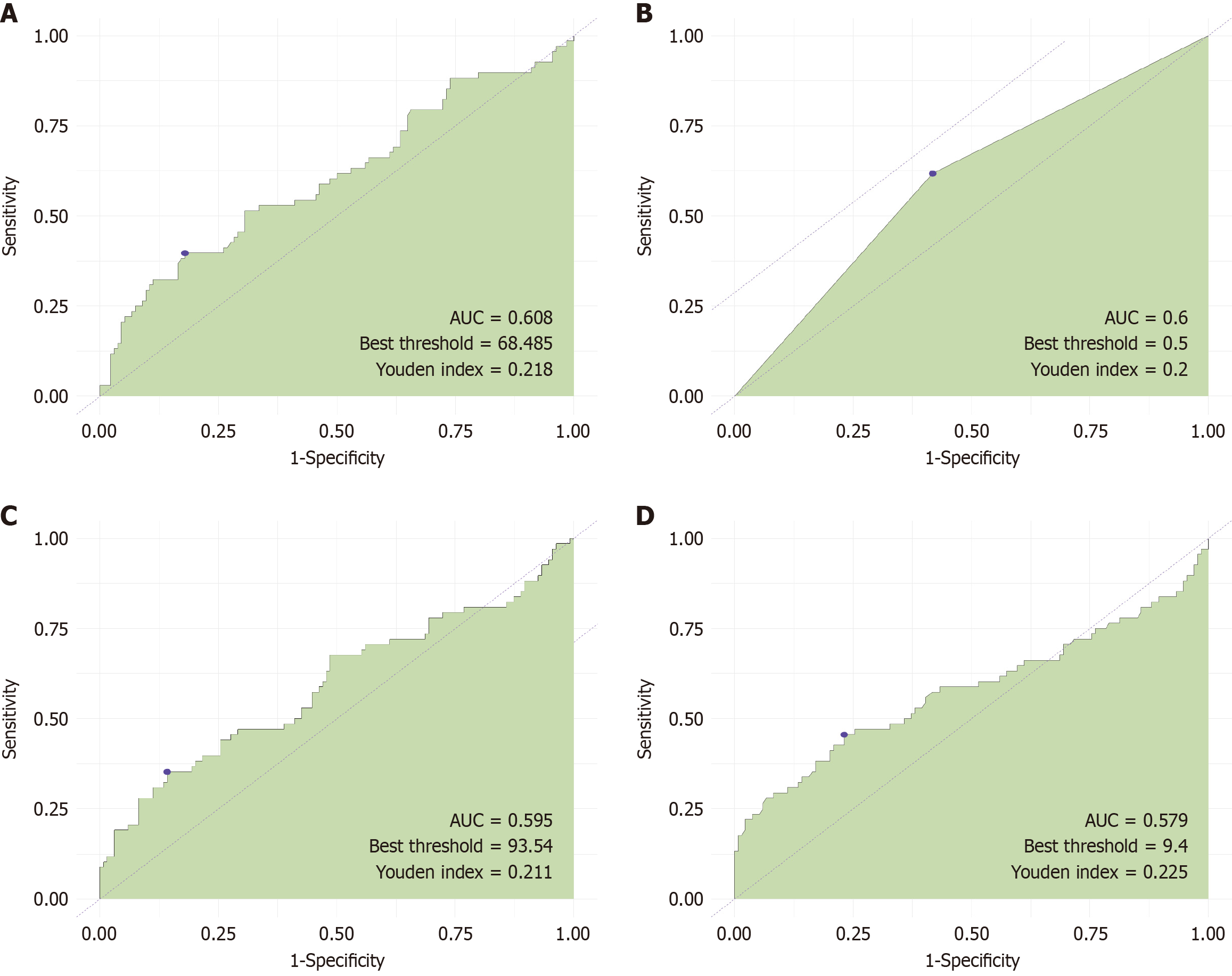
We evaluated the predictive value of four risk factors: Age, gender, CK, and CK-MB. The AUC values for these factors were 0.608, 0.6, 0.595, and 0.579, respectively. These results indicate that the predictive value of these four risk factors for asymptomatic ICI-associated myocarditis in patients with esophageal cancer after immunotherapy was low (Figure 3).
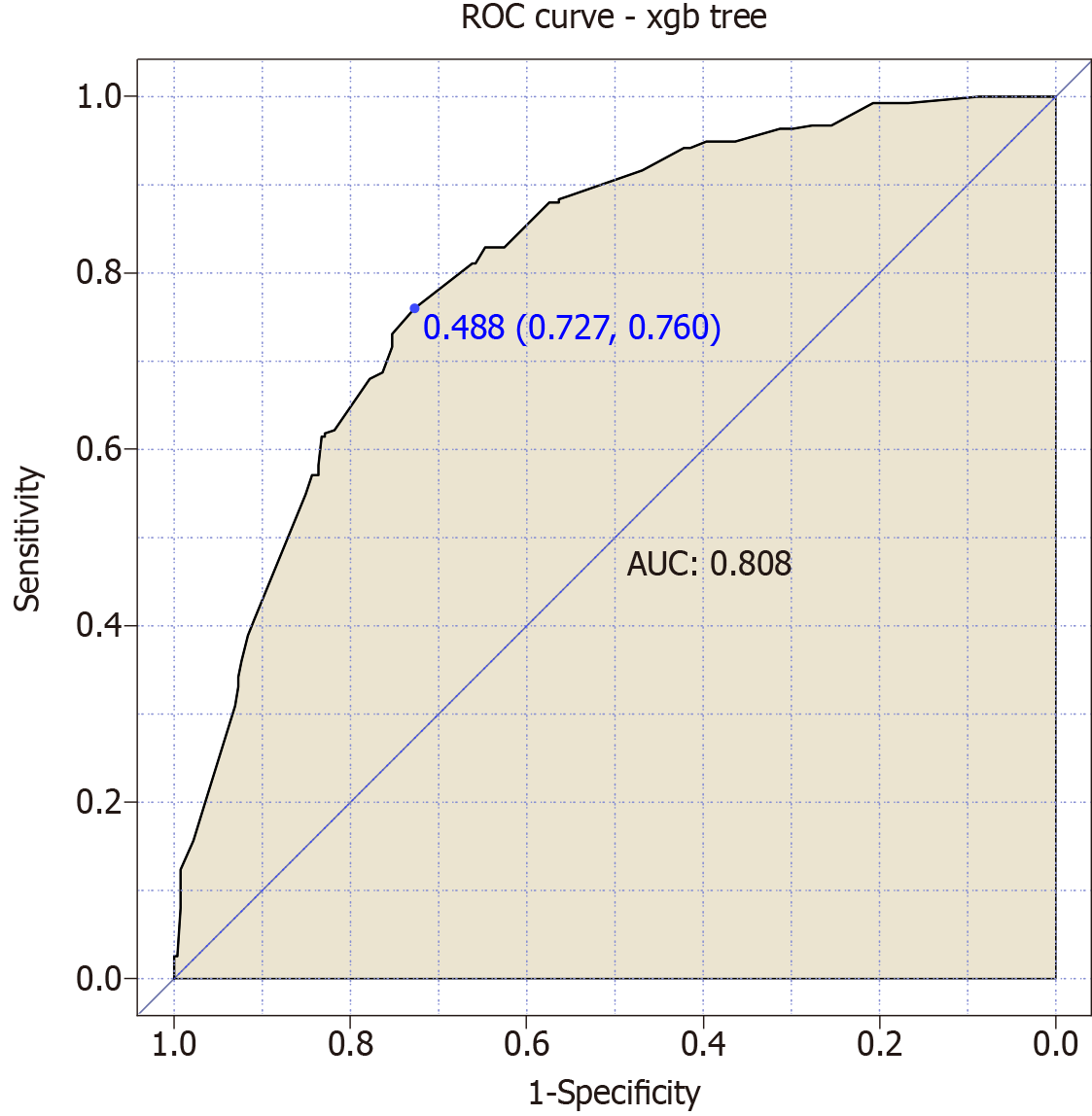
We combined the four risk factors to construct a joint predictive model for “asymptomatic ICI-associated myocarditis” in esophageal cancer patients following immunotherapy. The model achieved an AUC value of 0.808, indicating a highly significant predictive value for this condition (Figure 4).
In this retrospective cohort study, we aimed to identify risk factors and early diagnostic biomarkers for ICI-associated asymptomatic myocarditis in patients with esophageal cancer.
Our analysis shows that age and male sex were significant risk factors for developing myocarditis. The positive correlation between advanced age and increased myocarditis risk may be attributed to the natural decline in cardiac function that occurs with aging, as myocardial reserves and regenerative capacity diminish. These physiological changes may increase the vulnerability of older patients to immune-mediated myocardial injury. Additionally, the myocarditis risk observed in males was more than twice of females, potentially due to sex-based differences in immune response strength or genetic predispositions linked to the Y chromosome that affect cardiac stress responses. However, sex-specific immunological differences that may predispose males to exaggerated immune responses or impaired cardioprotective mechanisms during immunotherapy warrant further investigation. Our study observed a markedly higher incidence of myocarditis (33.66%) compared to the previously reported range of 0.27% to 3.1%. This discrepancy may be attributed to several factors. First, our study focused specifically on patients with esophageal cancer, who may exhibit distinct immune response and a heightened susceptibility to ICI-related myocarditis. Second, the retrospective design may have allowed for more comprehensive case identification through detailed data collection. Lastly, our definition of myocarditis may have been broader, encompassing subclinical cases that were likely missing in previous studies. Future prospective studies are warranted to validate these findings and more accurately determine the true incidence of ICI-related myocarditis.
Although our study did not reveal statistically significant differences in tumor biomarkers, routine blood test parameters, or detailed echocardiographic findings between patients with and without myocarditis, the elevated levels of CK-MB and CK in the occurrence group were noteworthy. CK-MB and CK are well-established indicators of myocardial injury, and their elevation in this study suggests the presence of subclinical myocardial damage associated with ICI administration. This damage is likely the result of inadvertent activation of autoimmune processes initially intended to enhance antitumor responses[16]. ICI-related myocarditis may share pathophysiological features with viral myocarditis, wherein immune overactivation leads to myocardial inflammation. However, further histological investigations are needed to confirm these correlations in the context of ICI therapy[17].
Our electrocardiogram findings, particularly the prolonged QRS duration observed in the occurrence group, highlight potential myocardial conduction disturbances that may precede the onset of clinical symptoms. A prolonged QRS duration reflets delayed ventricular depolarization, which may result from myocardial inflammation increasing resistance to electrical conduction[18]. If confirmed, QRS duration could serve as an early electrophysiological biomarker to identify patients predisposed to myocarditis during immunotherapy. Formal investigation of electrophysiological changes induced by checkpoint inhibitors may offer new avenues to improve cardiac monitoring protocols in clinical oncology[19].
Intriguingly, our risk factor analysis shows that while individual factors, such as age, gender, and CK and CK-MB levels, have limited predictive power alone, their combined use in a predictive model significantly improves myocarditis risk assessment. This suggests that ICI-related myocarditis is multifactorial, arising from the interplay of age-related physiological decline, sex-based immune response differences, and biochemical indicators of early myocardial distress[15,20-22]. This convergence likely diminishes certain patients’ resilience to immune perturbations induced by checkpoint inhibitors, leading to myocardial inflammation[23,24].
Our findings suggest the for of a stratified management approach in patients with esophageal cancer. For instance, older males with elevated CK-MB and CK levels may benefit from proactive cardiac monitoring and tailored adjustments to their immunotherapy regimen to reduce myocarditis risk. These factors should be carefully considered in treatment planning and could inform the development of monitoring algorithms that enhance clinical decision-making by improving vigilance around cardio-oncological risks in vulnerable patients.
The lack of significant differences in tumor biomarker levels and routine blood tests between groups underscores the specificity of cardiac biomarkers, such as CK and CK-MB, in detecting asymptomatic myocardial dysfunction amid standard oncological parameters[25-27]. These findings highlight the importance of incorporating cardiac-specific biomarkers in routine evaluations, particularly when myocardial involvement may go undetected by conventional oncological assays[28].
Our study provides a strong foundation for future prospective research and model refinement, incorporating additional variables from genetic, proteomic, or advanced imaging analyses to develop comprehensive risk profiles. Large cohort studies with cross-validation of our predictive model are needed to confirm its clinical applicability and reliability across diverse patient populations. Moreover, elucidating the molecular mechanisms underlying myocarditis in this context may uncover novel therapeutic targets to prevent immune-mediated cardiac injury during cancer treatment[29-31].
A key limitations of this study is its single-center, retrospective design. Conducting the research within one hospital and a specific patient population restricts the external validity and generalizability of our findings. The lack of data from multiple centers and diverse populations limits the robustness of the predictive model developed in this study. Future research should focus on validating these findings in larger, multi-center cohorts to enhance the applicability and reliability of the risk prediction model. Prospective studies involving diverse patient populations and varied healthcare settings will be crucial for further validation and refinement of our proposed model. Notably, while our study provides valuable insights into the risk factors and biomarkers associated with asymptomatic ICI-related myocarditis in patients with esophageal cancer, several limitations should be acknowledged. First, the retrospective design introduces potential selection bias and limits our ability to establish causal relationships between identified risk factors and the occurrence of myocarditis. Furthermore, the relatively small sample size, especially for patients diagnosed with myocarditis, may limit the generalizability and robustness of our results. Our use of de-identified data constrains the exploration of detailed patient-specific factors, such as medication histories and genetic predispositions, which can affect myocarditis risk. Additionally, since the inclusion criteria were based on available pathological data, undiagnosed conditions within our cohort may have been overlooked. Although we identified potential biomarkers and developed a predictive model, they require validation in larger prospective studies to confirm their efficacy and expand their applicability across diverse clinical settings. Future research should consider incorporating longitudinal follow-up and advanced imaging techniques to effectively monitor subclinical myocardial changes[32].
Our study conclusively demonstrates that age, gender, and specific cardiac biomarkers such as CK-MB and CK levels are significantly associated with increased risk of ICI-associated myocarditis in esophageal cancer patients. The development of a joint predictive model incorporating these factors enhances early diagnosis and personalized risk management. Future research should focus on validating this model in larger, multi-center cohorts and exploring additional biomarkers or imaging techniques to further refine risk stratification. These efforts will ultimately improve patient outcomes and contribute to safer cancer treatment modalities.
I would like to express my gratitude to all those helped me during the writing of this thesis. I acknowledge the help of my colleagues Cao HL and Yan HX. They have offered me suggestions in academic studies.
| 1. | Cheng CY, Hao WR, Cheng TH. Esophageal cancer: A global challenge requiring tailored strategies. World J Gastrointest Oncol. 2024;16:2881-2883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Reference Citation Analysis (3)] |
| 2. | Sheikh M, Roshandel G, McCormack V, Malekzadeh R. Current Status and Future Prospects for Esophageal Cancer. Cancers (Basel). 2023;15:765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 123] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 3. | Teng Y, Xia C, Cao M, Yang F, Yan X, He S, Cao M, Zhang S, Li Q, Tan N, Wang J, Chen W. Esophageal cancer global burden profiles, trends, and contributors. Cancer Biol Med. 2024;21:656-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 4. | Ogasawara M, Miyashita M, Yamagishi Y, Ota S. Immunotherapy employing dendritic cell vaccination for patients with advanced or relapsed esophageal cancer. Ther Apher Dial. 2020;24:482-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Qian J, Si Y, Zhou K, Tian Y, Guo Q, Zhao K, Yu J. Sarcopenia is associated with prognosis in patients with esophageal squamous cell cancer after radiotherapy or chemoradiotherapy. BMC Gastroenterol. 2022;22:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Chen Y, Yu R, Liu Y. Combine radiotherapy and immunotherapy in esophageal squamous cell carcinoma. Crit Rev Oncol Hematol. 2023;190:104115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 7. | Davern M, Lysaght J. Cooperation between chemotherapy and immunotherapy in gastroesophageal cancers. Cancer Lett. 2020;495:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 774] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 9. | Dolladille C, Ederhy S, Sassier M, Cautela J, Thuny F, Cohen AA, Fedrizzi S, Chrétien B, Da-Silva A, Plane AF, Legallois D, Milliez PU, Lelong-Boulouard V, Alexandre J. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol. 2020;6:865-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 371] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 10. | Poto R, Troiani T, Criscuolo G, Marone G, Ciardiello F, Tocchetti CG, Varricchi G. Holistic Approach to Immune Checkpoint Inhibitor-Related Adverse Events. Front Immunol. 2022;13:804597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 11. | Won T, Kalinoski HM, Wood MK, Hughes DM, Jaime CM, Delgado P, Talor MV, Lasrado N, Reddy J, Čiháková D. Cardiac myosin-specific autoimmune T cells contribute to immune-checkpoint-inhibitor-associated myocarditis. Cell Rep. 2022;41:111611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 12. | Kottwitz J, Bruno KA, Berg J, Salomon GR, Fairweather D, Elhassan M, Baltensperger N, Kissel CK, Lovrinovic M, Baltensweiler A, Schmied C, Templin C, Lima JAC, Landmesser U, Lüscher TF, Manka R, Heidecker B. Myoglobin for Detection of High-Risk Patients with Acute Myocarditis. J Cardiovasc Transl Res. 2020;13:853-863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Latcu SC, Novacescu D, Buciu VB, Dumitru CS, Ceausu RA, Raica M, Cut TG, Ilina R, Malita DC, Tarta C, Cumpanas AA. The Cavernous Nerve Injury Rat Model: A Pictorial Essay on Post-Radical Prostatectomy Erectile Dysfunction Research. Life (Basel). 2023;13:2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 14. | Nambi G, Alghadier M, Elnegamy TE, Basuodan RM, Alwhaibi RM, Vellaiyan A, Nwihadh NA, Aldhafian OR, Verma A, Pakkir Mohamed SH, Chevidikunnan MF, Khan F. Clinical (BMI and MRI) and Biochemical (Adiponectin, Leptin, TNF-α, and IL-6) Effects of High-Intensity Aerobic Training with High-Protein Diet in Children with Obesity Following COVID-19 Infection. Int J Environ Res Public Health. 2022;19:7194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Lehmann LH, Heckmann MB, Bailly G, Finke D, Procureur A, Power JR, Stein F, Bretagne M, Ederhy S, Fenioux C, Hamwy O, Funck-Brentano E, Romano E, Pieroni L, Münster JP, Allenbach Y, Anquetil C, Leonard-Louis S, Palaskas NL, Hayek SS, Katus HA, Giannitsis E, Frey N, Kaya Z, Moslehi J, Prifti E, Salem JE. Cardiomuscular Biomarkers in the Diagnosis and Prognostication of Immune Checkpoint Inhibitor Myocarditis. Circulation. 2023;148:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 75] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 16. | Mocan-Hognogi DL, Trancǎ S, Farcaş AD, Mocan-Hognogi RF, Pârvu AV, Bojan AS. Immune Checkpoint Inhibitors and the Heart. Front Cardiovasc Med. 2021;8:726426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Patel RP, Parikh R, Gunturu KS, Tariq RZ, Dani SS, Ganatra S, Nohria A. Cardiotoxicity of Immune Checkpoint Inhibitors. Curr Oncol Rep. 2021;23:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 18. | Ávila G. Fluorofenidone enhances cardiac contractility by stimulating CICR and Ca(V)1.2. Biochem Biophys Res Commun. 2023;681:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Hajem S, Ederhy S, Champiat S, Troalen F, Nolin-Lapalme A, Berhoune M, Cauquil C, Martin-Romano P, Baldini C, Laparra A, Vuagnat P, Hollebecque A, Mateus C, Besse B, Naltet C, Robert C, Marabelle A, Massard C, Lambotte O, Michot JM. Absence of significant clinical benefit for a systematic routine creatine phosphokinase measurement in asymptomatic patients treated with anti-programmed death protein (ligand) 1 immune checkpoint inhibitor to screen cardiac or neuromuscular immune-related toxicities. Eur J Cancer. 2021;157:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Lehmann LH, Cautela J, Palaskas N, Baik AH, Meijers WC, Allenbach Y, Alexandre J, Rassaf T, Müller OJ, Aras M, Asnani AH, Deswal A, Laufer-Perl M, Thuny F, Kerneis M, Hayek SS, Ederhy S, Salem JE, Moslehi JJ. Clinical Strategy for the Diagnosis and Treatment of Immune Checkpoint Inhibitor-Associated Myocarditis: A Narrative Review. JAMA Cardiol. 2021;6:1329-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 21. | Moslehi J, Lichtman AH, Sharpe AH, Galluzzi L, Kitsis RN. Immune checkpoint inhibitor-associated myocarditis: manifestations and mechanisms. J Clin Invest. 2021;131:e145186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 22. | Tan S, Day D, Nicholls SJ, Segelov E. Immune Checkpoint Inhibitor Therapy in Oncology: Current Uses and Future Directions: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2022;4:579-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 91] [Reference Citation Analysis (0)] |
| 23. | Ganassi M, Zammit PS. Involvement of muscle satellite cell dysfunction in neuromuscular disorders: Expanding the portfolio of satellite cell-opathies. Eur J Transl Myol. 2022;32:10064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 24. | Sanino G, Bosco M, Terrazzano G. Physiology of Midkine and Its Potential Pathophysiological Role in COVID-19. Front Physiol. 2020;11:616552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Johnson R, Nxele X, Cour M, Sangweni N, Jooste T, Hadebe N, Samodien E, Benjeddou M, Mazino M, Louw J, Lecour S. Identification of potential biomarkers for predicting the early onset of diabetic cardiomyopathy in a mouse model. Sci Rep. 2020;10:12352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Keramida K, Farmakis D, López Fernández T, Lancellotti P. Focused echocardiography in cardio-oncology. Echocardiography. 2020;37:1149-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Xiao H, Wang X, Li S, Liu Y, Cui Y, Deng X. Advances in Biomarkers for Detecting Early Cancer Treatment-Related Cardiac Dysfunction. Front Cardiovasc Med. 2021;8:753313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Guo Y, Sui JY, Kim K, Zhang Z, Qu XA, Nam YJ, Willette RN, Barnett JV, Knollmann BC, Force T, Lal H. Cardiomyocyte Homeodomain-Interacting Protein Kinase 2 Maintains Basal Cardiac Function via Extracellular Signal-Regulated Kinase Signaling. Circulation. 2019;140:1820-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Ibraheim H, Perucha E, Powell N. Pathology of immune-mediated tissue lesions following treatment with immune checkpoint inhibitors. Rheumatology (Oxford). 2019;58:vii17-vii28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Liu M, Lin Y, Qiao L, Chen J, Shi Q. Characteristics of cardiac involvement in immune-mediated necrotizing myopathy. Front Immunol. 2023;14:1094611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, Hare JM, Heidecker B, Heymans S, Hübner N, Kelle S, Klingel K, Maatz H, Parwani AS, Spillmann F, Starling RC, Tsutsui H, Seferovic P, Van Linthout S. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 710] [Article Influence: 142.0] [Reference Citation Analysis (0)] |
| 32. | Musella F, Librera M, Sibilio G, Boccalatte M, Tagliamonte G, Cavaglià E, Ferrara I, Puglia M, Dell'Aversana S, Ducci CB, Dellegrottaglie S, Savarese G, Scatteia A. Cardiovascular magnetic resonance parametric techniques to characterize myocardial effects of anthracycline therapy in adults with normal left ventricular ejection fraction: a systematic review and meta-analysis. Curr Probl Cardiol. 2024;49:102609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |