Published online May 14, 2025. doi: 10.3748/wjg.v31.i18.105495
Revised: March 14, 2025
Accepted: April 21, 2025
Published online: May 14, 2025
Processing time: 84 Days and 0.8 Hours
Knee osteoarthritis (KOA) is a chronic condition characterized by joint pain and dysfunction, driven by aging and obesity. Research indicates that the gut microbiota significantly influences KOA, potentially affecting inflammation and disease progression through the gut-joint axis. Traditional treatments like non-steroidal anti-inflammatory drugs offer symptom relief but have adverse effects. Emerging therapies like electroacupuncture (EA) and Tuina (TN) have shown promise in alleviating pain and improving joint function by targeting the gut microbiota.
To clarify the efficacy of EA with TN in treating KOA and its effect on gut micro
Sixty patients with KOA were allocated to EA or EA + TN (ET) group (n = 30 each). Seven acupoints were pun
The ET group showed higher rates of “effective” and “markedly effective” outcomes. The VAS score of the ET group remained significantly lower than that of the EA group (P < 0.001) immediately after treatment and 1 week post-treatment. The total WOMAC score (P < 0.001), pain (P = 0.191), stiffness (P = 0.015), and function scores (P < 0.001) decreased significantly in the ET group post-treatment. The gut microbiota analysis revealed no significant changes in alpha diversity in either group. Beta-diversity analysis indicated distinct patterns in the ET group before and after treatment. Significant changes in microbial abundance were detected in both groups, highlighting variations in Firmicutes, Actinobacteria, Proteobacteria, and Bacteroidetes.
ET outperforms EA alone in improving KOA pain, stiffness, and function, potentially via gut microbiota modu
Core Tip: This study introduces a novel therapeutic approach combining electroacupuncture and Tuina therapy to alleviate pain and enhance joint flexibility in knee osteoarthritis (KOA). It highlights the significant role of the gut microbiota in this process, demonstrating how electroacupuncture combined with Tuina can reduce pathogenic bacteria and increase beneficial ones to mitigate inflammatory responses, indirectly suggesting the influence of the gut-joint axis on KOA. This innovative perspective opens new avenues for understanding and treating inflammatory joint diseases, offering fresh therapeutic targets and strategies.
- Citation: Guo X, Guo L, Lu QZ, Xie H, Chen J, Su WL, Tian Y, Li XH, Miao HL, Zhang Y, Yang Y, Liao C, Deng JY, Yang YH, Tang CL, Liu HJ. Effect of electroacupuncture combined with Tuina therapy on gut microbiota in patients with knee osteoarthritis. World J Gastroenterol 2025; 31(18): 105495
- URL: https://www.wjgnet.com/1007-9327/full/v31/i18/105495.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i18.105495
Knee osteoarthritis (KOA) is a highly prevalent chronic disorder that manifests through three hallmark pathological processes: Gradual deterioration of articular cartilage integrity, osteophyte formation at joint margins, and persistent low-grade synovitis. It manifests clinically as pain, joint dysfunction, and deformity[1]. The incidence of KOA is rising globally, driven by an aging population and increasing obesity rates, resulting in a significant economic impact on patients and the healthcare system[2,3]. The pathogenesis of KOA is complex and involves a dynamic interplay of factors such as aging, metabolic dysregulation, injury, and inflammatory processes[4]. While non-steroidal anti-inflammatory drugs and corticosteroids remain key pharmacological interventions for symptom management in current clinical practice, extended administration of these agents correlates with elevated risks of treatment-related complications[5].
Emerging research has underscored the importance of the gut microbiota in diverse physiological processes, notably immune modulation, metabolism, and inflammatory regulation[6]. Under physiological conditions, the gut microbiota maintains a relative balance with the body and the external environment; once this balance is broken, it may cause intestinal dysbiosis and even diseases[7]. Increasing evidence suggests that the “gut-joint axis” may play a role in KOA[8], with the gut microbiota potentially influencing disease progression through mechanisms such as intestinal dysbiosis, cartilage degradation, and pain sensitization[9].
Extensive studies have shown that electroacupuncture (EA) can significantly alleviate clinical symptoms, such as pain and joint swelling, supporting its widespread clinical application[10-12]. Tuina (TN) therapy, grounded in the foundational theories of traditional Chinese medicine, combines the principles of modern anatomy, biomechanics, and imaging to treat KOA[13]. This integrated approach promotes the repair of periarticular tissues, nourishes articular cartilage, lubricates joints, and decelerates cartilage degeneration, thereby offering a complementary therapeutic avenue for KOA management[14]. Despite its clinical use, the potential advantages of EA combined with TN (ET) over EA alone remain unclear, as does the extent to which ET may ameliorate knee function and attenuate inflammation via modulation of the gut microbiota. Addressing these knowledge gaps could provide critical insights into the underlying mechanisms of KOA and lead to innovative therapeutic strategies.
The "gut-joint axis" highlights the role of bacterial byproducts like lipopolysaccharides (LPS), which can leak into the bloodstream from an impaired gut and trigger systemic inflammation that affects the joints, leading to conditions like osteoarthritis[15]. In patients with KOA, abnormalities in the diversity and structure of the gut microbiota can compromise intestinal barriers, allowing immune cells from the gut mucosa to migrate to sites of joint inflammation[16]. Emerging evidence indicates significant associations between gut microbial composition and pain intensity quantified by the Western Ontario and McMaster Universities osteoarthritis index (WOMAC) Pain Subscale, highlighting its potential as a modifiable biological modulator in KOA-associated pain pathogenesis[17].
This study investigated the efficacy of ET in treating KOA and its effect on gut microbiota regulation.
This study enrolled 60 patients diagnosed with KOA as part of a randomized controlled clinical trial conducted at the Chongqing Orthopedic Hospital of Traditional Chinese Medicine, Chongqing, China. Participants with KOA were randomized into either an EA group or an ET group, with 30 participants in each group, using the random number generation function in SPSS software (IBM SPSS Statistics, Version 15.0, for Windows; IBM, Armonk, NY, United States). Longitudinal stool specimens for microbiota dynamics analysis were obtained at baseline and after therapeutic intervention.
The inclusion criteria were as follows: A confirmed diagnosis of KOA; age between 40 and 80 years; disease duration of more than 6 months; and a pain intensity score of ≥ 3 on a visual analog scale (VAS) for most of the previous month. The exclusion criteria were: Gastrointestinal disorders; recent (within 8 weeks prior to sampling) use of antibiotics, probiotics, prebiotics, or any substances affecting the intestinal flora; other diseases associated with joint symptoms such as metabolic bone disease, acute trauma, gouty arthritis, rheumatoid arthritis, and other non-degenerative arthritis or joint deformities; pregnancy; and mental illness. Individuals who had undergone acupuncture therapy or enrolled in other research studies within 3 months prior to screening were excluded from participation.
Participants were not only briefed thoroughly on the research procedures but also assured of their unconditional right to withdraw. Of note, the consent forms specified that even if the participants opted out prematurely, their anonymized data would still contribute to outcome evaluations. The investigators adhered to the Good Clinical Practice guidelines throughout the study. Written informed consent was obtained from all participants before enrollment. This study received ethical approval from the Medical Ethics Review Committee of Chongqing Orthopedic Hospital of Traditional Chinese Medicine (GKYYIRB202309001) and was registered as a clinical trial on chictr.org.cn (ChiCTR2300076904).
All therapists in the study had Chinese medicine practitioner licenses and at least 5 years of clinical experience. Jiajian disposable sterile steel needles (size 0.30 mm × 50 mm, Wuxi Jiajian Medical Instruments Co., Ltd., China) were used. Both EA and ET therapies consisted of 12 sessions over 4 weeks (usually three sessions per week).
EA group: The acupuncture points were selected with reference to the Clinical Diagnosis and Treatment Guidelines for Orthopedics and Traumatology of Traditional Chinese Medicine-Knee Osteoarthritis. The obligatory acupoints included Zusanli (ST36), Yanglingquan (GB34), Yinlingquan (SP9), Dubi (ST35), Neixiyan (EX-LE5), Liangqiu (ST34), and Xuehai (SP10). Additionally, either a corresponding point or the Ashi point (the point of greatest pain) was selected based on the meridian associated with pain. The patients were placed in a supine position, and routine skin disinfection was performed. The needling techniques varied according to the acupoint: EX-LE5 was needled at a 45° oblique angle outward, ST35 was needled at a 45° oblique angle inward, and all other points were needled perpendicularly. Needles were stimulated manually for 10 seconds to elicit “De Qi” sensation. An electrical apparatus (Changzhou Yindi Electronic Medical Instruments Co. Ltd., Changzhou, China) was connected to the needles using alligator clips. The parameters were as follows: Frequency 2-100 Hz, intensity 2 mA, density wave, 20 minutes. The treatment was administered three times per week over four consecutive weeks.
ET group: TN therapy was administered after EA treatment. Placing the patient in the supine position, the therapist applied pressing and kneading techniques with the thumb to specific acupoints, including the Zusanli (ST36), Yanglingquan (GB34), Yinlingquan (SP9), Dubi (ST35), Neixiyan (EX-LE5), Liangqiu (ST34), Xuehai (SP10), and Heding (EX-LE2). Additionally, linear nodules or reactive points were targeted by pressing, kneading, and lateral flicking with the radial side of the thumb, which was performed for approximately five minutes per session. To relax the surrounding structures, rolling, kneading, and pinching techniques were used to release the quadriceps, adductor muscles, iliotibial tract, and medial and lateral collateral ligaments, as well as other soft tissues around the knee, for approximately 5 minutes per session. The patella was mobilized in various directions - upward, downward, medial, lateral, and diagonal (medial-up, medial-down, lateral-up, and lateral-down) - for 10-15 repetitions, focusing on the areas with restricted movement. The patella was gently pressed with the palm and kneaded to the left, right, and in a circular motion 10-15 times to rub it against the cartilage surface of the femoral condyle. The patella was fixed with five fingers and lifted repeatedly 5-10 times using force. Both hands were placed on the ankles, and the affected limb was pulled longitudinally with force for 1-2 minutes. The knee joint was then extended and bent to the maximum extent possible, followed by internal and external knee flexion movements.
Baseline assessment: The core baseline parameters comprised sex distribution, chronological age, and body mass index as fundamental demographic variables, VAS scores, and WOMAC scores.
Primary outcome: Pain severity was evaluated using the VAS, which served as the primary outcome measure. This scale quantifies pain from 0 to 10, where 0 denotes no pain, 1-3 signifies mild pain, 4-6 represents moderate pain, and 7-10 reflects severe pain. Pain severity was evaluated at three distinct time points: Baseline, immediately after treatment (T1), and 1 week after treatment (T2).
Secondary outcomes: The secondary outcomes included: (1) WOMAC scores: Knee joint pain was quantified using the WOMAC Pain Subscale comprising five items with scores ranging from 0 to 20 points. Additionally, joint stiffness and functional ability were assessed using the WOMAC Stiffness Subscale, which consists of two items scored from 0 to 8 points, and the WOMAC Functional Subscale, which includes 17 items scored from 0 to 68 points. WOMAC scores were evaluated at baseline and T1; and (2) Treatment efficacy: The efficacy index (%) was calculated as [(baseline WOMAC score - after treatment WOMAC score)/baseline WOMAC score] × 100% according to the WOMAC total scores with reference to the nimodipine method. Efficacy index ≥ 80% was considered cured; efficacy index ≥ 50% but < 80% was considered markedly effective; efficacy index ≥ 25% but < 50% was considered effective; and efficacy index < 25% was considered ineffective.
Two stool specimens were collected from each participant with KOA, one prior to and one following treatment, and were promptly frozen at -80 °C within 3 hours of collection. Total DNA from fecal samples was quantified using a NanoDrop spectrophotometer, and DNA quality was assessed via 1.2% agarose gel electrophoresis. Demographic and clinical data were collected during the clinical visits.
PCR was used to amplify the hypervariable V3-V4 segments of the 16S ribosomal RNA gene in bacterial genomic DNA. Vazyme VAHTS DNA Clean Beads were used to purify and recover the amplification products. The recovered PCR products were quantified using a Quant-iT PicoGreen dsDNA Assay Kit and measured using a BioTek FLX 800 microplate reader. Each sample was combined in appropriate proportions based on the quantity required for sequencing.
Following library preparation using the TruSeq Nano DNA LT Library Prep Kit (Illumina, San Diego, CA, United States), high-throughput sequencing was subsequently performed on an Illumina platform. Sequence denoising and operational taxonomic unit (OTU) clustering were performed using QIIME 2 DADA2 or the Vestra pipeline. QIIME 2 (version 2019.4) was subsequently used to analyze the species composition, alpha diversity, and beta diversity.
Continuous data were assessed for normality and the homogeneity of variance. Tukey’s test was applied for data that were normally distributed and met the assumptions of the χ2 test for variance. In cases where data were normally distributed but the variances were unequal, intergroup comparisons were performed using the paired-sample t-test. Statistical analyses were conducted using SPSS (Version 15.0, SPSS Inc., Chicago, IL, United States). P < 0.05 was used to define statistical significance. Continuous data are expressed as the mean ± SD and categorical outcomes as proportional frequencies.
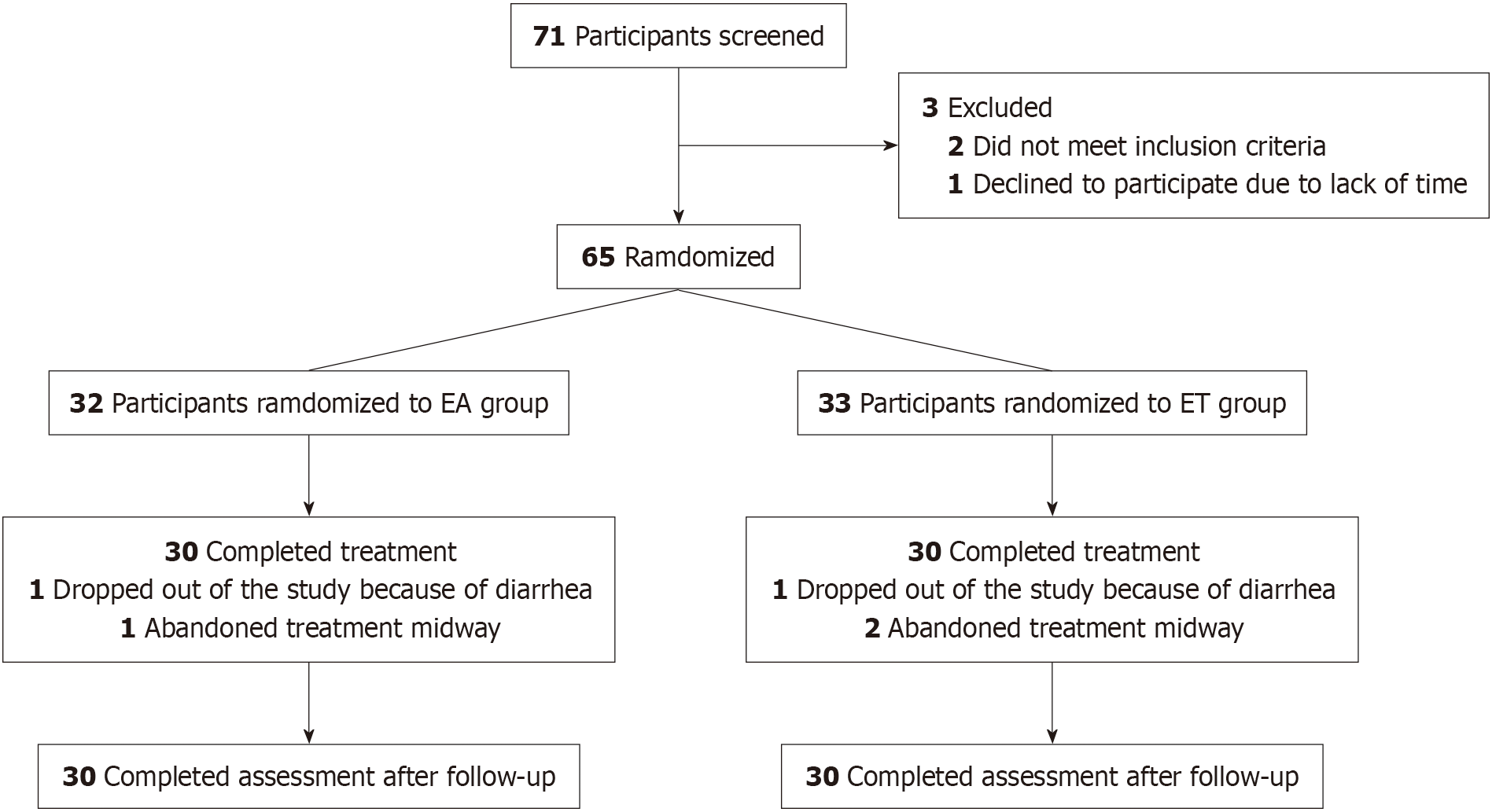
An initial assessment was conducted on 71 patients with KOA, 65 of whom were eligible for inclusion in this trial. Of these, 60 (92.3%) completed the trial, including 11 men (18.3%) and 49 women (81.7%). The mean ages of patients in the EA and ET groups were 64.25 ± 10.45 years and 63.22 ± 5.85 years, respectively. A total of 5 patients (7.7%) withdrew from the study for various reasons: In the EA group, 1 due to diarrhea and 1 discontinued treatment, while in the ET group, 1 withdrew due to diarrhea and 1 discontinued treatment. Details of the demographics of the included patients are presented in Table 1, and the numbers and reasons for withdrawal are depicted in the flowchart in Figure 1.
| Characteristic | EA group (n = 30) | ET group (n = 30) | P value |
| Age (years), mean ± SD | 64.25 ± 10.45 | 63.22 ± 5.85 | 0.794 |
| Women | 24 (88.89) | 25 (89.29) | 0.763 |
| BMI (kg/m2), mean ± SD | 23.94 ± 3.69 | 24.41 ± 2.02 | 0.733 |
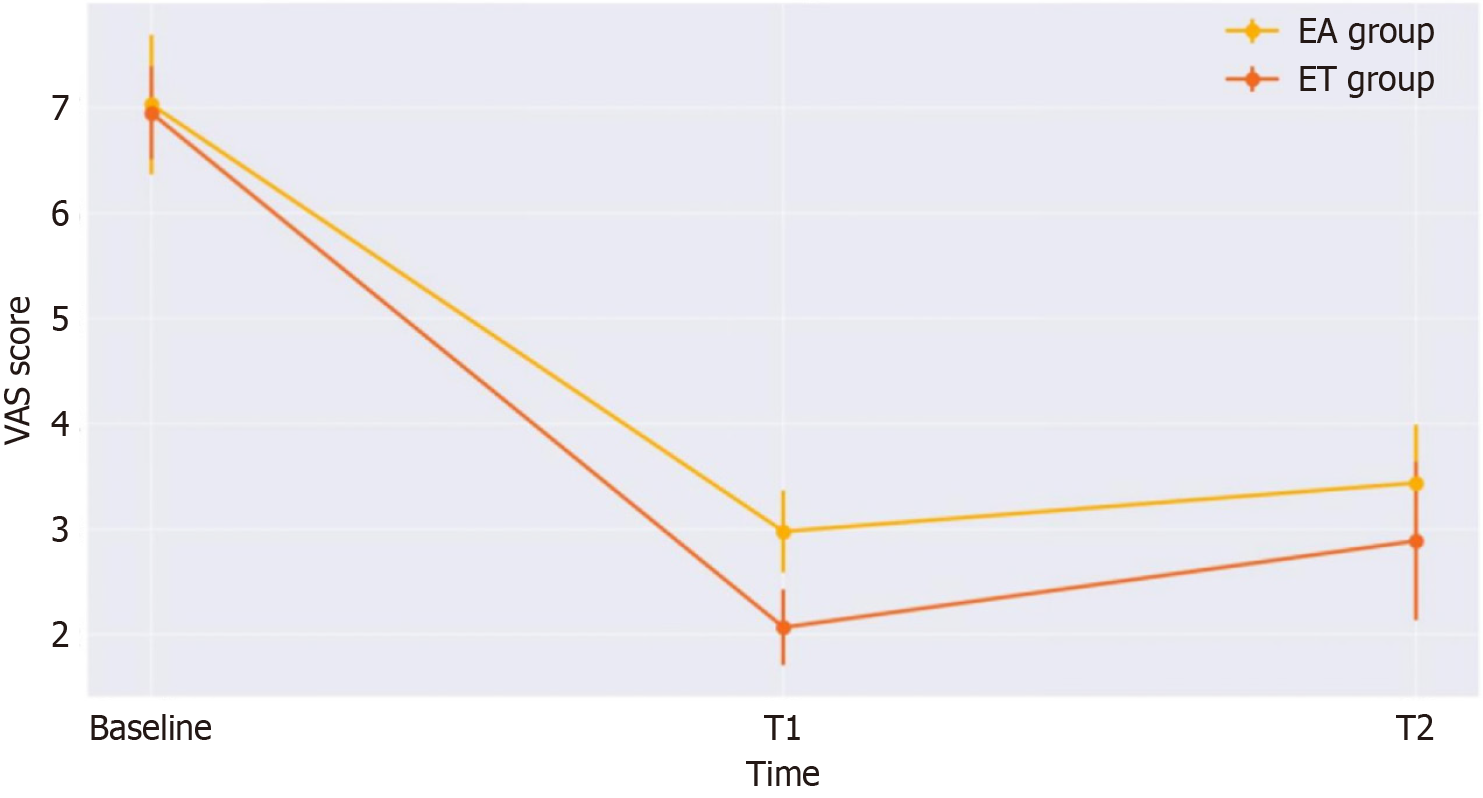
The results of the comparison of VAS scores between the EA and ET groups are shown in Table 2 and Figure 2. Initially, the VAS scores of the two groups were nearly identical. Comparisons between the groups showed that post-treatment VAS scores were significantly lower in the ET group than in the EA group (P < 0.001). Furthermore, at follow-up, the VAS scores of the ET group remained significantly lower than those of the EA group (P < 0.001).
| Outcome | EA group (n = 30) | ET group (n = 30) | P value |
| VAS | |||
| Baseline | 7.03 ± 0.66 | 6.95 ± 0.44 | 0.561 |
| T1 | 2.98 ± 0.39 | 2.07 ± 0.36 | < 0.001 |
| T2 | 3.44 ± 0.55 | 2.89 ± 0.75 | < 0.001 |
| WOMAC total scores | |||
| Baseline | 82.47 ± 6.40 | 81.00 ± 4.68 | 0.262 |
| T1 | 48.07 ± 8.22 | 38.4 ± 6.43 | < 0.001 |
| WOMAC pain | |||
| Baseline | 17.16 ± 2.16 | 16.8 ± 2.37 | 0.507 |
| T1 | 10.10 ± 2.09 | 9.01 ± 3.02 | 0.191 |
| WOMAC stiffness | |||
| Baseline | 6.50 ± 1.02 | 6.03 ± 0.91 | 0.132 |
| T1 | 4.13 ± 1.06 | 2.77 ± 0.80 | 0.015 |
| WOMAC function | |||
| Baseline | 58.80 ± 4.00 | 58.03 ± 3.27 | 0.555 |
| T1 | 33.80 ± 6.58 | 27.50 ± 4.85 | < 0.001 |
WOMAC scores: The WOMAC scores are shown in Table 2. In the two groups, the WOMAC total, pain, stiffness, and function scores were nearly identical (P > 0.05) at baseline. In comparisons between the groups at T1, the WOMAC total score (P < 0.001), WOMAC pain score (P = 0.191), WOMAC stiffness score (P = 0.015), and WOMAC function score (P < 0.001) were significantly lower in the ET group than in the EA group.
Treatment efficacy: The treatment outcomes for the two groups are detailed in Table 3. Of the 30 patients in the EA group, the treatment was markedly effective in 3 patients, effective in 25, and ineffective in 2, resulting in an effective rate of 93.33%. In the ET group, treatment was markedly effective in 23 patients, effective in 6, and ineffective in 1, resulting in an effective rate of 96.67%. The effective rate was significantly higher in the ET group (76.67%) than in the EA group (10.00%).
| Groups | Cured | Markedly effective | Effective | Ineffective | Effective rate |
| EA group (n = 30) | 0 | 3 | 25 | 2 | 93.33% |
| ET group (n = 30) | 0 | 23 | 6 | 1 | 96.67% |
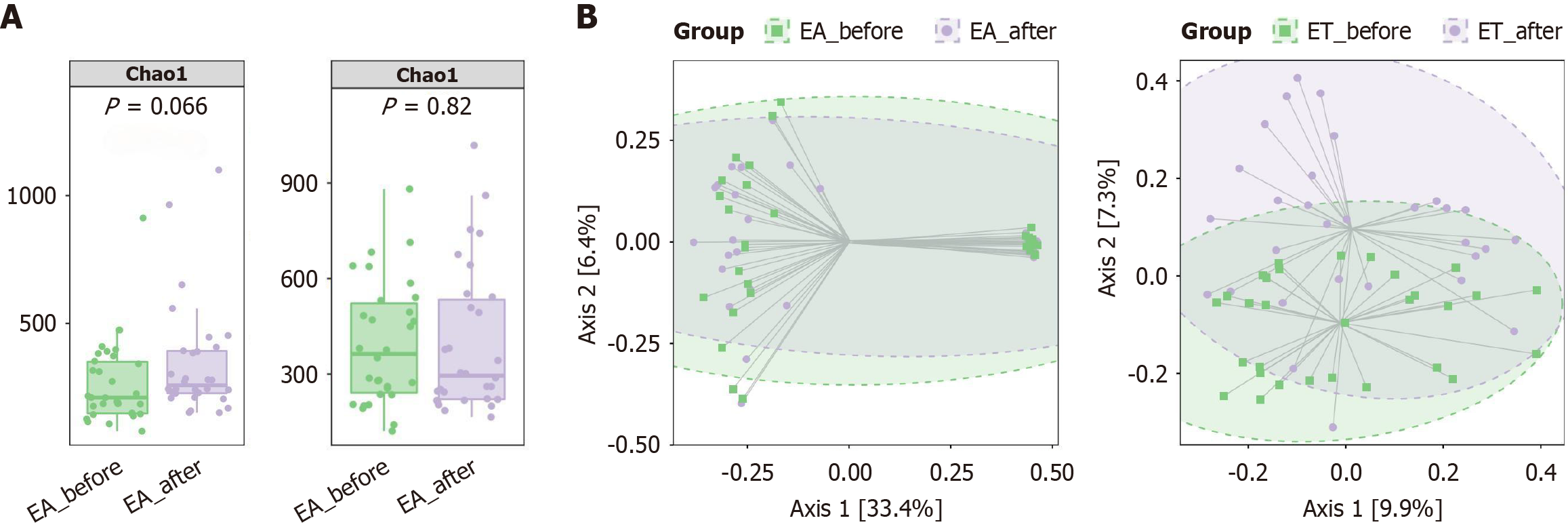
There were no significant differences (P > 0.05) in the Chao1 richness of the gut microbiota, as measured by the number of observed OTUs, between the EA-before and EA-after groups, and no significant differences (P > 0.05) between the ET-before and ET-after groups (Figure 3A). Based on the Bray-Curtis-based principal coordinated analyses, beta diversity between the ET-before and ET-after groups showed a clear pattern of independent grouping (P = 0.002). There was no significant difference in beta diversity between the EA-before and EA-after groups (Figure 3B).
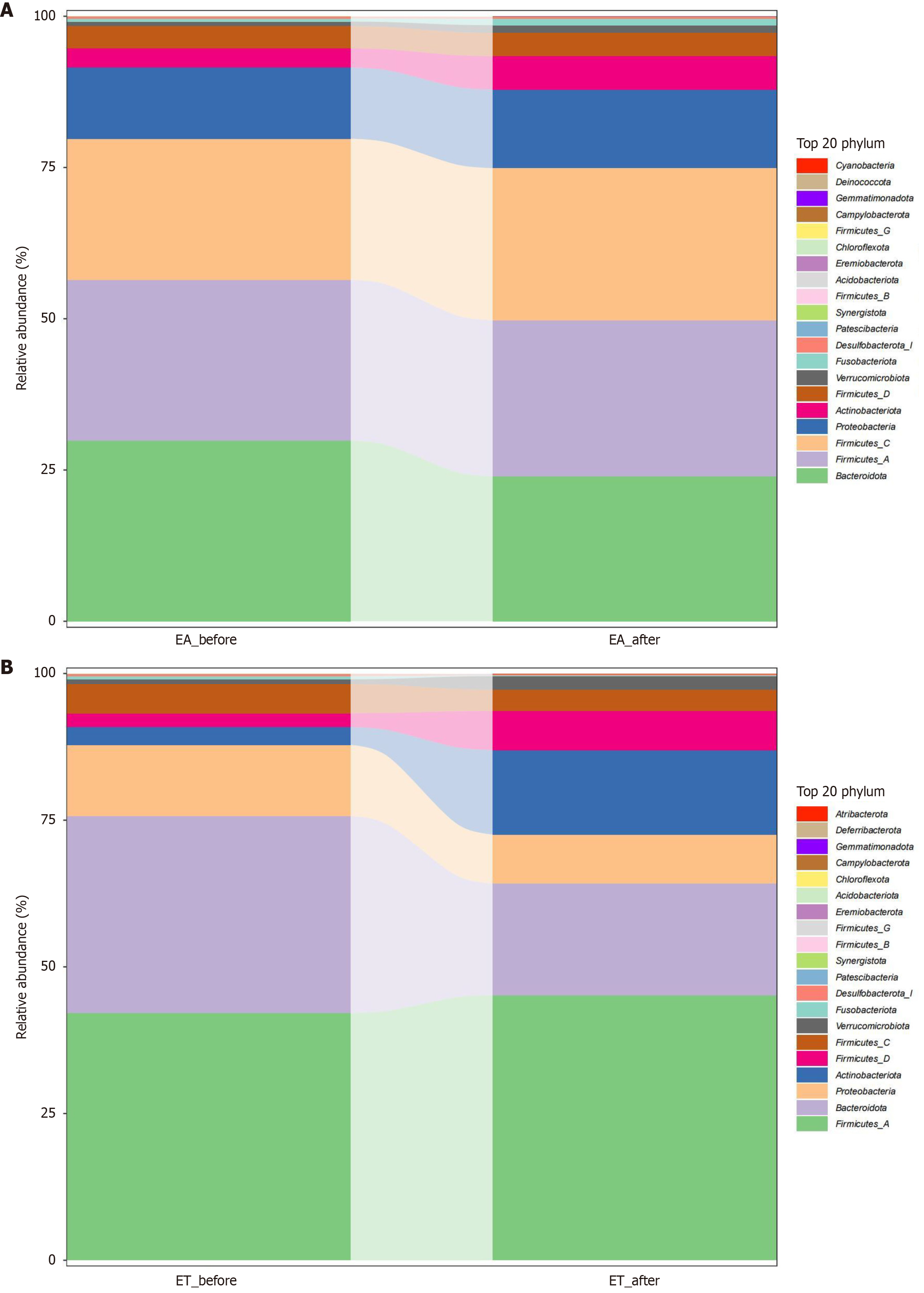
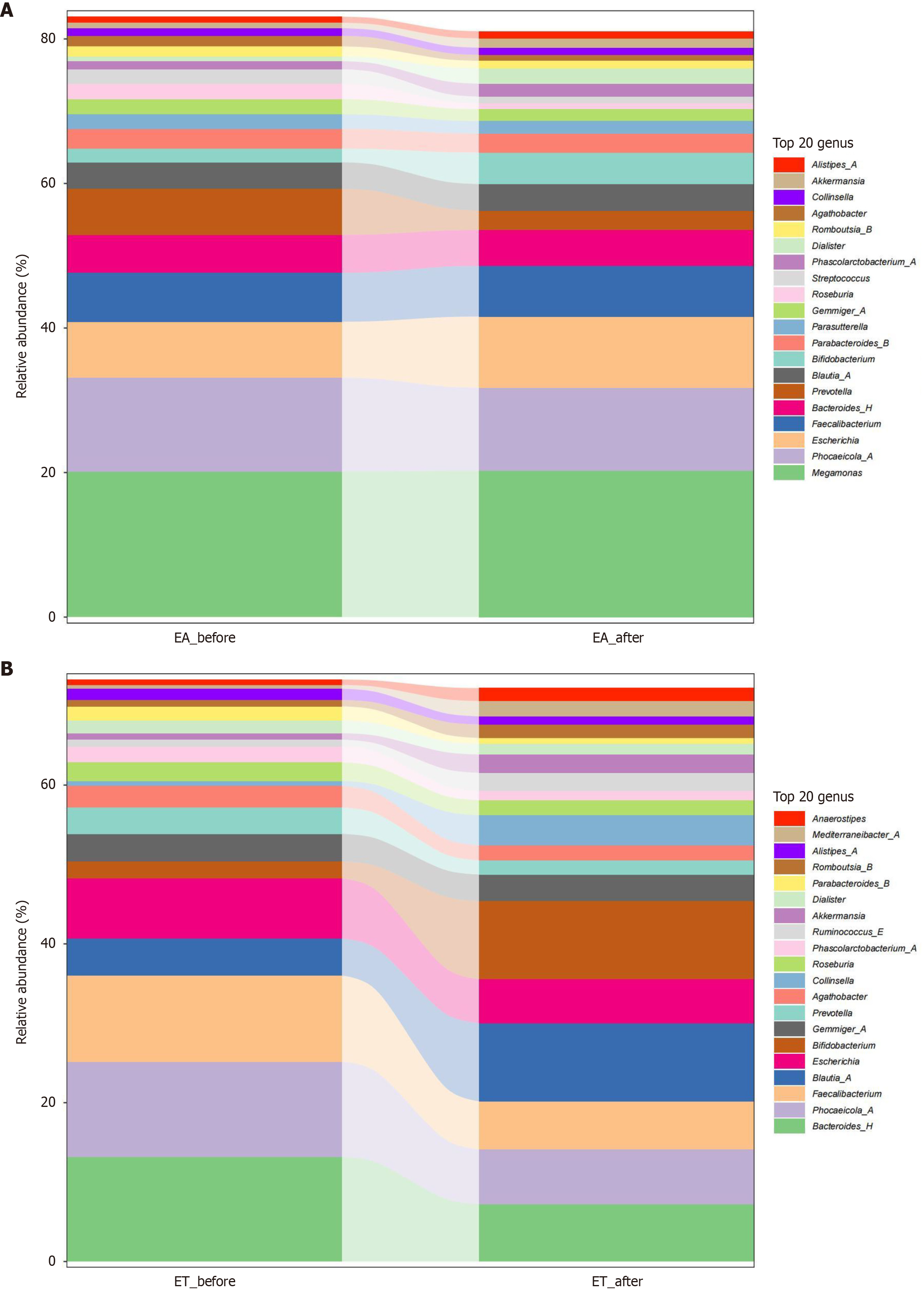
Differential analysis of phylum levels: At the phylum level, the 20 most abundant phyla in the EA group before and after treatment were Actinobacteria, Proteobacteria, FirmicutesC, FirmicutesA, and Bacteroidetes (Figure 4A). The top 20 relative abundances in the ET before and after treatment were Firmicutes D, Actinobacteria, Proteobacteria, Bacteroidetes, and FirmicutesA (Figure 4B).
Differential analysis of genus levels: At the genus level, the top 20 relative abundances in the EA-before and EA-after treatment groups included Bacteroides-H, Faecalibacterium, Escherichia, Phocaeicola-A, and Megamonas, (Figure 5A). The top 20 relative abundances in the ET-before and EA-after treatment included Escherichia, Blautia-A, Faecalibacterium, Phocaeicola-A, and Bacteroides-H (Figure 5B).
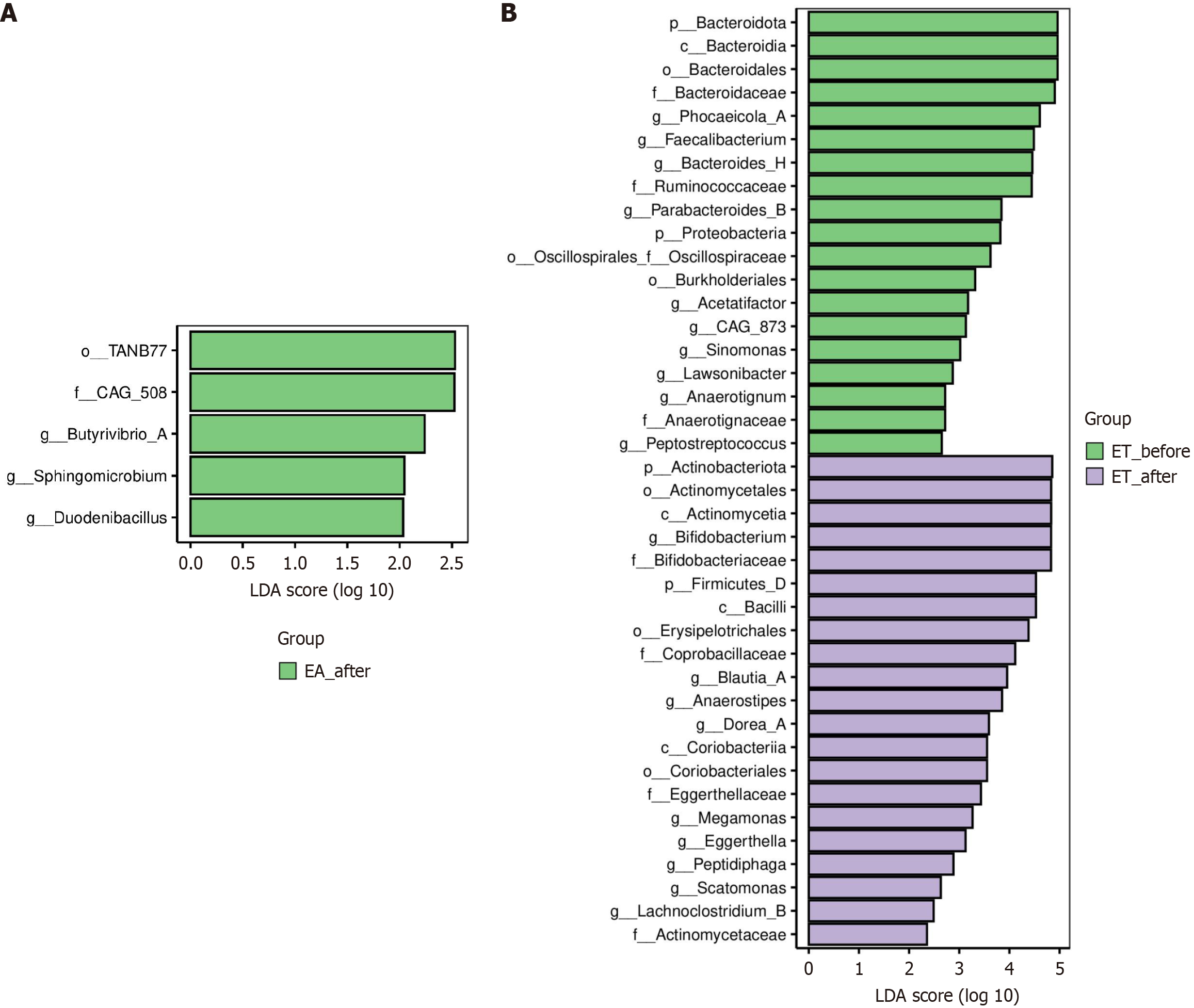
LefSe analysis was used to analyze marker species that were significantly different before and after the different treat
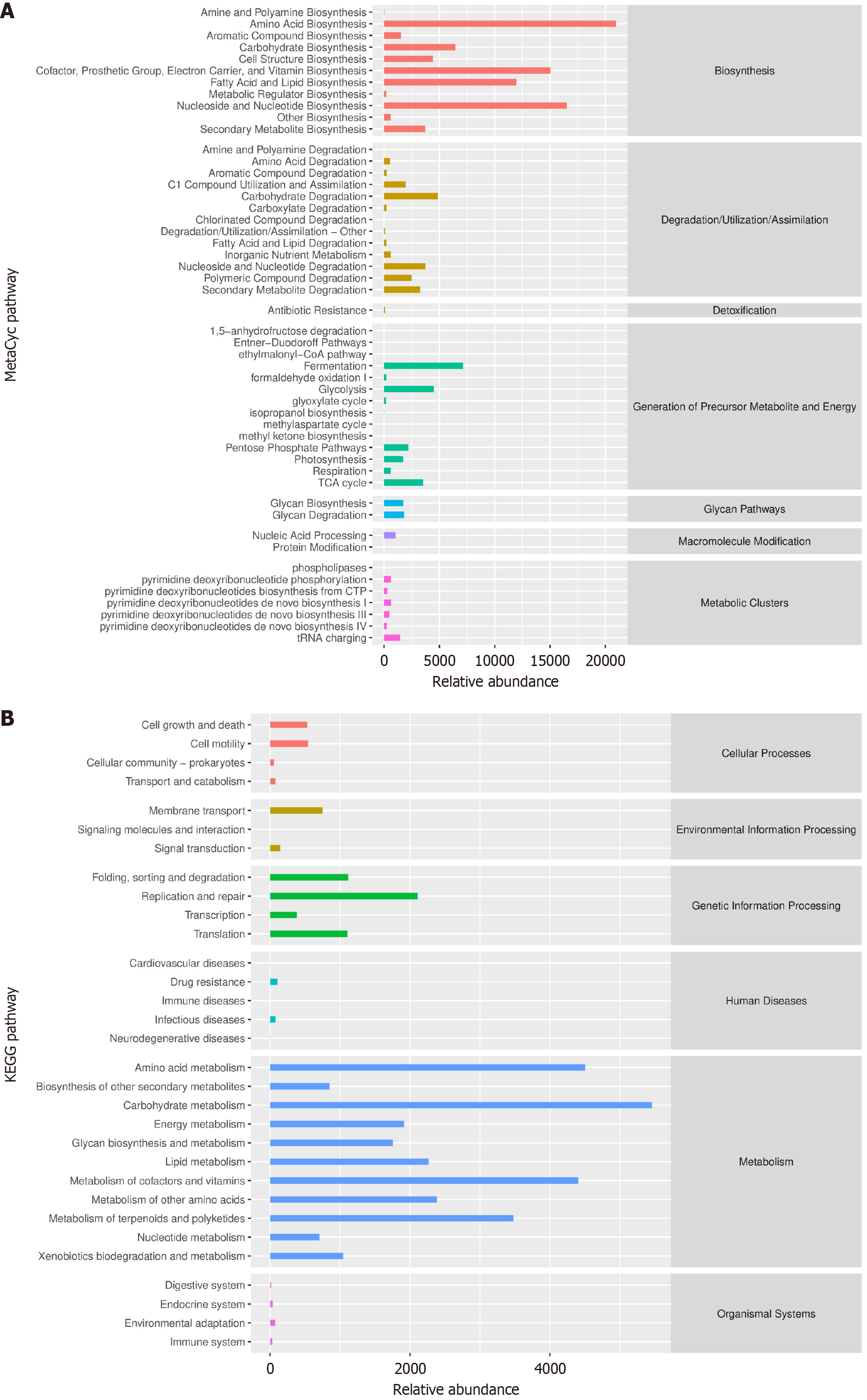
Enrichment analysis was performed on the marker species using the MetaCyc (Figure 7A) and Kyoto Encyclopedia of Genes and Genomes (Figure 7B) pathways to identify the metabolic pathways potentially highly correlated with these markers.
This study evaluated the effects of ET as a complementary therapy for KOA compared to those of EA, focusing on outcomes related to pain, stiffness, function, and microbiome composition. After 4 weeks of treatment, ET demonstrated superior pain reduction, improved joint stiffness, and restored function compared with EA in patients with KOA, with a markedly effective rate of 76.67% in the ET group and 10.00% in the EA group.
To further explore microbiome changes, we conducted 16S rRNA sequencing of samples collected before and after EA and ET treatments. Alpha diversity, which measures the variety of microbial species within individual samples, showed no significant changes after either EA or ET, which may reflect a greater variation in microbial diversity between individuals than between treatment groups. With advancements in microbiome analysis methods, these differences may become more detectable. Beta diversity analysis was used to observe differences in the microbiota structure between the different groups. Natural decomposition of the microbiota structure was performed using PCoA, and variability among samples was observed by locating, analyzing, and categorizing the samples. We found that ET modified the microbiome composition, whereas EA alone did not.
Recent studies have shown that increased metabolites of harmful bacteria in the gut can exacerbate the progression of KOA[18], and that some probiotics such as Bifidobacterium can control the progression of KOA by lowering the level of inflammation in the body[19]. These taxa are increasingly recognized for their capacity to enhance intestinal barrier integrity and suppress systemic inflammation via production of short-chain fatty acids and inhibition of Toll-like receptor signaling[20]. The human gut microbiome comprises four main bacterial phyla: Bacteroidetes, Proteobacteria, Actinobacteria, and Firmicutes. Firmicutes can help in polysaccharide fermentation in the intestines; the genus Bacillus play a key role in maintaining the intestinal microecological balance[21]. In the later stages of KOA, which is often accompanied by osteoporosis, it has been found that Bacteroides is positively associated with osteoporosis[18] and is enriched in mice with intestinal inflammation[22], making Bacteroides a potential causative agent of KOA. Bacteroides fragilis degrades mucin, causing disruption of intestinal mucosal barrier integrity. When the proportion of Bacteroides increases, competitive inhibition in the intestine is weakened and the growth of conditioned pathogens predominates, which can upregulate bacterial toxins and aggravate the release of inflammatory factors[23]. EA is believed to maintain species diversity and balance the number of species in the phyla Thick-walled Bacteria and Ascomycetes[24]. In addition, TN is reported to increase the relative abundance and ratio of the gut microbiota[25]. In our study, the lack of significant changes in alpha diversity may reflect the short treatment duration (4 weeks), which likely influenced specific microbial taxa rather than the overall community richness. Individual variability in baseline microbiota composition and the targeted nature of ET in modulating key functional taxa (e.g., Bifidobacterium and Blautia), rather than broad diversity shifts, may also explain the stability of alpha diversity. This result aligns with reports showing that therapeutic interventions often affect microbial structure (beta diversity) and metabolic pathways before altering alpha diversity. We also found that ET treatment was able to increase the abundance of Firmicutes in the patients with KOA and significantly decreased the abundance of Bacteroidota, thus improving the Firmicutes/Bacteroidota ratio. This suggests that the therapeutic effects of ET may be related to increasing the intestinal barrier to protect functional bacteria, decreasing the release of intestinal endotoxins, and reducing inflammation.
Proteobacteria were found to be closely associated with osteoarthritis as a potential biomarker, and after different treatments, the abundance of Proteobacteria was reduced, and osteoarthritis symptoms were significantly reduced[26-28]. When the intestinal barrier is compromised, an increase in the abundance of Proteobacteria and inflammatory factors introduced into the bloodstream increases the level of inflammatory factors in the body and aggravates osteoarthritis[27-29]. We found that the abundance of Proteobacteria was significantly reduced in patients with KOA following ET treatment.
At the genus level, Blautia, part of the “functional core group of the gut microbiota,” plays a protective role by protecting the intestinal mucosal barrier, reducing inflammation levels, and improving gastrointestinal motility[30]. Bifidobacterium is an important intestinal probiotic that combats pathogens by producing bacteriocins and organic acids[28]. Quantitative microbial profiling revealed a pronounced depletion of Proteobacteria, with taxonomic analysis confirming this phylum-level diminution.
The gut microbiota demonstrates biosynthetic capacity through generation of diverse bioactive compounds, ranging from enzymatic proteins to short-chain fatty acids and immunomodulatory metabolites. When the composition of the gut microbiota is dysregulated, pathogenic bacteria increase in the intestines and continuously release LPS into the blood circulation, inducing inflammation[31]. LPS were detected in the joint fluids of patients with KOA, demonstrating the involvement of these endotoxins in the pathogenesis of KOA. Notably, synovial macrophage activation intensity and osteophyte progression severity in KOA demonstrated significant positive associations with circulatory LPS/lipopolysaccharide-binding protein concentrations in both systemic circulation and synovial compartments[32]. Of note, a microbial DNA signature was found in human and mouse cartilage, and changes in this signature were associated with the progression of human KOA[33]. The experimental data demonstrated that the gut microbiota mechanistically contributes to KOA pathogenesis through immune cell-mediated transport pathways, encompassing both direct cellular trafficking and indirect signaling cascades.
This study on the efficacy of combining EA with TN therapy for KOA suggests potential benefits related to gut microbiota modulation. However, three primary limitations were identified in the current study: (1) Absence of comparative controls; (2) Restricted cohort dimensions, and (3) Compressed temporal scope of experimental observation. These factors limit the ability to fully assess the long-term effects and generalize the findings. Future studies should incorporate a control group, extend the duration of follow-up, and increase the sample size. This approach provides a baseline comparison with no treatment, enables the assessment of long-term therapeutic effects, and reduces the possibility of type II errors.
Our findings suggest that ET alleviates pain and improves joint flexibility/function in patients with KOA. Mechanistically, ET may mitigate inflammatory responses through gut microbiota modulation, as evidenced by the reduction in pathogenic bacteria (e.g., Proteobacteria) and increased abundance of beneficial taxa (e.g., Bifidobacterium). However, while our clinical data associate microbiota shifts with symptom improvement, causality remains unproven. Future studies should confirm whether ET-driven microbial changes directly mediate therapeutic effects in KOA.
We extend our sincere thanks to the patients and doctors who participated in and supported our study.
| 1. | Katz JN, Arant KR, Loeser RF. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA. 2021;325:568-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 1214] [Article Influence: 303.5] [Reference Citation Analysis (0)] |
| 2. | Steinberg J, Southam L, Roumeliotis TI, Clark MJ, Jayasuriya RL, Swift D, Shah KM, Butterfield NC, Brooks RA, McCaskie AW, Bassett JHD, Williams GR, Choudhary JS, Wilkinson JM, Zeggini E. A molecular quantitative trait locus map for osteoarthritis. Nat Commun. 2021;12:1309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 3. | Sharma L. Osteoarthritis of the Knee. N Engl J Med. 2021;384:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 583] [Article Influence: 145.8] [Reference Citation Analysis (0)] |
| 4. | Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 657] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 5. | Liang Z, Zhou J, Quan R, Gao S, Li RH, Li SW, Mi BH, Zhang YQ, Lin N, Chen WH. [Clinical study of Osteoking combined with non-steroidal anti-inflammatory drugs in treatment of knee osteoarthritis]. Zhongguo Shiyan Fangjixue Zazhi. 2023;29:80-86. [DOI] [Full Text] |
| 6. | Graziani C, Talocco C, De Sire R, Petito V, Lopetuso LR, Gervasoni J, Persichilli S, Franceschi F, Ojetti V, Gasbarrini A, Scaldaferri F. Intestinal permeability in physiological and pathological conditions: major determinants and assessment modalities. Eur Rev Med Pharmacol Sci. 2019;23:795-810. [PubMed] [DOI] [Full Text] |
| 7. | Zhang YT, Sun YM, Zhang JH, Huo Y, Li X, Li WB, Zhao AP, Wang R. [Interaction between gut microbiota and drugs]. Zhongguo Yaolixue Tongbao. 2020;36:1650-1655. [DOI] [Full Text] |
| 8. | Brandtzaeg P. Review article: Homing of mucosal immune cells--a possible connection between intestinal and articular inflammation. Aliment Pharmacol Ther. 1997;11 Suppl 3:24-37; discussion 37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Jiang S, Shen B. [Research progress on the relationship between gut microbiota dysbiosis and osteoarthritis]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2023;37:371-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Zhu R, Yuan PW, Dong B, Zheng J. [Overview of acupuncture treatment for knee osteoarthritis in recent five years]. Zhongguo Yiyao Daobao. 2021;18:35-38. [DOI] [Full Text] |
| 11. | Zhang YM, Liu JC, Tan Q, Xiong Y, Yang HQ, Yang ZY, Cheng QP. [Research progress on traditional Chinese medicine treatments and nursing care for knee osteoarthritis]. Zhongguo Zhongyiyao Xiandai Yuancheng Jiaoyu. 2023;21:192-194. [DOI] [Full Text] |
| 12. | Chen J, Liu A, Zhou Q, Yu W, Guo T, Jia Y, Yang K, Niu P, Feng H. Acupuncture for the Treatment of Knee Osteoarthritis: An Overview of Systematic Reviews. Int J Gen Med. 2021;14:8481-8494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Yang BQ. [Finite element analysis of knee joint stress of patients with kneearthralgia treated by adjusting balance manipulation]. MSc Thesis, Gansu University of Chinese Medicine. 2023. [DOI] [Full Text] |
| 14. | Xu H, Xiao LB, Kang BX, Xu XR, Zhong S, Qiu GW, Shi Q. [Clinical efficacy and safety of Chinese Tuina in treating knee osteoarthritis: Meta-analysis]. Shaanxi Zhongyi. 2019;40:1807-1813. [DOI] [Full Text] |
| 15. | Longo UG, Lalli A, Bandini B, de Sire R, Angeletti S, Lustig S, Ammendolia A, Budhiparama NC, de Sire A. Role of the Gut Microbiota in Osteoarthritis, Rheumatoid Arthritis, and Spondylarthritis: An Update on the Gut-Joint Axis. Int J Mol Sci. 2024;25:3242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 16. | Boer CG, Radjabzadeh D, Medina-Gomez C, Garmaeva S, Schiphof D, Arp P, Koet T, Kurilshikov A, Fu J, Ikram MA, Bierma-Zeinstra S, Uitterlinden AG, Kraaij R, Zhernakova A, van Meurs JBJ. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat Commun. 2019;10:4881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 17. | Chen L, Huang Z, Li Q, Chen C, Luo Y, Kang P. Activated intestinal microbiome-associated tryptophan metabolism upregulates aryl hydrocarbon receptor to promote osteoarthritis in a rat model. Int Immunopharmacol. 2023;118:110020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 18. | Cho KH, Na HS, Jhun J, Woo JS, Lee AR, Lee SY, Lee JS, Um IG, Kim SJ, Park SH, Cho ML. Lactobacillus (LA-1) and butyrate inhibit osteoarthritis by controlling autophagy and inflammatory cell death of chondrocytes. Front Immunol. 2022;13:930511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 19. | Porter NT, Martens EC. The Critical Roles of Polysaccharides in Gut Microbial Ecology and Physiology. Annu Rev Microbiol. 2017;71:349-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 171] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 20. | Quinn-Bohmann N, Wilmanski T, Sarmiento KR, Levy L, Lampe JW, Gurry T, Rappaport N, Ostrem EM, Venturelli OS, Diener C, Gibbons SM. Microbial community-scale metabolic modelling predicts personalized short-chain fatty acid production profiles in the human gut. Nat Microbiol. 2024;9:1700-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Reference Citation Analysis (0)] |
| 21. | Ling CW, Miao Z, Xiao ML, Zhou H, Jiang Z, Fu Y, Xiong F, Zuo LS, Liu YP, Wu YY, Jing LP, Dong HL, Chen GD, Ding D, Wang C, Zeng FF, Zhu HL, He Y, Zheng JS, Chen YM. The Association of Gut Microbiota With Osteoporosis Is Mediated by Amino Acid Metabolism: Multiomics in a Large Cohort. J Clin Endocrinol Metab. 2021;106:e3852-e3864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 22. | Li X, Peng X, Qiao B, Peng M, Deng N, Yu R, Tan Z. Gut-Kidney Impairment Process of Adenine Combined with Folium sennae-Induced Diarrhea: Association with Interactions between Lactobacillus intestinalis, Bacteroides acidifaciens and Acetic Acid, Inflammation, and Kidney Function. Cells. 2022;11:3261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Stojanov S, Berlec A, Štrukelj B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms. 2020;8:1715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 1052] [Article Influence: 210.4] [Reference Citation Analysis (0)] |
| 24. | Liu GH, Zhuo XC, Huang YH, Liu HM, Wu RC, Kuo CJ, Chen NH, Chuang LP, Lin SW, Chen YL, Yang HY, Lee TY. Alterations in Gut Microbiota and Upregulations of VPAC2 and Intestinal Tight Junctions Correlate with Anti-Inflammatory Effects of Electroacupuncture in Colitis Mice with Sleep Fragmentation. Biology (Basel). 2022;11:962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Qin YH, Wu YC, Lin FC, Yang XC, Hao F. [Clinical Study on the Therapy of Rubbing Abdomen and Pinching along the Spine for Cervical Spondylosis of Vertebral Artery Type]. Xinzhongyi. 2019;51:217-220. [DOI] [Full Text] |
| 26. | Wang L, Wang Y, Zhang P, Song C, Pan F, Li G, Peng L, Yang Y, Wei Z, Huang F. Gut microbiota changes in patients with spondyloarthritis: A systematic review. Semin Arthritis Rheum. 2022;52:151925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Yilmaz B, Juillerat P, Øyås O, Ramon C, Bravo FD, Franc Y, Fournier N, Michetti P, Mueller C, Geuking M, Pittet VEH, Maillard MH, Rogler G; Swiss IBD Cohort Investigators, Wiest R, Stelling J, Macpherson AJ. Publisher Correction: Microbial network disturbances in relapsing refractory Crohn's disease. Nat Med. 2019;25:701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Xu T, Yang D, Liu K, Gao Q, Liu Z, Li G. Miya Improves Osteoarthritis Characteristics via the Gut-Muscle-Joint Axis According to Multi-Omics Analyses. Front Pharmacol. 2022;13:816891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 29. | Zhong W, Wu K, Long Z, Zhou X, Zhong C, Wang S, Lai H, Guo Y, Lv D, Lu J, Mao X. Gut dysbiosis promotes prostate cancer progression and docetaxel resistance via activating NF-κB-IL6-STAT3 axis. Microbiome. 2022;10:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 107] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 30. | Zhang J, Guo Z, Xue Z, Sun Z, Zhang M, Wang L, Wang G, Wang F, Xu J, Cao H, Xu H, Lv Q, Zhong Z, Chen Y, Qimuge S, Menghe B, Zheng Y, Zhao L, Chen W, Zhang H. A phylo-functional core of gut microbiota in healthy young Chinese cohorts across lifestyles, geography and ethnicities. ISME J. 2015;9:1979-1990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 338] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 31. | Li Y, Si H, Ma Y, Li S, Gao L, Liu K, Liu X. Vitamin D3 affects the gut microbiota in an LPS-stimulated systemic inflammation mouse model. Microbes Infect. 2023;25:105180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med. 2024;19:275-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 332] [Article Influence: 332.0] [Reference Citation Analysis (0)] |
| 33. | Dunn CM, Velasco C, Rivas A, Andrews M, Garman C, Jacob PB, Jeffries MA. Identification of Cartilage Microbial DNA Signatures and Associations With Knee and Hip Osteoarthritis. Arthritis Rheumatol. 2020;72:1111-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |