Published online May 14, 2025. doi: 10.3748/wjg.v31.i18.105530
Revised: February 27, 2025
Accepted: April 24, 2025
Published online: May 14, 2025
Processing time: 107 Days and 7.6 Hours
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) is a procedure used for patients with initially unresectable colorectal liver metastases (CRLM). However, the procedure has been reported to be asso
To assess the safety and feasibility of full laparoscopic ALPPS in patients with CRLM.
A retrospective analysis was conducted on all consecutive patients with CRLM who underwent full laparoscopic ALPPS at the Sixth Affiliated Hospital of Sun Yat-sen University between March 2021 and July 2024.
Fifteen patients were included, 13 with synchronous liver metastases. Nine patients had more than five liver tumors, with the highest count being 22. The median diameter of the largest lesion was 2.8 cm on preoperative imaging. No extrahepatic metastases were observed. RAS mutations were detected in nine patients, and 14 underwent preoperative chemotherapy. The median increase in future liver remnant volume during the interstage interval was 47.0%. All patients underwent R0 resection. Overall complication rates were 13.3% (stage 1) and 53.3% (stage 2), while major complication rates (Clavien-Dindo ≥ IIIa) were 13.3% (stage 1) and 33.3% (stage 2). No mortality occurred in either stage. The median hospital stay after stage 2 was 10 days.
Full laparoscopic ALPPS for CRLM is safe and feasible, with the potential for reduced morbidity and mortality, offering radical resection opportunities for patients with initially unresectable CRLM.
Core Tip: This study evaluates the feasibility and safety of full laparoscopic associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) in colorectal liver metastases. Among 15 patients, the procedure achieved a 100% radical resection rate with significant future liver remnant hypertrophy (47% median increase). Complication rates were low, particularly in stage 1, with no mortality reported. Laparoscopic modifications minimized invasiveness and enhanced surgical outcomes. Post-ALPPS liver regeneration was substantial, demonstrating the potential for repeat interventions. These findings support full laparoscopic ALPPS as a promising strategy for advanced colorectal liver metastases, addressing the limitations of traditional hypertrophy techniques like portal venous embolization or portal venous ligation.
- Citation: Zheng ZY, Zhang L, Li WL, Dong SY, Song JL, Zhang DW, Huang XM, Pan WD. Laparoscopic associating liver partition and portal vein ligation for staged hepatectomy for colorectal liver metastases: A single-center experience. World J Gastroenterol 2025; 31(18): 105530
- URL: https://www.wjgnet.com/1007-9327/full/v31/i18/105530.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i18.105530
Colorectal cancer is the third most common malignant tumor worldwide, with the liver being the most frequent site of distant metastases[1]. Among these patients, 15%-25% are diagnosed with synchronous liver metastases, while another 15%-25% develop metachronous liver metastases. Due to significant improvements in 5-year overall survival, surgery remains the mainstay of curative therapy for patients with colorectal liver metastases (CRLM)[2,3]. However, owing to the widespread distribution or vascular involvement of metastases, achieving a sufficient future liver remnant (FLR) may not be possible, increasing the risk of post-hepatectomy liver failure (PHLF).
Several techniques have been developed to stimulate liver hypertrophy in patients with insufficient FLR before hepatectomy, with two-stage hepatectomy (TSH) being widely used in contemporary practice[4-6]. The first stage involves portal venous ligation (PVL) or portal venous embolization (PVE) to stimulate FLR hypertrophy, typically 4-8 weeks before the second stage of hepatectomy. This technique can increase the FLR volume by 27%-39%[6]. However, a drawback of this technique is the risk of insufficient FLR hypertrophy and tumor progression during the waiting period[4], which may result in tumor resection failure.
In 2012, Schnitzbauer et al[7] published an innovative method of liver resection called associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). A notable advantage of ALPPS is the rapid increase in FLR within a short period of approximately 2 weeks, enabling radical resection and minimizing the risk of tumor progression during the waiting period[7-13]. Additionally, the primary indication for ALPPS is CRLM, as it is considered safer for secondary than for primary liver tumors[12,14-16]. However, significant morbidity and mortality have been reported with ALPPS, making this procedure controversial[7-17]. In recent years, various modifications, including partial ALPPS, mini-ALPPS, and ablation-assisted ALPPS, have been developed to reduce the risks associated with this procedure[18]. Additionally, laparoscopic or robotic surgery offers a minimally invasive alternative to reduce the risks of ALPPS[18-23]. Nevertheless, the technical challenges of performing two complex laparoscopic liver surgeries within a short timeframe have limited the widespread adoption of the full laparoscopic ALPPS procedure. This study aimed to assess the feasibility and safety of full laparoscopic ALPPS in patients with CRLM.
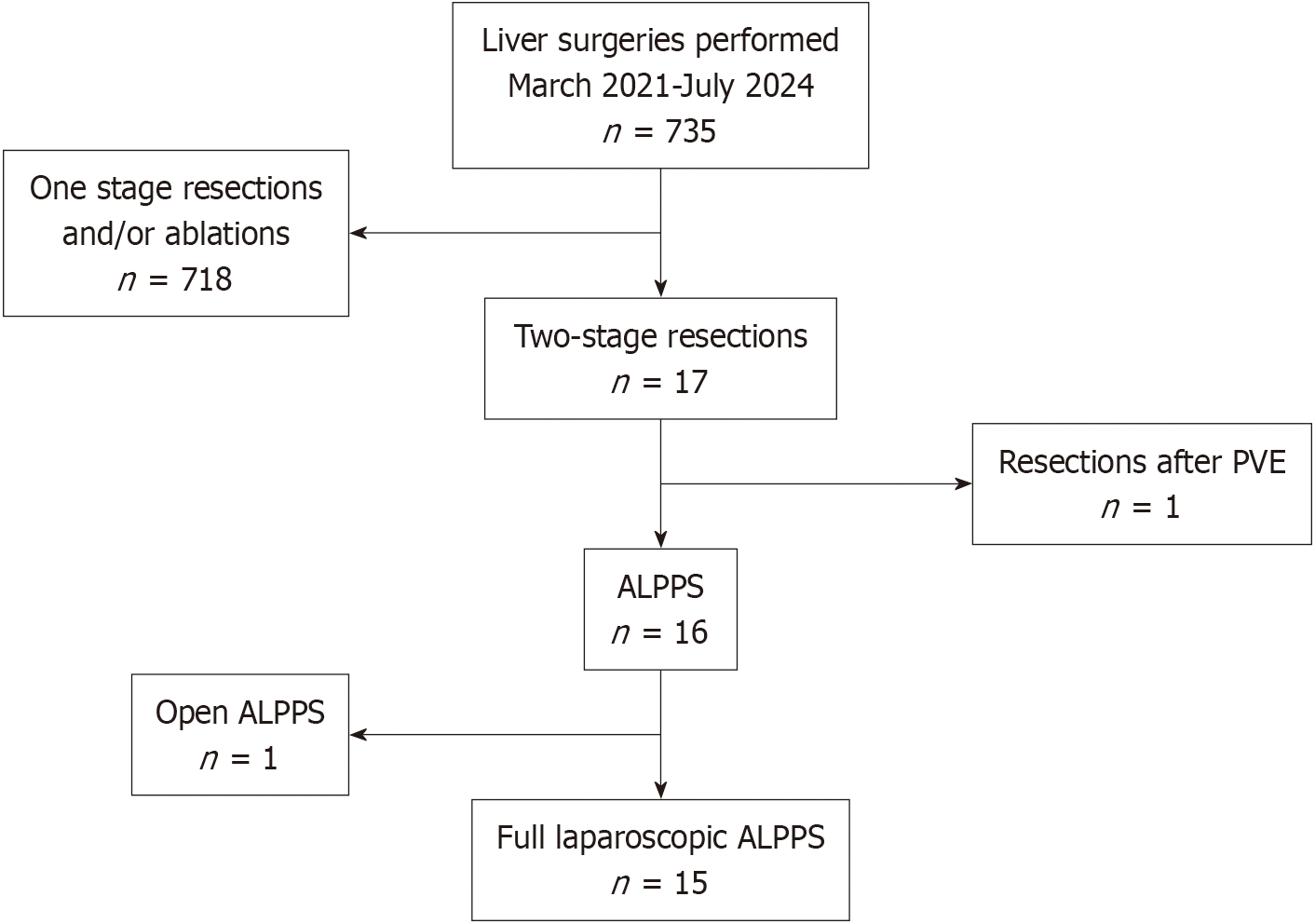
A retrospective analysis was conducted on all consecutive patients with CRLM who visited the Sixth Affiliated Hospital of Sun Yat-sen University from March 2021 to July 2024. The inclusion criteria were as follows: (1) Patients with CRLM confirmed by clinical or histological evidence; (2) Patients underwent liver surgeries, whether through open or laparoscopic approaches; and (3) Complete and detailed laboratory and clinical information. The exclusion criteria were as follows: (1) Extrahepatic metastases; (2) Patients with other primary malignant tumors; (3) Patients underwent one stage resections and/or ablations; (4) Patients underwent two-stage resections after PVE/PVL; and (5) Patients underwent open ALPPS (Figure 1).
All patients underwent a detailed clinical work-up, including computed tomography (CT) and/or magnetic resonance imaging, and their therapeutic schedules were evaluated by a multidisciplinary team prior to surgeries. The indication for ALPPS was insufficient FLR, as determined by CT measurements prior to stage 1 procedures. An insufficient FLR was defined as a remaining liver volume without tumors of less than 30% in patients without underlying liver diseases or less than 40% in patients with liver diseases, such as hepatic steatosis, cirrhosis, or liver damage after chemotherapy following the final surgery[24]. All surgeries were performed by the same surgeon (Wei-Dong Pan).
The FLR volumetric analyses were performed using Vitrea 4.6 Workstation (Vital Images, Minnetonka, MN, United States). Firstly, the portal venous phases of four-phase contrast-enhanced CT scans were obtained to facilitate optimal identification of Couinaud segments and resection planes tailored to individual patient anatomy. Secondly, axial CT slices were manually traced, with interpolations of intermediate slices performed by the software. All contours, including extrapolated slices, were reviewed and corrected by two independent researchers to minimize interobserver variability. Finally, the software generated 3D reconstructions integrating vascular/ductal anatomy, with automated volume calculation. Key exclusions comprised the gallbladder and inferior vena cava (IVC), while intrahepatic structures were retained to reflect functional liver mass.
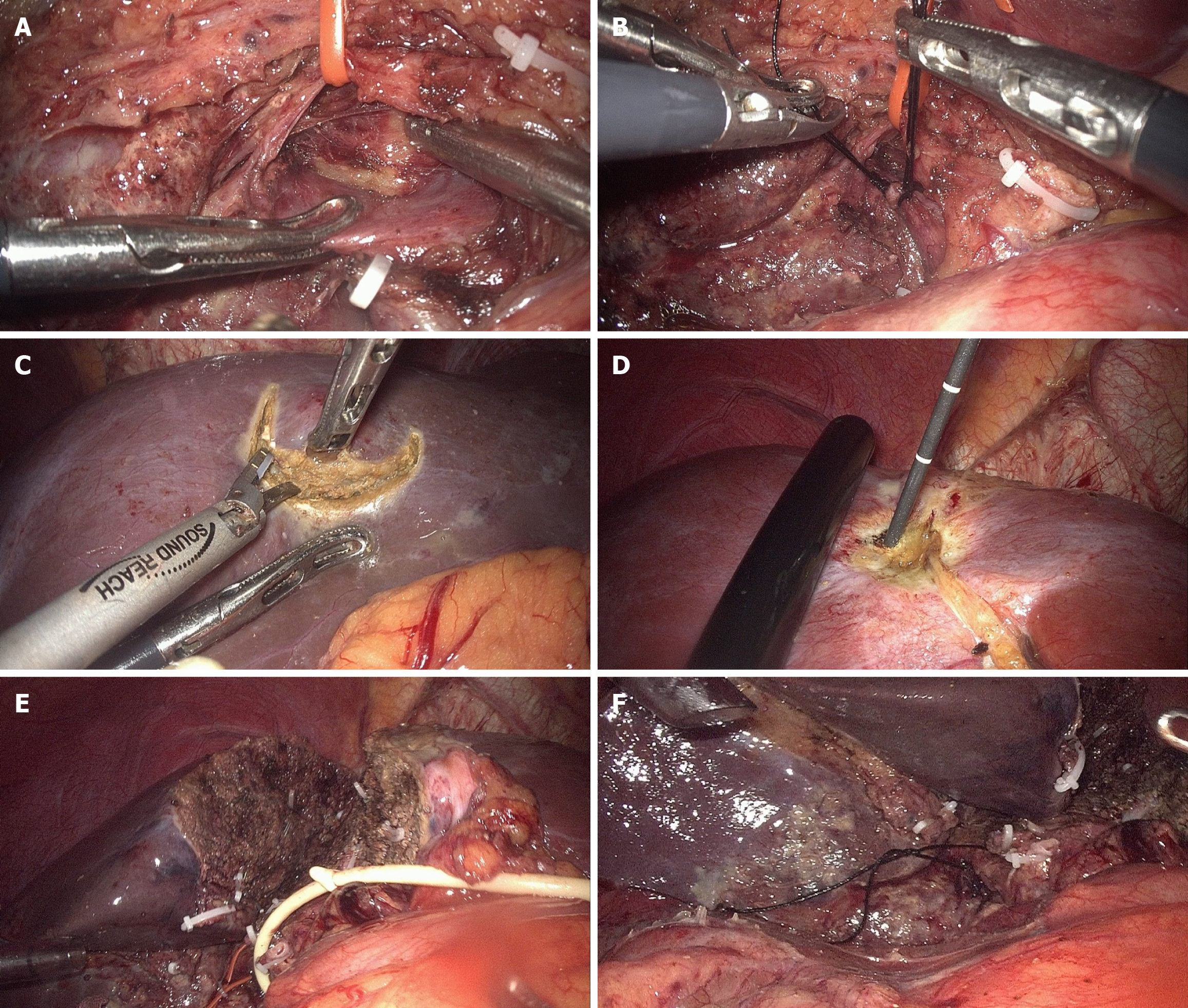
In stage 1 laparoscopic ALPPS, intraoperative ultrasound was initially performed to locate tumors in the FLR and confirm whether they involved the middle hepatic vein (MHV). If the tumors involved the MHV, the liver parenchyma was transected on the left side of the MHV in the subsequent procedure; otherwise, it was transected on the right side. Additionally, the transection was required to be at least 1 cm away from the tumor. The gallbladder was removed during stage 1. The right hepatic artery (RHA) was identified and preserved. The right portal vein was ligated without transection using double nonabsorbable sutures. Tumors in the FLR identified by ultrasound were resected and/or treated with microwave ablation according to their size and location. Subsequently, the liver parenchyma was partially transected[25] in situ to the anterior plane of the right Glisson pedicle and caudate lobe without exposing the posthepatic veins or IVC. The MHV was transected on the cephalic side when tumors were involved. Finally, the RHA was marked with loose sutures for identification during stage 2 (Figure 2). Interstage chemotherapy was not conducted. Before stage 2 of ALPPS, the volume growth and tumor progression of the FLR were assessed using CT at least 8 days after phase 1. Subsequent surgeries were performed only if the FLR hypertrophy was deemed adequate. Additional resection and/or ablation of progressive tumors in the FLR were performed if detected.
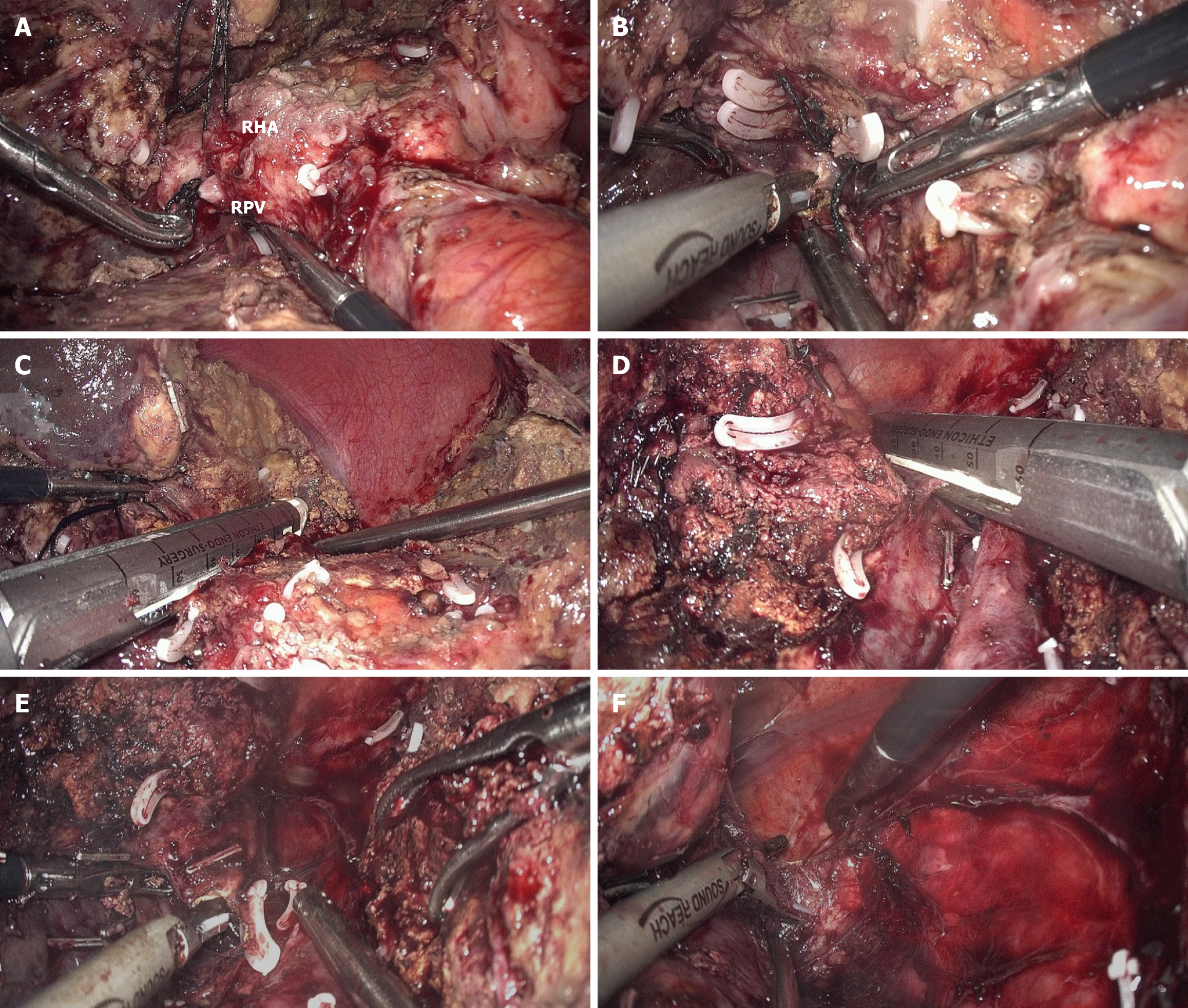
At the start of stage 2 of laparoscopic ALPPS, adhesions between the divided liver segments were separated using gentle blunt dissection. After separating the liver hilar adhesions, the RHA and right portal vein were identified and transected. The right Glisson’s pedicle was then divided using an endostapler after transecting the lowest part of the caudate lobe and several posthepatic veins. The residual parenchyma was subsequently divided anterior to the IVC until the right hepatic vein was transected using an endostapler. Finally, complete mobilization of the right liver from the retroperitoneum and diaphragm was performed (Figure 3). The specimen was removed through a para-midline incision.
Demographic variables, including primary colorectal cancer, liver metastases, Fong’s clinical risk score[26], and comprehensive ALPPS preoperative risk assessment (CAPRA) score[14], were recorded. Left colonic and rectal tumors were classified as left-sided primary tumors. The FLR, standardized total liver volume (sTLV), and FLR/sTLV before stages 1 and 2 were documented. sTLV was calculated based on the body surface area[27,28]. Increased velocity was defined as the increase in FLR volume divided by the number of days between the stage 1 operation and CT evaluation prior to stage 2. Intraoperative and postoperative variables were documented. Complications were assessed using the Clavien-Dindo classification[29] and quantitatively analyzed with a comprehensive complication index (CCI)[30], calculated using a web-based calculator (https://www.cci-calculator.com). PHLF was defined based on the criteria of the International Study Group of Liver Surgery[31]. Mortality was defined as death within 90 days of surgery.
Continuous variables were expressed as medians and ranges, while categorical variables were reported as counts and percentages. Given the descriptive nature of this study, no statistical tests were conducted. Statistical analyses were conducted using the SPSS Statistics version 22 software (IBM Corp., Armonk, NY, United States).
Complete laparoscopic ALPPS was performed in 15 patients between March 2021 and July 2024. The median age of the patients was 57 years, and 53.3% (n = 8) were male. Seven patients (46.7%) presented with left-sided primary tumors, six (40.0%) had right-sided tumors, and two (13.3%) presented with multiple anatomic localizations bilaterally. Primary tumors were resected in most patients (n = 11, 73.3%). No extrahepatic metastases were observed. Thirteen (86.7%) patients presented with synchronous liver metastases. Nine patients (60.0%) had more than five liver tumors, with a maximum of 22. The median diameter of the largest lesion was 2.8 cm on preoperative imaging. RAS mutations were detected in nine patients (60.0%). Preoperative chemotherapy, either neoadjuvant or conversion, was administered to 14 (93.3%) patients. The median CAPRA score was 2.48. Detailed information on demographic and tumor characteristics is presented in Table 1.
| Variable | Overall (n = 15) | |
| Age, years | 57 (37-70) | |
| Gender | Male | 8 (53.3) |
| Female | 7 (46.7) | |
| BMI, kg/m2 | 22.0 (18.6-26.2) | |
| ASA score | 2 | 14 (93.3) |
| 3 | 1 (6.7) | |
| Primary tumor | ||
| Location | Left | 7 (46.7) |
| Right | 6 (40.0) | |
| Bilateral | 2 (13.3) | |
| Primary tumor resected | 11 (73.3) | |
| Liver metastases | ||
| Timing of liver metastases | Synchronous | 13 (86.7) |
| Metachronous | 2 (13.3) | |
| Number of tumors | 1-5 | 6 (40.0) |
| 6-10 | 4 (26.7) | |
| 11-20 | 4 (26.7) | |
| > 20 | 1 (6.7) | |
| Bilobar disease | 8 (53.3) | |
| Max tumor size, mm | 2.8 (1.4-6.5) | |
| MHV involvement | 7 (46.7) | |
| RAS mutation | 9 (60.0) | |
| CEA before ALPPS, ng/mL | 9.1 (2.0-179.2) | |
| CRS | 2 | 5 (33.3) |
| 3 | 8 (53.3) | |
| 4 | 2 (13.3) | |
| CAPRA score | 2.48 (0.7-4.54) | |
| Extrahepatic metastases | 0 (0.0) | |
| Chemotherapy1 | ||
| Preoperative chemotherapy | 14 (93.3) | |
| Cycles of chemotherapy | 8 (1-30) | |
| Chemotherapy response | PR | 7 (50.0) |
| SD | 3 (21.4) | |
| PD | 4 (28.6) |
All patients successfully underwent full laparoscopic ALPPS without conversion to open surgery. Concomitant left-sided colorectal resection was performed in one patient (6.7%) during stage 1 ALPPS. In stage 1 ALPPS, seven patients (46.7%) underwent FLR tumor procedures, of which three (20.0%) underwent microwave ablation, and 4 (26.7%) underwent both wedge resection and microwave ablation. The median interstage interval was 17 days. Extended right hepatectomies were performed in seven patients (46.7%) during stage 2 ALPPS due to tumor involvement of the MHV. All patients underwent R0 resection. Detailed operative characteristics are presented in Table 2.
| Variable | Overall (n = 15) | |
| Stage 1 of ALPPS | ||
| Associated procedures | Concomitant colorectal resection | 1 (6.7) |
| Ablation in FLR | 3 (20.0) | |
| Resection and ablation in FLR | 4 (26.7) | |
| Operation time, minute | 255 (170-430) | |
| Blood loss, mL | 100 (50-600) | |
| Blood transfusion | 1 (6.7) | |
| Conversion to open | 0 (0.0) | |
| Interstage interval, days | 17 (13-24) | |
| Stage 2 of ALPPS | ||
| Type of hepatectomy | Right hepatectomy | 8 (53.3) |
| Extended right hepatectomy | 7 (46.7) | |
| Resection/ablation in FLR | 0 (0.0) | |
| Operation time, minute | 195 (171-409) | |
| Blood loss, mL | 100 (20-1000) | |
| Blood transfusion | 2 (13.3) | |
| Conversion to open | 0 (0.0) | |
| R0 resection | 15 (100.0) |
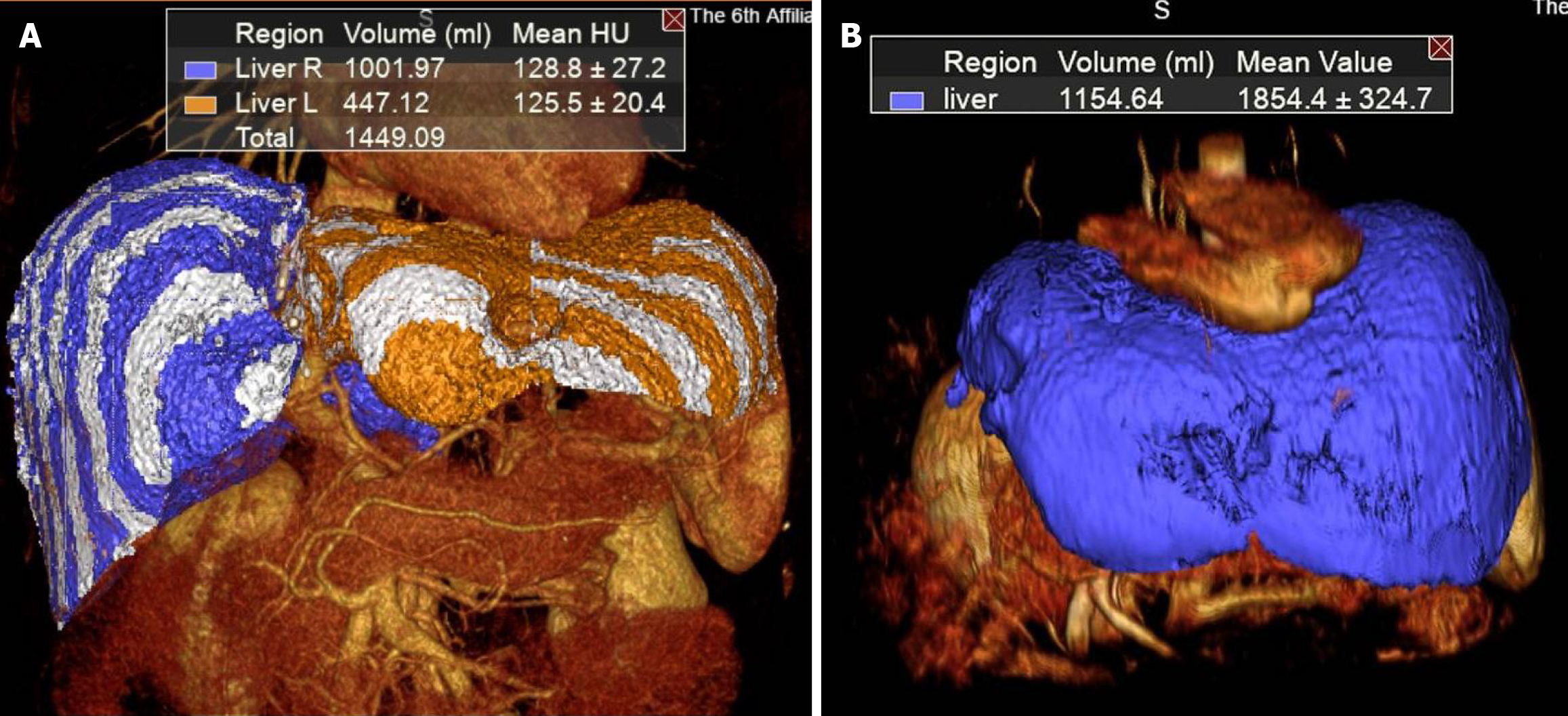
The median FLR volume before stage 1 ALPPS was 356.0 mL, while the median FLR/sTLV ratio was 33.3%. A significant increase in the median FLR volume was observed, reaching 478.1 mL before stage 2, with the median FLR/sTLV ratio increasing to 41.5%. Furthermore, the median increase rate of FLR volume during the interstage interval was 47.0%, while the median increased velocity was 11.2 mL/day. Detailed information regarding the volumetric data is presented in Table 3. Furthermore, the FLR volume of case 4 was calculated 3 months after the stage 2 operation to evaluate FLR regeneration in the post-ALPPS period (Figure 4).
| Variable | Overall (n = 15) |
| FLR pre stage 1, mL | 356.0 (202.6-458.3) |
| FLR/sTLV pre stage 1, % | 33.3 (17.8-41.2) |
| FLR pre stage 2, mL | 478.1 (380.9-642.3) |
| FLR/sTLV pre stage 2, % | 41.5 (33.4-57.8) |
| Increase volume, mL | 173.9 (30.7-247.8) |
| Increase rate, % | 47.0 (7.5-97.3) |
| Time between stage 1 and imaging, days | 10 (8-21) |
| Increased velocity, mL/day | 11.2 (3.4-26.6) |
Postoperative short-term outcomes are summarized in Table 4. The overall complication rate in stage 1 was 13.3% (n = 2), both of which were major complications (Clavien-Dindo ≥ 3a), presenting primarily with pleural effusion and ascites. One of these two patients developed a biliary fistula after stage 1, which was resolved in stage 2. The median CCI score in stage 1 was 0. The overall complication rate in stage 2 was 53.3% (n = 8), higher than that in stage 1. The major complication rate in stage 2 was 33.3% (n = 5), exceeding the rate in stage 1. After stage 2 ALPPS, three patients developed biliary fistula, and one had mild PHLF (International Study Group of Liver Surgery grade A). The median CCI score for stage 2 was 8.7. No reoperations or mortality occurred in either stage. The median hospital stay after stage 2 was 10 days.
| Variable | Overall (n = 15) | |
| Stage 1 of ALPPS | ||
| Complication type | Total | 2 (13.3) |
| Pleural effusion | 2 (13.3) | |
| Ascites | 2 (13.3) | |
| Biliary fistula | 1 (6.7) | |
| Cardiac insufficiency | 1 (6.7) | |
| Complication grade | Minor (Clavien-Dindo 1-2) | 1 (6.7) |
| Major (Clavien-Dindo 3-5) | 2 (13.3) | |
| CCI score | 0.0 (0.0-50.0) | |
| Mortality | 0 (0.0) | |
| Stage 2 of ALPPS | ||
| Complication type | Total | 8 (53.3) |
| Wound infection | 2 (13.3) | |
| Pleural effusion | 3 (20.0) | |
| Ascites | 2 (13.3) | |
| Biliary fistula | 3 (20.0) | |
| PHLF (ISGLS grade A) | 1 (6.7) | |
| Cardiac insufficiency | 1 (6.7) | |
| Complication grade | Minor (Clavien-Dindo 1-2) | 4 (26.7) |
| Major (Clavien-Dindo 3-5) | 5 (33.3) | |
| CCI score | 8.7 (0.0-45.4) | |
| Mortality | 0 (0.0) | |
| Hospital stay after stage 2, days | 10 (5-26) |
The indications for ALPPS include liver tumors with insufficient FLR, such as hepatocellular carcinoma, intrahepatic cholangiocarcinoma, and metastatic tumors, including CRLM[32]. A series of studies have shown CRLM to be the best indication for ALPPS[12,14-16,33,34]. In general, patients with CRLM do not have severe underlying liver diseases, such as cirrhosis or portal hypertension. Thus, stimulating a rapid increase in FLR volume may be beneficial in improving the resection rate of stage 2 operations.
CRLM is often accompanied by a high tumor burden, with widespread distribution or vascular involvement, leading to insufficient FLR volume and initial unresectability. As a more conventional strategy, PVE/PVL can increase FLR volume. However, the risks of insufficient hypertrophy and tumor progression often result in the failure of tumor resection after PVE/PVL[4]. In recent years, the LIGRO trial, the first global randomized controlled trial comparing ALPPS with TSH in patients with advanced CRLM, was completed, providing the strongest evidence for the feasibility of ALPPS[10]. The resection rate was significantly higher with ALPPS than with TSH (92% vs 57%; P < 0.0001). Another large cohort study on ALPPS from 22 centers over a 10-year period, published in 2020, reported a 96% resection rate in patients with CRLM[12]. Additionally, several studies have reported the results of minimally invasive strategies for ALPPS, with a 100% resection rate[18,23,25,35,36]. In this study, all patients successfully underwent full laparoscopic ALPPS with radical resection, achieving a high resection rate of 100%, similar to the results of previous studies.
Sufficient FLR volume is a prerequisite for the successful completion of ALPPS. Therefore, the primary reason for the high resection rates is the rapid growth of FLR volume. In this study, the median increase in FLR volume was 47.0%, which is consistent with the results of several other studies[10-12,17,18,23,35,36]. However, the factors contributing to rapid liver regeneration remain unclear. Chemotherapy with oxaliplatin and/or irinotecan is the standard systemic therapy for CRLM. However, the hepatotoxic effects of these drugs, including sinusoidal obstruction syndrome induced by oxaliplatin and steatohepatitis induced by irinotecan, have raised growing concerns. Previous studies have reported that chemotherapeutic agents are a factor in reducing liver regeneration[15,37]. However, the results of these studies are inconsistent. Recently, a cohort study using data from the International ALPPS Registry was conducted to identify factors related to FLR growth[38]. Interestingly, they reported that anthropometric characteristics, such as height, weight, FLR size, and sex, were key factors affecting FLR growth rather than chemotherapy. In this study, most patients underwent preoperative chemotherapy, with a maximum of 30 cycles administered to one patient (case 2). However, the rate of increase in this patient population was 49.6%, higher than the median rate of 47.0%. This indicates that differences in liver regeneration among individuals may be multifactorial, and further research in this area is needed.
In our experience, several key strategies have been emphasized to ensure the effectiveness of surgery and reduce intraoperative injuries. First, FLR tumors should be completely resected and/or ablated during stage 1 to ensure that the FLR achieves a no evidence of disease status after stage 1. This outcome relies on accurate localization using CT or magnetic resonance imaging before stage 1 and detailed scanning with laparoscopic intraoperative ultrasound. If FLR tumors are missed during stage 1, a repeat detail scanning should be performed during stage 2. If a condition of disappearing liver metastases occurs[39,40], early initiation of adjuvant therapy is recommended, followed by close monitoring. Second, the liver parenchyma was partially transected in stage 1 instead of being completely transected. This technique, known as “partial ALPPS”, was first developed by the Zurich’s group, though they performed it using an open approach[25]. They reported that partial ALPPS provided hypertrophic benefits comparable to those of classical ALPPS when the parenchymal transection was at least 50%. Additionally, partial ALPPS results in lower perioperative morbidity and mortality. In practice, the parenchyma was transected to the anterior plane of the right Glisson pedicle and caudate lobe, avoiding exposure of the posthepatic veins and IVC. This procedure is beneficial for maintaining the original anatomical gap between the liver and the IVC, reducing adhesions to the IVC during stage 2, which can otherwise lead to intraoperative vascular injuries. Moreover, the enhanced visualization provided by laparoscopy facilitates precise transection and helps prevent biliary injuries[35]. Furthermore, the liver should be transected in situ without mobilizing the right liver during stage 1 to minimize adhesions to the IVC and diaphragm in stage 2. Finally, a loose suture was placed on the RHA at the end of stage 1 to prevent confusion dissection and reduce the risk of hilum injury during stage 2. In conclusion, as one study stated, the main principle is to “keep the first step small, reduce liver partition, avoid liver mobilization, and postpone the second step until recovery of liver function”[35].
The high morbidity and mortality associated with ALPPS have been a subject of controversy since the first study on this complex procedure. In the first report by Schnitzbauer et al[7] in 2012, the complication and mortality rates were 68% and 12% respectively. Other authors have reported similar results[10-12,17], highlighting the security risks associated with ALPPS. However, in these studies, ALPPS was performed using an open approach, resulting in increased surgical invasiveness as well as high morbidity and mortality rates. In recent years, minimally invasive modifications and techniques have been applied to ALPPS, proving beneficial for reducing complication and mortality rates[18,23,25,35,36]. In this study, the incidence of biliary fistulas was 20% after completion of ALPPS, consistent with findings in other reports[23,35]. Patients with biliary fistulas were successfully treated with endoscopic biliary stent implantation and/or percutaneous drainage. Notably, the incidence of PHLF was 6.7%, which is lower than that reported in other studies[23]. Furthermore, morbidities in the two stages of ALPPS were quantitatively analyzed, revealing that the median CCI was 0 in stage 1 and 8.7 in stage 2. The CCI is calculated based on the complication grading by the Clavien-Dindo classification and reflects the severity of the overall complication burden on the patient, ranging from 0 (no complication) to 100 (death)[30]. The CCI results in this study demonstrate the safety of full laparoscopic ALPPS, especially in stage 1 operations. Similarly, the mortality rate in this study was 0, further demonstrating the safety of this minimally invasive approach. CAPRA predicts 90-day mortality preoperatively and offers a reliable method for selecting patients suitable for ALPPS prior to surgery[14]. It is evaluated using seven variables: Age, body surface area, primary liver tumors, comorbidities (such as severe cardiovascular disease, mild or severe diabetes, renal disease), and ALPPS types. In this study, the median CAPRA score was 2.48, indicating a probability of 90 days mortality of less than 7%[14].
CRLM has a high recurrence rate, which remains significantly high even after ALPPS with R0 resection. In this study, recurrence data were not analyzed due to inadequate follow-up time. The 1-year recurrence rate in the ALPPS group of the LIGRO trial was 53.8%[41]. Additionally, in a large 10-year cohort study on ALPPS, the hepatic recurrence rate was 60%[12]. However, they also reported that patients could achieve remarkable long-term survival after ALPPS, particularly those who underwent repeat liver surgery and/or ablation of recurrent hepatic lesions. Therefore, the FLR volume at the time of recurrence is crucial, as it is related to the feasibility of repeat surgery and/or ablation. Although many studies have focused on the increase in FLR during the interstage period of ALPPS, few reports have addressed liver regeneration after the completion of ALPPS. In this study, FLR volume was calculated for case 4 at 3 months post-ALPPS. Case 4 involved a patient with up to 22 tumors distributed bilaterally in the liver, including six on the left side and 16 on the right. Additionally, the tumors involved the MHV, resulting in an FLR volume of 352.2 mL (29.3%) prior to ALPPS. During the ALPPS period, the patient underwent local resections and ablations for the FLR in stage 1, followed by extended right hepatectomy in stage 2, preserving a liver volume of 447.1 mL (36.9%). Remarkably, the liver volume increased to 1154.6 mL at 3 months post-ALPPS, equivalent to normal liver volume (Figure 3). This phenomenon shows great potential for liver regeneration after ALPPS, offering the possibility of repeat surgery and/or ablation when tumors recur.
This study has some limitations. First, the primary limitation was the retrospective observational design without a comparative analysis. The level of evidence used in this study was low. Although selection bias was inevitable, consecutive patients were included to mitigate potential selection bias. Second, the single-center design and specific disease features resulted in a limited number of participants. Due to differences in clinical management and technical measures, the results of this study may not be fully applicable to other centers. Finally, this study lacked information on long-term survival due to inadequate follow-up time. However, some studies have demonstrated the effectiveness of ALPPS in promoting long-term survival[12,41-46].
Full laparoscopic ALPPS for CRLM is safe and feasible, with the potential for reduced morbidity and mortality. However, due to the technical complexity of this procedure, it cannot be guaranteed that each center can perform successfully and safely. It requires surgeons to be highly experienced with laparoscopic hepatectomy. Nevertheless, it must be acknowledged that this procedure can provide an opportunity for radical resection for patients with initially unresectable CRLM. Therefore, multicenter prospective comparative clinical studies should be conducted in the future.
This study is supported by National Key Clinical Discipline. We thank all the colleagues who assisted in clinical information collection.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55828] [Article Influence: 7975.4] [Reference Citation Analysis (132)] |
| 2. | de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 590] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 3. | Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 279] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 4. | van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM, van Delden OM. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013;36:25-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 333] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 5. | Adam R, Miller R, Pitombo M, Wicherts DA, de Haas RJ, Bitsakou G, Aloia T. Two-stage hepatectomy approach for initially unresectable colorectal hepatic metastases. Surg Oncol Clin N Am. 2007;16:525-536, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Pandanaboyana S, Bell R, Hidalgo E, Toogood G, Prasad KR, Bartlett A, Lodge JP. A systematic review and meta-analysis of portal vein ligation versus portal vein embolization for elective liver resection. Surgery. 2015;157:690-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, Kroemer A, Loss M, Rümmele P, Scherer MN, Padberg W, Königsrainer A, Lang H, Obed A, Schlitt HJ. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 933] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 8. | Alvarez FA, Ardiles V, de Santibañes M, Pekolj J, de Santibañes E. Associating liver partition and portal vein ligation for staged hepatectomy offers high oncological feasibility with adequate patient safety: a prospective study at a single center. Ann Surg. 2015;261:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 9. | Bertens KA, Hawel J, Lung K, Buac S, Pineda-Solis K, Hernandez-Alejandro R. ALPPS: challenging the concept of unresectability--a systematic review. Int J Surg. 2015;13:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Sandström P, Røsok BI, Sparrelid E, Larsen PN, Larsson AL, Lindell G, Schultz NA, Bjørnbeth BA, Isaksson B, Rizell M, Björnsson B. ALPPS Improves Resectability Compared With Conventional Two-stage Hepatectomy in Patients With Advanced Colorectal Liver Metastasis: Results From a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial). Ann Surg. 2018;267:833-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 11. | Bednarsch J, Czigany Z, Sharmeen S, van der Kroft G, Strnad P, Ulmer TF, Isfort P, Bruners P, Lurje G, Neumann UP. ALPPS versus two-stage hepatectomy for colorectal liver metastases--a comparative retrospective cohort study. World J Surg Oncol. 2020;18:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Petrowsky H, Linecker M, Raptis DA, Kuemmerli C, Fritsch R, Kirimker OE, Balci D, Ratti F, Aldrighetti L, Voskanyan S, Tomassini F, Troisi RI, Bednarsch J, Lurje G, Fard-Aghaie MH, Reese T, Oldhafer KJ, Ghamarnejad O, Mehrabi A, Abraham MET, Truant S, Pruvot FR, Hoti E, Kambakamba P, Capobianco I, Nadalin S, Fernandes ESM, Kron P, Lodge P, Olthof PB, van Gulik T, Castro-Benitez C, Adam R, Machado MA, Teutsch M, Li J, Scherer MN, Schlitt HJ, Ardiles V, de Santibañes E, Brusadin R, Lopez-Lopez V, Robles-Campos R, Malagó M, Hernandez-Alejandro R, Clavien PA. First Long-term Oncologic Results of the ALPPS Procedure in a Large Cohort of Patients With Colorectal Liver Metastases. Ann Surg. 2020;272:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 13. | Chen Z, Shen S, Xie W, Liao J, Feng S, Li S, Tan J, Kuang M. Comparison of clinical efficacy between LAPS and ALPPS in the treatment of hepatitis B virus-related hepatocellular carcinoma. Gastroenterol Rep (Oxf). 2023;11:goad060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Capobianco I, Oldhafer KJ, Fard-Aghaie MH, Robles-Campos R, Brusadin R, Petrowsky H, Linecker M, Mehrabi A, Hoffmann K, Li J, Heumann A, Hernandez-Alejandro R, Tun-Abraham ME, Jovine E, Serenari M, Bjornsson B, Sandström P, Alikhanov R, Efanov M, Muiesan P, Schlegel A, van Gulik TM, Olthof PB, Stavrou GA, Serna-Higuita LM, Königsrainer A, Nadalin S. Development and internal validation of the Comprehensive ALPPS Preoperative Risk Assessment (CAPRA) score: is the patient suitable for Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS)? Hepatobiliary Surg Nutr. 2022;11:52-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Schadde E, Raptis DA, Schnitzbauer AA, Ardiles V, Tschuor C, Lesurtel M, Abdalla EK, Hernandez-Alejandro R, Jovine E, Machado M, Malago M, Robles-Campos R, Petrowsky H, Santibanes ED, Clavien PA. Prediction of Mortality After ALPPS Stage-1: An Analysis of 320 Patients From the International ALPPS Registry. Ann Surg. 2015;262:780-5; discussion 785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 16. | Oldhafer KJ, Stavrou GA, van Gulik TM; Core Group. ALPPS--Where Do We Stand, Where Do We Go?: Eight Recommendations From the First International Expert Meeting. Ann Surg. 2016;263:839-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Ratti F, Schadde E, Masetti M, Massani M, Zanello M, Serenari M, Cipriani F, Bonariol L, Bassi N, Aldrighetti L, Jovine E. Strategies to Increase the Resectability of Patients with Colorectal Liver Metastases: A Multi-center Case-Match Analysis of ALPPS and Conventional Two-Stage Hepatectomy. Ann Surg Oncol. 2015;22:1933-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Michal K, Sau M, Tamara GMH, Long JR. A better route to ALPPS: minimally invasive vs open ALPPS. Surg Endosc. 2020;34:2379-2389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Xiao L, Li JW, Zheng SG. Totally laparoscopic ALPPS in the treatment of cirrhotic hepatocellular carcinoma. Surg Endosc. 2015;29:2800-2801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Surjan RC, Makdissi FF, Basseres T, Leite D, Charles LF, Bezerra RO, Schadde E, Machado MA. First totally laparoscopic ALPPS procedure with selective hepatic artery clamping: Case report of a new technique. Medicine (Baltimore). 2016;95:e4236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Shafaee Z, Kazaryan AM, Marvin MR, Cannon R, Buell JF, Edwin B, Gayet B. Is laparoscopic repeat hepatectomy feasible? A tri-institutional analysis. J Am Coll Surg. 2011;212:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Goh BK, Lee SY, Chan CY, Wong JS, Cheow PC, Chung AY, Ooi LL. Early experience with robot-assisted laparoscopic hepatobiliary and pancreatic surgery in Singapore: single-institution experience with 20 consecutive patients. Singapore Med J. 2018;59:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Magistri P, Guidetti C, Catellani B, Caracciolo D, Odorizzi R, Frassoni S, Bagnardi V, Guerrini GP, Di Sandro S, Di Benedetto F. Robotic ALPPS for primary and metastatic liver tumours: short-term outcomes versus open approach. Updates Surg. 2024;76:435-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Khan AS, Garcia-Aroz S, Ansari MA, Atiq SM, Senter-Zapata M, Fowler K, Doyle MB, Chapman WC. Assessment and optimization of liver volume before major hepatic resection: Current guidelines and a narrative review. Int J Surg. 2018;52:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 25. | Petrowsky H, Györi G, de Oliveira M, Lesurtel M, Clavien PA. Is partial-ALPPS safer than ALPPS? A single-center experience. Ann Surg. 2015;261:e90-e92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 26. | Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309-18; discussion 318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2800] [Article Influence: 107.7] [Reference Citation Analysis (1)] |
| 27. | Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1521] [Cited by in RCA: 2016] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 28. | Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM, Lerut J, Materne R, Wang X, Encarnacion A, Herron D, Mathey C, Ferrari G, Charnsangavej C, Do KA, Denys A. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 464] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 29. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24841] [Article Influence: 1182.9] [Reference Citation Analysis (0)] |
| 30. | Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 1297] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 31. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1726] [Article Influence: 123.3] [Reference Citation Analysis (0)] |
| 32. | Hernandez-Alejandro R, Ruffolo LI, Alikhanov R, Björnsson B, Torres OJM, Serrablo A. Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) procedure for colorectal liver metastasis. Int J Surg. 2020;82S:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Schadde E, Ardiles V, Robles-Campos R, Malago M, Machado M, Hernandez-Alejandro R, Soubrane O, Schnitzbauer AA, Raptis D, Tschuor C, Petrowsky H, De Santibanes E, Clavien PA; ALPPS Registry Group. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg. 2014;260:829-36; discussion 836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 349] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 34. | Schnitzbauer AA, Schadde E, Linecker M, Machado MA, Adam R, Malago M, Clavien PA, de Santibanes E, Bechstein WO. Indicating ALPPS for Colorectal Liver Metastases: A Critical Analysis of Patients in the International ALPPS Registry. Surgery. 2018;164:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Machado MA, Makdissi FF, Surjan RC, Basseres T, Schadde E. Transition from open to laparoscopic ALPPS for patients with very small FLR: the initial experience. HPB (Oxford). 2017;19:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Melandro F, Giovanardi F, Hassan R, Larghi Laureiro Z, Ferri F, Rossi M, Mennini G, Pawlik TM, Lai Q. Minimally Invasive Approach in the Setting of ALPPS Procedure: a Systematic Review of the Literature. J Gastrointest Surg. 2019;23:1917-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 37. | Pagano D, Gruttadauria S. Impact of future remnant liver volume on post-hepatectomy regeneration in non-cirrhotic livers. Front Surg. 2014;1:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Lopez-Lopez V, Linecker M, Cruz J, Brusadin R, Lopez-Conesa A, Machado MA, Hernandez-Alejandro R, Voskanyan AS, Li J, Balci D, Adam R, Ardiles V, De Santibañes E, Tomassini F, Troisi RI, Lurje G, Truant S, Pruvot FR, Björnsson B, Stojanovic M, Montalti R, Cayuela V, Kozyrin I, Cai X, de Vicente E, Rauchfuss F, Lodge P, Ratti F, Aldrighetti L, Oldhafer KJ, Malago M, Petrowsky H, Clavien PA, Robles-Campos R. Liver growth prediction in ALPPS - A multicenter analysis from the international ALPPS registry. Liver Int. 2022;42:2815-2829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Melstrom LG, Warner SG, Wong P, Sun V, Raoof M, Singh G, Chavin KD, Fong Y, Adam R, Hugh TJ. Management of disappearing colorectal liver metastases: an international survey. HPB (Oxford). 2021;23:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Kuhlmann K, van Hilst J, Fisher S, Poston G. Management of disappearing colorectal liver metastases. Eur J Surg Oncol. 2016;42:1798-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Røsok BI, Høst-Brunsell T, Brudvik KW, Carling U, Dorenberg E, Björnsson B, Lothe RA, Bjørnbeth BA, Sandström P. Characterization of early recurrences following liver resection by ALPPS and two stage hepatectomy in patients with colorectal liver-metastases and small future liver remnants; a translational substudy of the LIGRO-RCT. HPB (Oxford). 2019;21:1017-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Hasselgren K, Røsok BI, Larsen PN, Sparrelid E, Lindell G, Schultz NA, Bjørnbeth BA, Isaksson B, Larsson AL, Rizell M, Björnsson B, Sandström P. ALPPS Improves Survival Compared With TSH in Patients Affected of CRLM: Survival Analysis From the Randomized Controlled Trial LIGRO. Ann Surg. 2021;273:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 43. | Maupoey Ibáñez J, Montalvá Orón EM, Boscà Robledo A, Camacho Ramírez A, Hernando Sanz A, Granero Castro P, Alegre Delgado A, López-Andújar R. From conventional two-stage hepatectomy to ALPPS: Fifteen years of experience in a hepatobiliary surgery unit. Hepatobiliary Pancreat Dis Int. 2021;20:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Björk D, Hasselgren K, Røsok BI, Larsen PN, Sparrelid E, Lindell G, Schultz NA, Bjørnbeth BA, Isaksson B, Lindhoff Larsson A, Rizell M, Björnsson B, Sandström P. Long-Term Follow-Up of Patients with Advanced Colorectal Liver Metastasis: A Survival Analysis from the Randomized Controlled Multicenter Trial LIGRO. Ann Surg Open. 2024;5:e455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 45. | Alvarez FA, Ardiles V, Chara C, de Santibañes M, Sánchez Clariá R, Pekolj J, de Santibañes E. Adjuvant chemotherapy is associated with better oncological outcomes after ALPPS for colorectal liver metastases. Updates Surg. 2024;76:855-868. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 46. | Wang QQ, Quan XL, Zhang Y, Shu GM. Long-term survival of ALPPS procedure for hepatocellular carcinoma with tumor thrombus in the right branch of portal vein: A case report. Asian J Surg. 2024;47:3203-3204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |