Published online Jun 28, 2025. doi: 10.3748/wjg.v31.i24.105823
Revised: April 14, 2025
Accepted: June 9, 2025
Published online: June 28, 2025
Processing time: 132 Days and 23.7 Hours
The clinical effectiveness of magnetically controlled capsule endoscopy (MCE) is well established. However, problems, such as abdominal distension, insufficient gastric filling, and prolonged gastric retention time, persist with MCE gastric pre
To compare gastric filling using a carbonated soft drink with that using pure water during MCE.
We performed an open-label randomised controlled trial at the Endoscopy Centre of Changhai Hospital in Shanghai. Patients aged 18-80 years, with or without gastrointestinal symptoms, sche
From December 3, 2020 to May 17, 2021, 252 patients (141 men), aged 18-77 years, were assigned to the C (n = 126) and W (n = 126) groups. For the primary outcome, 123 patients in the C group achieved a gastric filling score of ≥ 4 (97.62% vs 80.16%, P < 0.0001). More patients in the C group had the highest gastric filling scores within the first 5 min (78.57% vs 29.37%, P < 0.0001) and 10 minutes (54.76% vs 13.49%, P < 0.0001) after the capsule entered the stomach. More patients in the W group required extra liquid for gastric refilling (1.59% vs 16.67%, P < 0.0001). Transpyloric passage of the capsule under magnetic control was successfully performed in 43 patients in the C group (P < 0.0001), accompanied by a shorter gastric transit time (53.27 ± 53.83 minutes vs 71.12 ± 52.19 minutes, P = 0.001).
Carbonated soft drinks demonstrated superior and more sustained gastric filling compared with those of water alone, with the potential to promote gastric emptying.
Core Tip: Gastric preparation is closely related to the gastric visualisation, image quality, completion rate, and diagnostic efficacy in the widely used magnetically controlled capsule endoscopy (MCE). The gastric preparation method needs continuous optimisation. Drinking 550 mL of carbonated soft drink before MCE obtained a better gastric filling result for at least 10 minutes compared with drinking 1000 mL pure water, combined with a potential promotion of gastric emptying. This study provided a preliminary clinical assessment of a novel gastric preparation regimen for MCE. Carbonated soft drink performed better and sustained gastric filling compared with pure water for gastric preparation.
- Citation: Zhu JH, Liu X, Zhou W, Xu XN, Sheng WD, Han YL, Qiu XO, Liu YW, Qian YY, Liao Z, Li ZS. Carbonated soft drink for gastric preparation for magnetically controlled capsule endoscopy: An open-label randomized controlled trial. World J Gastroenterol 2025; 31(24): 105823
- URL: https://www.wjgnet.com/1007-9327/full/v31/i24/105823.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i24.105823
As a patient-friendly alternative method, magnetically controlled capsule endoscopy (MCE) has bridged the gap in gastric examination within the field of capsule endoscopy and has been increasingly used in different populations in recent years[1]. MCE achieves an accuracy of 93.4%, offers a simplified operation process and cost savings, and is con
Because of the size and unusual anatomy of the stomach, gastric preparation is of critical importance, as it is closely related to gastric visualisation, completion rates, image quality, and diagnostic efficacy[3]. Adequate gastric filling can mitigate the challenges posed by mucosal folds and peristalsis of the gastrointestinal tract[4]. After various attempts, including the use of gas-producing powder, defoamer, and protease[2], patients are typically required to drink 800-1000 mL of water in a short time for gastric filling[5]. Although the diagnostic yield (DY) and clinical effectiveness of MCE have been confirmed, issues related to gastric preparation persist, including abdominal distension, insufficient gastric filling, and prolonged gastric retention time, all of which warrant further investigation[3,4].
Carbon dioxide is widely used in clinical practice as an insufflation gas, particularly in the field of digestive endoscopy[6-9], offering shorter examination times, less luminal distension, reduced abdominal pain, and greater patient acceptance owing to its rapid absorption through the bowel mucosa[10]. Based on prior experience with the application of gas-producing powder in MCE[2], we proposed a novel gastric preparation method using dimethicone and a carbonated soft drink.
The CO2 released from carbonated soft drinks fills the gastric cavity. Considering the synergistic effect of gas and liquid, patients do not need to consume 1000 mL of pure water as per routine procedures. After testing different volumes, we found that gastric filling with 400-500 mL of carbonated soft drink was comparable to that achieved with 800-1000 mL of pure water[11]. This study aimed to evaluate gastric filling using a carbonated soft drink compared with pure water in patients undergoing MCE.
We performed an open-label randomised controlled trial at the Endoscopy Centre of Changhai Hospital in Shanghai. Patients aged 18-80 years, with or without gastrointestinal symptoms, scheduled for MCE were consecutively recruited. The exclusion criteria were as follows: Dysphagia; suspected or known gastrointestinal obstruction, stenosis, or fistula; unwillingness to undergo or intolerance to abdominal surgery (including endoscopic procedures) in case of capsule retention; presence of electronic devices or magnetic metals (including pacemakers, cochlear implants, drug infusion pumps, or nerve stimulators) except for magnetic resonance imaging-compatible devices; inability to cooperate because of physical or psychological disorders; pregnancy[5]; requirement for only a small bowel or gastric examination; and inability to perform gastric preparation as required. Patients who provided informed consent were randomly assigned to the carbonated soft drink group (C group) or water group (W group) in a 1:1 ratio at the time of the examination appointment. The study was performed according to the principles of the 1975 Declaration of Helsinki (6th revision, 2008), as reflected in the a priori approval by the Ethics Committee of Changhai Hospital (CHCE2020-117). The study was registered at ClinicalTrials.gov (NCT04479423). All authors had access to the study data, reviewed and approved the final manuscript, and read the CONSORT 2010 statement, which was prepared and revised according to the CONSORT 2010 statement.
The MCE system (Ankon Technologies Co. Ltd., Shanghai, China) is an emerging diagnostic modality in clinical practice that enables the examination of both the stomach and small bowel. Bowel preparation was performed at 8:00 pm the day before the examination and at 4:00 am the following day with 1 L of PEG (Wanhe Pharmaceutical Co. Ltd., Shenzhen, China).
After an overnight fast (> 8 hours), the patients were required to ingest 400 mg of dimethicone (Menarini Pharmaceutical Co. Ltd., Firenze, Italy) suspended in 100 mL of water. Thirty minutes later, patients in the W group ingested 1000 mL of water to distend the stomach. Patients in the C group were provided with 550 mL of carbonated soft drink (Sprite Zero®, Sprite™ soft drink, The Coca-Cola Company, Australia; ingredients include water, carbon dioxide, citric acid, potassium citrate, sodium benzoate, aspartame, acesulfame, sucralose, and edible essence) and were instructed to drink it quickly while minimising burping.
The endoscopist then examined and observed the gastric mucosa using standard operative procedures. Additional water ingestion was required after the last record of gastric filling in patients with poor gastric filling. When the gastric filling score was < 3, the participants were asked to shake their bodies to promote gas release. In the absence of significant improvement in filling, an additional 200 mL of water was consumed to ensure the effectiveness of the examination. After the gastric examination, the capsule was guided close to the pylorus using an external magnet. Once the pylorus opened, peristalsis propelled the capsule into the duodenum, facilitating its quick passage through the pylorus and providing sufficient time for small bowel examination. ‘Small bowel mode’ was then activated to enable a complete examination of the small bowel.
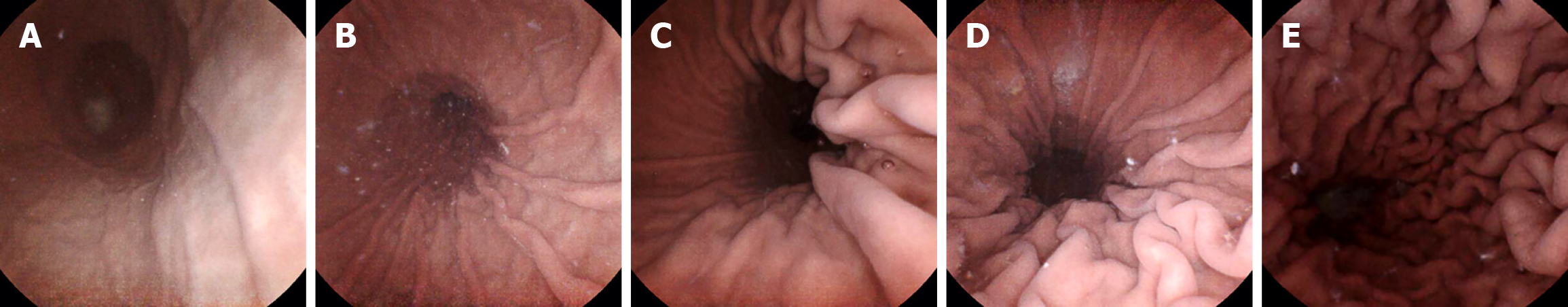
The primary endpoint was the number of patients with a gastric filling score ≥ 4 within 5 minutes after the capsule entered the stomach. Gastric filling was assessed through the degree of extension of the folds of the greater curvature of the stomach[12]. A five-point grading scale was used to define gastric filling as excellent, good, moderate, fair, or poor (Figure 1). A higher score indicated better gastric filling.
Secondary endpoints included the gastric filling score at 0-5 minutes and 5-10 minutes, gastric transit time (GTT), gastric examination time (GET), small bowel transit time (SBTT), DY, the proportion of patients requiring liquid supplementation, gastric cleanliness, abdominal fullness, and satisfaction. The definitions are as follows: GTT: Duration from the last oesophageal image to the first duodenal image; GET: Time taken for a complete examination of the stomach. We also focused on the number of patients in whom capsules reached the duodenum under magnetic control. SBTT: Time from the first duodenal image to the caecal image. DY: Detection of focal gastric and small bowel lesions, including polyps, ulcers, gastric fundus varices, and submucosal tumours, as well as diffuse lesions, such as superficial, atrophic, and erosive gastritis.
Gastric cleanliness was quantified using the gastric cleanliness score (GCS), which assessed six primary anatomical landmarks of the stomach (cardia, fundus, body, angulus, antrum, and pylorus). A four-point grading scale was used to define cleanliness as poor, fair, good, or excellent (scores ranging 1-4)[5].
As a subjective index, abdominal fullness was assessed using a visual analogue scale (range: 0-10)[12]. Similarly, comfort was scored from 0 to 10, with 10 representing the highest level of comfort. The pre-procedure perception and post-procedure satisfaction questionnaire[13] was completed after the examination to record patient satisfaction. Safety was evaluated after a 2-week follow-up period to monitor adverse events, including asphyxia caused by capsule aspiration, swallowing disorders, and capsule retention, which was defined as the capsule remaining in the digestive tract for at least 2 weeks or requiring intervention to aid its passage[14].
Basic patient characteristics were collected before randomisation. Enrolled patients were required to report abdominal fullness and complete a pre-procedure perception questionnaire immediately after gastric preparation.
A single endoscopist (Zhou W) examined all enrolled patients and recorded the GET. The other two endoscopists independently reviewed the images, established diagnoses, and evaluated study-related parameters. When the scores given by the two endoscopists were inconsistent, the patient's images were reviewed by a third endoscopist. The final score was used for statistical analysis after discussion among the three. After the examination, patients were asked to complete a post-procedure satisfaction questionnaire. GTT and SBTT were assessed using the ESNavi software. Each patient was followed up 2 weeks after the examination via telephone and the hospital information system for the collection of adverse events.
Randomisation and allocation were managed by the staff involved in the trial. A computer-generated list of random numbers, created using the SPSS software, was produced and managed using sealed, opaque, numbered envelopes labelled "W" or "C", which were distributed to patients according to the order of gastric preparation. After enrolment, patients were randomly assigned to receive either 1000 mL of pure water or 550 mL of carbonated soft drink by a trained research nurse, according to the label on the envelope. The endoscopists and outcome assessors were blinded to the assigned treatments. Allocation was not revealed until the end of the trial or in the event of a serious adverse event.
Data were analysed according to the intention-to-treat principle and on a per-protocol basis. Based on the results of gastric filling with carbonated soft drink in the pilot study at Changhai Hospital (100% vs 94%) and assuming a two-sided type I error of 0.025, 126 patients per treatment group were required to provide a power of 80%. Quantitative data, including age, body mass index, abdominal fullness, gastric filling score, GCS, GTT, SBTT, and satisfaction questionnaire score, are presented as mean ± SD or median (interquartile range). Categorical data, including gastrointestinal symptoms, medical and personal history, and DY, are presented as frequencies (percentages). χ2 tests, t tests, analysis of covariance, Fisher’s exact test, and nonparametric tests were conducted to analyse differences in outcome measures. All reported P values were two-sided, and differences with P < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS (version 22.0, IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY, United States: IBM Corp.). A statistical review was conducted before submission.
From December 3, 2020 to May 17, 2021, 440 patients were referred for MCE examination, and 188 patients were excluded: Three patients had a surgical history that altered gastrointestinal anatomy, two patients were suspected of having intestinal obstruction, 25 required only small bowel examination, 55 required only gastric examination, 16 did not meet the inclusion criteria (six were over 80 years old, and 10 were under 18 years old), and 87 declined to participate (Figure 2).
A total of 252 patients (141 men), aged 18-77 years, were randomly assigned to groups C (n = 126) and W (n = 126). No statistical differences were observed in baseline characteristics and indications between the two groups (Table 1). No capsule retention or adverse events related to capsule endoscopy were observed during the 2-week follow-up period. All patients completed the examinations as required.
| C group (n = 126) | W group (n = 126) | P value | |
| Male | 74 (58.73) | 67 (53.17) | 0.374 |
| Age, year | 46.90 ± 13.20 | 47.49 ± 13.32 | 0.722 |
| BMI, kg/m2 | 23.28 ± 3.19 | 23.53 ± 3.75 | 0.579 |
| Indications | 0.713 | ||
| Health checkup | 24 (19.05) | 27 (21.43) | |
| Abdominal distention | 27 (21.43) | 29 (23.02) | |
| Abdominal pain | 35 (27.78) | 29 (23.02) | |
| Diarrhea | 10 (7.94) | 8 (6.35) | |
| Acid reflux | 7 (5.56) | 10 (7.94) | |
| Belching | 4 (3.17) | 0 (0.00) | |
| Elevated tumor markers | 3 (2.38) | 5 (3.97) | |
| Vomiting | 4 (3.17) | 5 (3.97) | |
| Hematochezia | 7 (5.56) | 6 (4.76) | |
| Others1 | 5 (3.97) | 7 (5.56) | |
| History of abdominal surgery | 14 (11.11) | 22 (17.46) | 0.150 |
| Smoking | 29 (23.02) | 30 (23.81) | 0.882 |
| Drinking | 32 (25.40) | 23 (18.25) | 0.170 |
| Hp infection | 0.665 | ||
| No infection | 51 (40.48) | 42 (33.33) | |
| Untreated | 13 (10.32) | 18 (14.29) | |
| Under treatment | 6 (4.76) | 4 (3.17) | |
| Cured | 12 (9.52) | 14 (11.11) | |
| Unknown | 44 (34.92) | 48 (38.10) | |
| Anticoagulation | 7 (5.56) | 6 (4.76) | 0.776 |
| Diabetes | 6 (4.76) | 6 (4.76) | 1.000 |
| Hypertension | 16 (12.70) | 26 (20.63) | 0.091 |
For the primary endpoint, 123 patients in C group and 101 patients in W group scored > 4 points (97.62% vs 80.16%, P < 0.0001). The median gastric filling score at 0-5 and 5-10 minutes after the capsule entered the stomach was 5.0 (5.0-5.0) and 5.0 (4.0-5.0) in the C group, which was statistically higher than 4.0 (4.0-5.0) and 4.0 (3.0-4.0) in the W group (P0-5 < 0.0001,
| C group (n = 126) | W group (n = 126) | P value | |
| Primary outcome | 123 (97.62) | 101 (80.16) | < 0.0001 |
| 0-5 minutes, median (IQR) | 5 (5-5) | 4 (4-5) | < 0.0001 |
| 5 | 99 (78.57) | 37 (29.37) | |
| 4 | 24 (19.05) | 64 (50.79) | |
| 3 | 2 (1.59) | 18 (14.29) | |
| 2 | 1 (0.79) | 7 (5.56) | |
| 5-10 minutes, median (IQR) | 5 (4-5) | 4 (3-4) | < 0.0001 |
| 5 | 69 (54.76) | 17 (13.49) | |
| 4 | 38 (30.16) | 56 (44.44) | |
| 3 | 9 (7.14) | 39 (30.95) | |
| 2 | 1 (0.79) | 11 (8.73) |
Patients in the C group had a shorter GTT (53.27 ± 53.83 minutes vs 71.12 ± 52.19 minutes, P = 0.001). Although no significant differences were observed between the groups in terms of GET (11.70 ± 2.76 minutes vs 11.26 ± 2.84 minutes, P = 0.219) or SBTT (4.84 ± 1.71 minutes vs 5.00 ± 1.82 minutes, P = 0.587), transpyloric passage of the capsule under magnetic control was successfully performed in more patients in the C group (43 vs 15, P < 0.0001; Table 3).
| C group (n = 126) | W group (n = 126) | P value | |
| GET, minute, mean ± SD | 11.70 ± 2.76 | 11.26 ± 2.84 | 0.219 |
| GTT, minute, mean ± SD | 53.27 ± 53.83 | 71.12 ± 52.19 | 0.001 |
| SBTT, hour, mean ± SD | 4.84 ± 1.71 | 5.00 ± 1.82 | 0.587 |
| Patients with capsule reaching duodenum under magnetic control | 43 (34.13) | 15 (11.90) | < 0.0001 |
| Patients with liquid for gastric refilling | 2 (1.59) | 21 (16.67) | < 0.0001 |
| Gastric cleanliness score, median (IQR) | |||
| Cardia | 4 (4-4) | 4 (4-4) | 0.451 |
| Fundus | 3 (3-3) | 3 (3-4) | < 0.0001 |
| Angulus | 4 (4-4) | 4 (4-4) | 0.356 |
| Body | 4 (3-4) | 4 (3-4) | 0.750 |
| Antrum | 4 (4-4) | 4 (4-4) | 0.202 |
| Pylorus | 4 (4-4) | 4 (4-4) | 0.695 |
| Abdominal fullness, mean ± SD | 7.85 ± 1.86 | 7.72 ± 1.70 | 0.551 |
| Comfortableness, mean ± SD | 7.91 ± 2.30 | 8.40 ± 1.65 | 0.054 |
| Total satisfaction score, median (IQR) | 2 (0-12) | 2 (0-10) | 0.882 |
| Gastric lesions | 24 (19.05) | 24 (19.05) | 0.331 |
| Polyp | 13 | 15 | |
| Ulcer | 1 | 4 | |
| Submucosal mass | 10 | 5 | |
| Small bowel lesions | 10 (7.94) | 18 (14.29) | 0.102 |
| Polyp | 1 | 0 | |
| Ulcer | 9 | 18 | |
| Others | 3 (2.38)1 | 1 (0.79)2 | 0.622 |
| Total | 37 (29.37) | 43 (34.13) | 0.173 |
The mean abdominal fullness scores in the two groups were 7.85 (SD, 1.86) and 7.72 (SD, 1.70), respectively (P = 0.551). Satisfaction (2.0, 2.0, P = 0.882) and comfort scores (7.91 vs 8.40, P = 0.054) reported by patients showed no significant differences.
The median GCS at primary gastric anatomical landmarks in both groups showed similar results, with "excellent" scores except for the fundus, where both groups achieved a "good" rating (P < 0.0001).
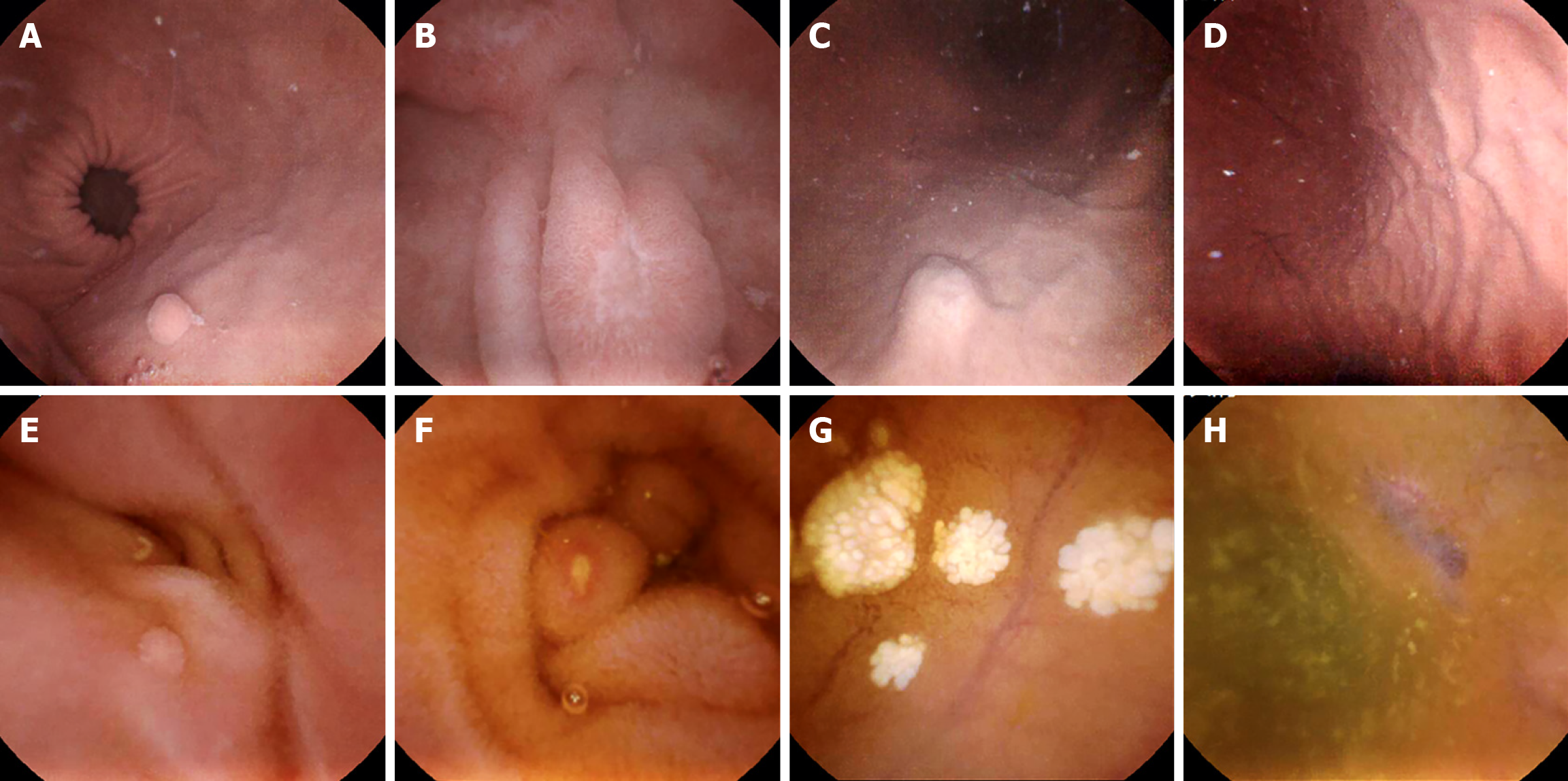
In this study, 80 cases (37 in C group and 43 in W group) were identified as positive focal lesions. Both groups had 24 patients diagnosed with gastric lesions, with 10 and 18 patients identified as having small bowel lesions in the C and W groups, respectively. No obvious differences were observed in the per-patient detection rates. For the safety evaluation, the capsules were evacuated intact, and no capsule retention occurred (Table 3, Figure 3).
Multivariable analyses of outcomes demonstrating statistical significance revealed additional clinical correlations beyond group allocation effects. The gastric fundus cleanliness score was associated with body weight (P = 0.017) and anticoagulant use (P < 0.001; Supplementary Table 1). Good gastric filling (gastric filling score of ≥ 4 within 5 minutes after the capsule entered the stomach) showed sex-specific (P = 0.010) and smoking-related (P < 0.001) variations (Supplementary Table 2). Magnetic capsule duodenal arrival rates demonstrated associations with body weight (P = 0.012), diabetes (P = 0.044), and hypertension (P = 0.030; Supplementary Table 3). GTT correlated with body weight (P = 0.010; Supplementary Table 4), whereas abdominal fullness exhibited sex differences (P = 0.014; Supplementary Table 5). Patient-reported comfort was linked to age (P = 0.005) and smoking history (P = 0.044; Supplementary Table 6).
This study provided a preliminary clinical assessment of a novel gastric preparation method for MCE. With similar examination compliance, drinking 550 mL of carbonated soft drink before MCE resulted in better gastric filling for at least 10 minutes compared with drinking 1000 mL of pure water, combined with a potential promotion of gastric emptying. Although the results showed relatively poor cleanliness at the gastric fundus, the GTT of the capsule was accelerated in C group, ensuring more time for small bowel examination.
Sufficient evidence that MCE is a promising method for the diagnosis of gastrointestinal diseases exists[12], and it could be used as a multifunctional unit with treatment functions at specified sites in the gastrointestinal tract in the near future[15]. Effective gastrointestinal preparation ensures complete and accurate visualisation. However, the optimal strategy for gastric preparation remains controversial. Patients are required to drink 800-1000 mL of pure water before MCE for gastric filling, which can be challenging, as some patients cannot complete this in a short time. In this study, replacing water with a carbonated soft drink appears to be a feasible option. For patients, reducing liquid intake will decrease the gastric preparation time, increase patient compliance, and enhance the willingness to repeat the MCE examination[14]. The potential role of carbonated soft drinks in promoting gastrointestinal motility may reduce the risk of capsule retention in the stomach and incomplete examination of the small bowel.
The improved gastric filling capacity of carbonated soft drinks in our study can be explained by the fact that gastric acid promotes the release of CO2 from carbonated soft drinks, which is primarily discharged from the stomach through burping. For better gastric filling and visualisation, the patients were asked to change their body position to promote gas release and reduce burping during the examination. The safety and advantages of using CO2 during endoscopy have been confirmed in previous studies[16,17]. Rapid absorption of CO2 and its discharge from the stomach alleviated the discomfort experienced by patients after gastric examination. However, the beneficial use of CO2 in upper gastrointestinal endoscopy and sedated patients remains controversial[18,19].
Another interesting finding of the study was that gastric emptying in C group appeared to be promoted, as reflected by the shorter GTT and higher proportion of patients whose capsules reached the duodenum under magnetic control compared to the pure W group. This may be explained by stronger gastrointestinal movement and higher intragastric pressure. In addition, the continuous release of CO2 into the gastric cavity during examination not only helps maintain gastric filling[20] but also increases the pressure difference between the two sides of the pylorus, which may facilitate pyloric opening for faster gastric emptying. As early as 1952, relevant studies confirmed that gastric emptying time is influenced by gastric contents and that carbonated water generally accelerates gastric evacuation[21]. Suzuki et al[22] showed that different gastric-contraction rhythms were induced by oral carbonic stimulation alone and carbonated water ingestion, and substantial augmentations were found after carbonated water ingestion. Nevertheless, the somatosensory perception brought about by carbonated water is shaped by the interaction between chemical and mechanical stimuli[23], and the underlying mechanism needs to be explored under the removal of interference from food additives in further studies.
Because of the stomach’s anatomy, cleaning the fundus completely using the defoamer is difficult in the upright position, which indicates a difference in cleanliness between the six primary anatomical landmarks of the stomach[24,25]. The mixing of mucus and small bubbles released from carbonated soft drinks may interfere with gastric cleanliness at the fundus. Concurrently, exogenous HCO3- directly neutralises gastric acid, inducing a transient pH elevation that inhibits pepsin activity and reduces mucolytic degradation. The removal of mucus by pronase has been shown to eliminate this problem[26]. Nevertheless, the visualisation of the gastric mucosa was comparable between the two groups, given the results of gastric cleanliness and DY.
This study has a few methodological limitations. First, we found unsatisfactory gastric fundus cleanliness in C group, which may be related to the interaction between gastric fundus mucus and bubbles. Although this had no significant impact on DY, improving the cleanliness of the gastric fundus requires further exploration. Second, soft drink con
With advances in fully automated technology[30], bioinformatics, telemedicine, and artificial intelligence, the indications for MCE have been further expanded. With the increasing use of capsule endoscopy, especially in early cancer screening for asymptomatic populations, increasing the efficiency and tolerability of gastric preparation is of paramount importance[14,27]. In summary, our study innovatively investigated and demonstrated the feasibility and efficacy of carbonated soft drinks for gastric preparation under MCE examination and suggested a potential trend towards enhanced gastric emptying. These findings may help develop a new gastric preparation regimen, particularly for patients who struggle to drink a large amount of water in a short period, including older adults, children, and individuals with poor gastrointestinal motility.
The authors are grateful to Quan-Cai Cai for providing valuable guidance on the statistical analysis and to the Foreign Language Teaching and Research Department of the Naval Medical University for providing writing assistance for this article.
| 1. | Cao Q, Deng R, Pan Y, Liu R, Chen Y, Gong G, Zou J, Yang H, Han D. Robotic wireless capsule endoscopy: recent advances and upcoming technologies. Nat Commun. 2024;15:4597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 2. | Liao Z, Duan XD, Xin L, Bo LM, Wang XH, Xiao GH, Hu LH, Zhuang SL, Li ZS. Feasibility and safety of magnetic-controlled capsule endoscopy system in examination of human stomach: a pilot study in healthy volunteers. J Interv Gastroenterol. 2012;2:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Cave DR, Hakimian S, Patel K. Current Controversies Concerning Capsule Endoscopy. Dig Dis Sci. 2019;64:3040-3047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Shamsudhin N, Zverev VI, Keller H, Pane S, Egolf PW, Nelson BJ, Tishin AM. Magnetically guided capsule endoscopy. Med Phys. 2017;44:e91-e111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Jiang B, Pan J, Qian YY, He C, Xia J, He SX, Sha WH, Feng ZJ, Wan J, Wang SS, Zhong L, Xu SC, Li XL, Huang XJ, Zou DW, Song DD, Zhang J, Ding WQ, Chen JY, Chu Y, Zhang HJ, Yu WF, Xu Y, He XQ, Tang JH, He L, Fan YH, Chen FL, Zhou YB, Zhang YY, Yu Y, Wang HH, Ge KK, Jin GH, Xiao YL, Fang J, Yan XM, Ye J, Yang CM, Li Z, Song Y, Wen MY, Zong Y, Han X, Wu LL, Ma JJ, Xie XP, Yu WH, You Y, Lu XH, Song YL, Ma XQ, Li SD, Zeng B, Gao YJ, Ma RJ, Ni XG, He CH, Liu YP, Wu JS, Liu J, Li AM, Chen BL, Cheng CS, Sun XM, Ge ZZ, Feng Y, Tang YJ, Li ZS, Linghu EQ, Liao Z; Capsule Endoscopy Group of the Chinese Society of Digestive Endoscopy. Clinical guideline on magnetically controlled capsule gastroscopy (2021 edition). J Dig Dis. 2023;24:70-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Lo SK, Fujii-Lau LL, Enestvedt BK, Hwang JH, Konda V, Manfredi MA, Maple JT, Murad FM, Pannala R, Woods KL, Banerjee S; ASGE Technology Committee. The use of carbon dioxide in gastrointestinal endoscopy. Gastrointest Endosc. 2016;83:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Maple JT, Banerjee S, Barth BA, Bhat YM, Desilets DJ, Gottlieb KT, Pfau PR, Pleskow DK, Siddiqui UD, Tokar JL, Wang A, Song LM, Rodriguez SA; GE Technology Assessment Committee. Methods of luminal distention for colonoscopy. Gastrointest Endosc. 2013;77:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Zhang WY, Jiang XP, Miao L, Chen FC, Huang ZM, Huang XL. Efficacy and safety of carbon dioxide insufflation versus air insufflation for endoscopic retrograde cholangiopancreatography: A meta-analysis update. Clin Res Hepatol Gastroenterol. 2017;41:217-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Li X, Dong H, Zhang Y, Zhang G. CO2 insufflation versus air insufflation for endoscopic submucosal dissection: A meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0177909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Dellon ES, Hawk JS, Grimm IS, Shaheen NJ. The use of carbon dioxide for insufflation during GI endoscopy: a systematic review. Gastrointest Endosc. 2009;69:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Zhu JH, Qian YY, Liu X, Jiang B, Liao Z, Li ZS. [Gastric preparation with Sprite Zero ® for magnetically controlled capsule endoscopy]. Zhonghua Xiaohua Neijing Zazhi. 2022;39:972-977. [DOI] [Full Text] |
| 12. | Geropoulos G, Aquilina J, Kakos C, Anestiadou E, Giannis D. Magnetically Controlled Capsule Endoscopy Versus Conventional Gastroscopy: A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2021;55:577-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | de Franchis R, Eisen GM, Laine L, Fernandez-Urien I, Herrerias JM, Brown RD, Fisher L, Vargas HE, Vargo J, Thompson J, Eliakim R. Esophageal capsule endoscopy for screening and surveillance of esophageal varices in patients with portal hypertension. Hepatology. 2008;47:1595-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Leung FW. Methods of reducing discomfort during colonoscopy. Dig Dis Sci. 2008;53:1462-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Vasilakakis MD, Koulaouzidis A, Marlicz W, Iakovidis DK. The future of capsule endoscopy in clinical practice: from diagnostic to therapeutic experimental prototype capsules. Prz Gastroenterol. 2020;15:179-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Kim SY, Chung JW, Park DK, Kwon KA, Kim KO, Kim YJ, Kim JH. Comparison of carbon dioxide and air insufflation during consecutive EGD and colonoscopy in moderate-sedation patients: a prospective, double-blind, randomized controlled trial. Gastrointest Endosc. 2017;85:1255-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Wang WL, Wu ZH, Sun Q, Wei JF, Chen XF, Zhou DK, Zhou L, Xie HY, Zheng SS. Meta-analysis: the use of carbon dioxide insufflation vs. room air insufflation for gastrointestinal endoscopy. Aliment Pharmacol Ther. 2012;35:1145-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Nakagawara H, Takahashi H, Ogawa M, Endo Y, Watanabe Y, Hirayama M, Ryuzaki H, Moriyama M, Iriguchi Y. The influence of esophagogastroduodenoscopy using carbon dioxide insufflation on abdominal ultrasonographic imaging efficiency. J Med Ultrason (2001). 2020;47:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Lord AC, Riss S. Is the type of insufflation a key issue in gastro-intestinal endoscopy? World J Gastroenterol. 2014;20:2193-2199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Murray K, Placidi E, Schuring EA, Hoad CL, Koppenol W, Arnaudov LN, Blom WA, Pritchard SE, Stoyanov SD, Gowland PA, Spiller RC, Peters HP, Marciani L. Aerated drinks increase gastric volume and reduce appetite as assessed by MRI: a randomized, balanced, crossover trial. Am J Clin Nutr. 2015;101:270-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Lolli G, Greenberg LA, Lester D. The influence of carbonated water on gastric emptying. N Engl J Med. 1952;246:490-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Suzuki M, Mura E, Taniguchi A, Moritani T, Nagai N. Oral Carbonation Attenuates Feeling of Hunger and Gastric Myoelectrical Activity in Young Women. J Nutr Sci Vitaminol (Tokyo). 2017;63:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Wise PM, Wolf M, Thom SR, Bryant B. The influence of bubbles on the perception carbonation bite. PLoS One. 2013;8:e71488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Mackintosh CE, Kreel L. Anatomy and radiology of the areae gastricae. Gut. 1977;18:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 38] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Zhang Y, Zhang Y, Huang X. Development and Application of Magnetically Controlled Capsule Endoscopy in Detecting Gastric Lesions. Gastroenterol Res Pract. 2021;2021:2716559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Fujii T, Iishi H, Tatsuta M, Hirasawa R, Uedo N, Hifumi K, Omori M. Effectiveness of premedication with pronase for improving visibility during gastroendoscopy: a randomized controlled trial. Gastrointest Endosc. 1998;47:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Tsompanaki E, Thanapirom K, Papatheodoridi M, Parikh P, Chotai de Lima Y, Tsochatzis EA. Systematic Review and Meta-analysis: The Role of Diet in the Development of Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2023;21:1462-1474.e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Mullee A, Romaguera D, Pearson-Stuttard J, Viallon V, Stepien M, Freisling H, Fagherazzi G, Mancini FR, Boutron-Ruault MC, Kühn T, Kaaks R, Boeing H, Aleksandrova K, Tjønneland A, Halkjær J, Overvad K, Weiderpass E, Skeie G, Parr CL, Quirós JR, Agudo A, Sánchez MJ, Amiano P, Cirera L, Ardanaz E, Khaw KT, Tong TYN, Schmidt JA, Trichopoulou A, Martimianaki G, Karakatsani A, Palli D, Agnoli C, Tumino R, Sacerdote C, Panico S, Bueno-de-Mesquita B, Verschuren WMM, Boer JMA, Vermeulen R, Ramne S, Sonestedt E, van Guelpen B, Holgersson PL, Tsilidis KK, Heath AK, Muller D, Riboli E, Gunter MJ, Murphy N. Association Between Soft Drink Consumption and Mortality in 10 European Countries. JAMA Intern Med. 2019;179:1479-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 29. | Hernández-López R, Canto-Osorio F, Vidaña-Pérez D, Torres-Ibarra L, Rivera-Paredez B, Gallegos-Carrillo K, Velazquez R, Ramírez P, Barrientos-Gutiérrez T, Salmerón J, López-Olmedo N. Soft drink and non-caloric soft drink intake and their association with blood pressure: the Health Workers Cohort Study. Nutr J. 2022;21:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 30. | Xiao YF, Wu ZX, He S, Zhou YY, Zhao YB, He JL, Peng X, Yang ZX, Lv QJ, Yang H, Bai JY, Fan CQ, Tang B, Hu CJ, Jie MM, Liu E, Lin H, Koulaouzidis A, Zhao XY, Yang SM, Xie X. Fully automated magnetically controlled capsule endoscopy for examination of the stomach and small bowel: a prospective, feasibility, two-centre study. Lancet Gastroenterol Hepatol. 2021;6:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |