Published online May 14, 2025. doi: 10.3748/wjg.v31.i18.104206
Revised: March 29, 2025
Accepted: April 23, 2025
Published online: May 14, 2025
Processing time: 143 Days and 21.9 Hours
Diagnosis of inflammatory bowel disease and assessment of disease activity are fundamentally reliant on endoscopy. Nonetheless, it is costly and invasive, high
To examine the correlation of biomarkers with endoscopic activity, evaluate their diagnostic significance, and develop models to forecast endo
We performed a retrospective single-center analysis of 365 patients with ulcerative colitis (UC), 319 with Crohn’s disease (CD) and 100 controls at the First Affiliated Hospital of Zhengzhou University from January 2022 to September 2024. The following biomarkers were analyzed: White blood cell, hemoglobin (Hb), platelet (PLT), neutrophil (N), lymphocyte (L), hematocrit (HCT), eosi
Serum N, PLT, GLB, CRP, ESR, CAR, CLR, PLR, PAR, and NLR levels were significantly elevated (P < 0.001 or P < 0.05) in the UC and CD groups compared to controls, whereas Hb, HCT, L, ALB, and AGR were reduced (P < 0.001 or P < 0.05). Aside from L and eosinophil, substantial differences were observed between mild and severe activity in UC and CD (P < 0.001 or P < 0.05). UC and CD patients who exhibited an endoscopic response after 14 weeks of treatment had elevated CRP, CAR, and CLR levels at baseline compared to endoscopic nonresponders (P < 0.01 or P < 0.05). The UC nomogram model utilizing ESR, CAR, and PAR, along with the CD nomogram model employing AGR and PAR, demonstrate predictive significance and clinical applicability for assessing endoscopic activity.
White blood cell, Hb, HCT, PLT, N, CRP, ESR, ALB, GLB, AGR, CAR, CLR, PLR, PAR and NLR are significantly correlated with the endoscopic activity of UC and CD. Patients with UC and CD exhibiting elevated CRP, CAR, and CLR levels are more inclined to respond to treatment. Our nomogram models can precisely forecast endoscopic activity.
Core Tip: This is a retrospective single-center observational study to examine the correlation of serum biomarkers with endoscopic activity of ulcerative colitis (UC) and Crohn’s disease (CD), evaluate their diagnostic significance, and develop nomogram models to forecast endoscopic activity. Many markers such as white blood cell, hemoglobin, hematocrit, platelet (PLT), neutrophil, C-reactive protein (CRP), erythrocyte sedimentation rate, albumin (ALB), globulin, ALB/globulin, CRP/ALB, CRP/lymphocyte, PLT/lymphocyte, PLT/ALB, and neutrophil/lymphocyte are associated with endoscopic activity in patients with UC and CD. We can predict endoscopic severity of UC and CD by using the nomogram models.
- Citation: Liu X, Pan LX, Pei JX, Pu T, Wen HT, Zhao Y. Role of serological biomarkers in evaluating and predicting endoscopic activity in inflammatory bowel disease. World J Gastroenterol 2025; 31(18): 104206
- URL: https://www.wjgnet.com/1007-9327/full/v31/i18/104206.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i18.104206
Patients with inflammatory bowel disease (IBD) generally undergo alternating phases of remission and active disease, rendering the evaluation of disease activity essential for effective treatment[1]. Endoscopy is the definitive method for assessing disease activity in IBD. However, its invasive characteristics and substantial expense restrict its practicality for regular monitoring. Thus, there is an increasing demand for more accessible and economical indicators to evaluate disease activity[2]. Serological indices, commonly utilized in clinical practice, are readily accessible and may reveal abnormalities closely linked to disease activity and prognosis in patients with IBD[3]. This study systematically evaluated various potential serum biomarkers to determine the most clinically significant serological indicators for assessing endoscopic activity in IBD and their implications for treatment and prognosis. Additionally, predictive models were created to assess the efficacy of serum markers in forecasting endoscopic activity in patients with IBD.
We enrolled 365 patients with ulcerative colitis (UC), 319 patients with Crohn’s disease (CD), and 100 control patients at the First Affiliated Hospital of Zhengzhou University from January 2022 to September 2024. We gathered patients with gastrointestinal polyps to serve as controls. Demographic characteristics, clinical information, laboratory findings, and endoscopic activity were retrospectively extracted from the electronic medical records system. Utilizing gender, age, and body mass index (BMI), we employed propensity score matching to screen 330 UC patients, 270 CD patients, and 85 control subjects. We assessed the variance of several biomarkers [white blood cell (WBC), hemoglobin (Hb), platelet (PLT), neutrophil (N), lymphocyte (L), eosinophil (EOS), hematocrit (HCT), albumin (ALB), globulin (GLB), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), ALB/GLB (AGR), CRP/ALB (CAR), CRP/L (CLR), PLT/L (PLR), PLT/ALB (PAR), N/L (NLR)] in the peripheral blood of individuals with UC, CD, and healthy controls.
With the initial cohort of 365 UC and 319 CD patients, we constructed two nomograms to forecast endoscopic activity and assess their precision and clinical applicability. Fifty UC and fifty CD patients were monitored at week 14 post-treatment, after which we assessed the disparity in baseline levels between the endoscopic responders and non-responders.
The Mayo endoscopic subscore (MES) and Simple Endoscopic Score for CD (SES-CD) scores were utilized to assess the endoscopic activity of IBD. Mild endoscopic activity was defined as MES = 1 or SES-CD = 3-6, moderate activity as MES = 2 or SES-CD = 7-15, and severe activity as MES = 3 or SES-CD ≥ 16. Endoscopic responses were characterized by a reduction of MES by at least 1 point or a reduction of SES-CD exceeding 50% at 14 weeks of follow-up.
We performed data analysis and management utilizing IBM SPSS 26.0 software. Continuous variables were represented as mean ± SD or median (interquartile range), whereas discrete variables were displayed as counts and percentages. The normality of continuous variables was evaluated using the Kolmogorov-Smirnov test. We employed the independent t-test for variables that follow a normal distribution. The Mann-Whitney U-test was employed for continuous variables that are not normally distributed. The χ2 test was utilized to compare discrete variables. We employed R 4.2.1 to develop the nomogram prediction model and utilized receiver operating characteristic (ROC) curves, calibration curves, and decision curve analysis (DCA) to assess the model’s differentiation, calibration, and clinical utility. A P value of less than 0.05 was deemed statistically significant.
Following propensity score matching, we evaluated 330 patients with UC, 270 patients with CD, and 85 healthy control subjects, revealing no statistically significant differences in baseline characteristics among the three groups. The MES score indicated that 37.0% of patients had mild UC, 29.7% had moderate UC, and 33.3% had severe UC. The distribution of CD patients classified as mild, moderate, and severe was 50.7%, 40.0%, and 9.3%, respectively, based on the SES-CD score. The patient demographics for UC, CD, and healthy controls are presented in Table 1.
| UC | CD | HCs | P value1 | P value2 | P value3 | |
| Number of subjects, n | 330 | 270 | 85 | - | - | - |
| Age, years, median (IQR) | 48 (33, 59) | 47 (31, 54) | 49 (41, 60) | 0.084 | 0.062 | 0.108 |
| BMI, kg/m2, median (IQR) | 23.84 (19.61, 24.37) | 22.88 (18.40, 25.09) | 24.59 (22.26, 28.73) | 0.132 | 0.075 | 0.144 |
| Gender | ||||||
| Male | 174 (52.7) | 156 (57.8) | 44 (51.8) | 0.480 | 0.327 | 0.129 |
| Female | 156 (47.3) | 114 (42.2) | 41 (48.2) | |||
| Duration of illness, years, median (IQR) | 2 (0.6, 5) | 1 (0.3, 3) | - | |||
| Endoscopic activity | ||||||
| Mild | 122 (37.0) | 137 (50.7) | - | |||
| Moderate | 98 (29.7) | 108 (40.0) | - | |||
| Severe | 110 (33.3) | 25 (9.3) | - |
The comparison of the 17 serum biomarkers among the UC group, CD group, and healthy controls is presented in Supplementary Table 1. The median values of WBC, PLT, N, GLB, CRP, ESR, CLR, CAR, PAR, and PLR were elevated in the UC group compared to healthy controls, whereas Hb, HCT, L, ALB, and AGR were diminished. In the CD group, the median values of PLT, N, GLB, CRP, ESR, CLR, CAR, PAR, and PLR exceeded those of healthy controls (P < 0.001 or P < 0.05), while Hb, HCT, L, ALB, and AGR were diminished. L, EOS, CRP, CLR, and CAR exhibited significant differences in mild to moderate UC and CD.
In comparison to patients with mild UC, Hb, HCT, ALB, AGR were significantly diminished in moderate and severe UC, whereas white WBC, PLT, N, CRP, ESR, GLB, CAR, CLR, PAR, PLR and NLR were significantly elevated, with EOS and L showing no significant difference. However, only PLT, CRP, ESR, ALB, AGR, CAR, CLR, PAR, and NLR exhibited significant differences between moderate and severe endoscopic activity. N, CRP, ESR, ALB, GLB, AGR, CAR, CLR, and PAR exhibited significant differences across all three groups. Furthermore, Hb and HCT exhibited significant variations solely in cases of mild and moderate endoscopic activity. PLT, PLR, and NLR exhibited significant differences solely in cases of moderate and severe endoscopic activity.
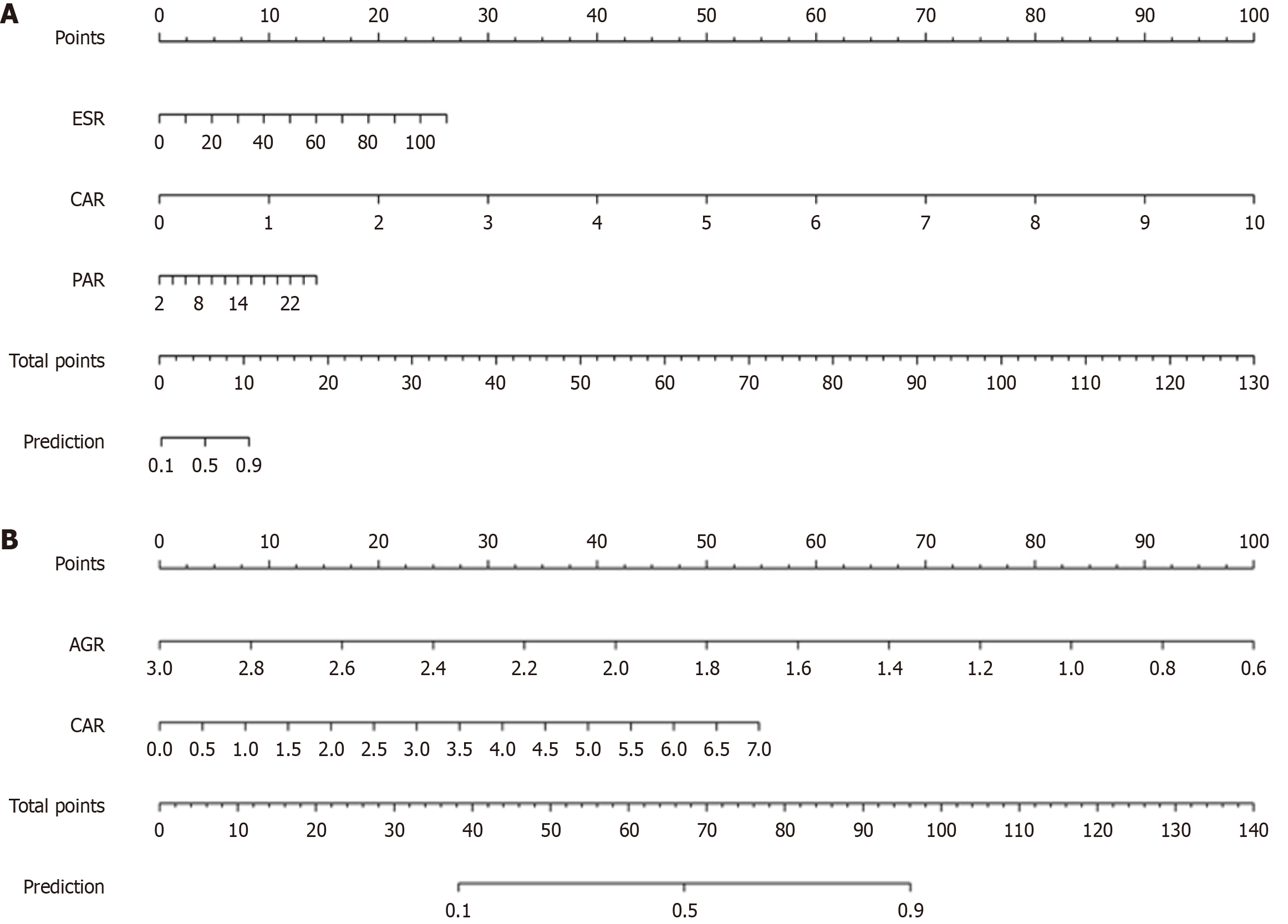
The establishment of the predictive model for UC endoscopic activity: We randomly partitioned 365 UC into a training set (n = 255) and a validation set (n = 110) in a 7:3 ratio. There were no significant differences in age, sex, course of disease, BMI and serum indexes between the training set and the validation set (P > 0.05). The patients were categorized into mild endoscopic activity (MES = 0, 1) and moderate to severe endoscopic activity (MES = 2, 3). We plotted ROC, calibration, and DCA curves of the training set and internal validation set to evaluate the accuracy and clinical utility of the predictive model. The disparities in WBC, Hb, HCT, ESR, AGR, CAR, CLR, PLR, PAR, and NLR between the two groups were statistically significant. The regression analysis indicated that ESR [odds ratio (OR) = 1.09, P = 0.0015], CAR (OR = 381.71, P = 0.0018), and PAR (OR = 1.27, P = 0.0072) were independent risk factors for endoscopic activity in UC (Table 2). The nomogram model is illustrated in Figure 1A. According to the scores of ESR, CAR and PAR corresponding to the nomogram model, the “prediction” in the sum of scores is the probability that the patient’s endoscopic activity is moderate to severe.
| Standard error | Wald-value | OR (95%CI) | P value | |
| CAR | 1.9005 | 9.7846 | 381.71 (9.21-15827.23) | 0.0018 |
| ESR | 0.0262 | 10.0550 | 1.09 (1.03-1.14) | 0.0015 |
| PAR | 0.0896 | 7.2274 | 1.27 (1.07-1.52) | 0.0072 |
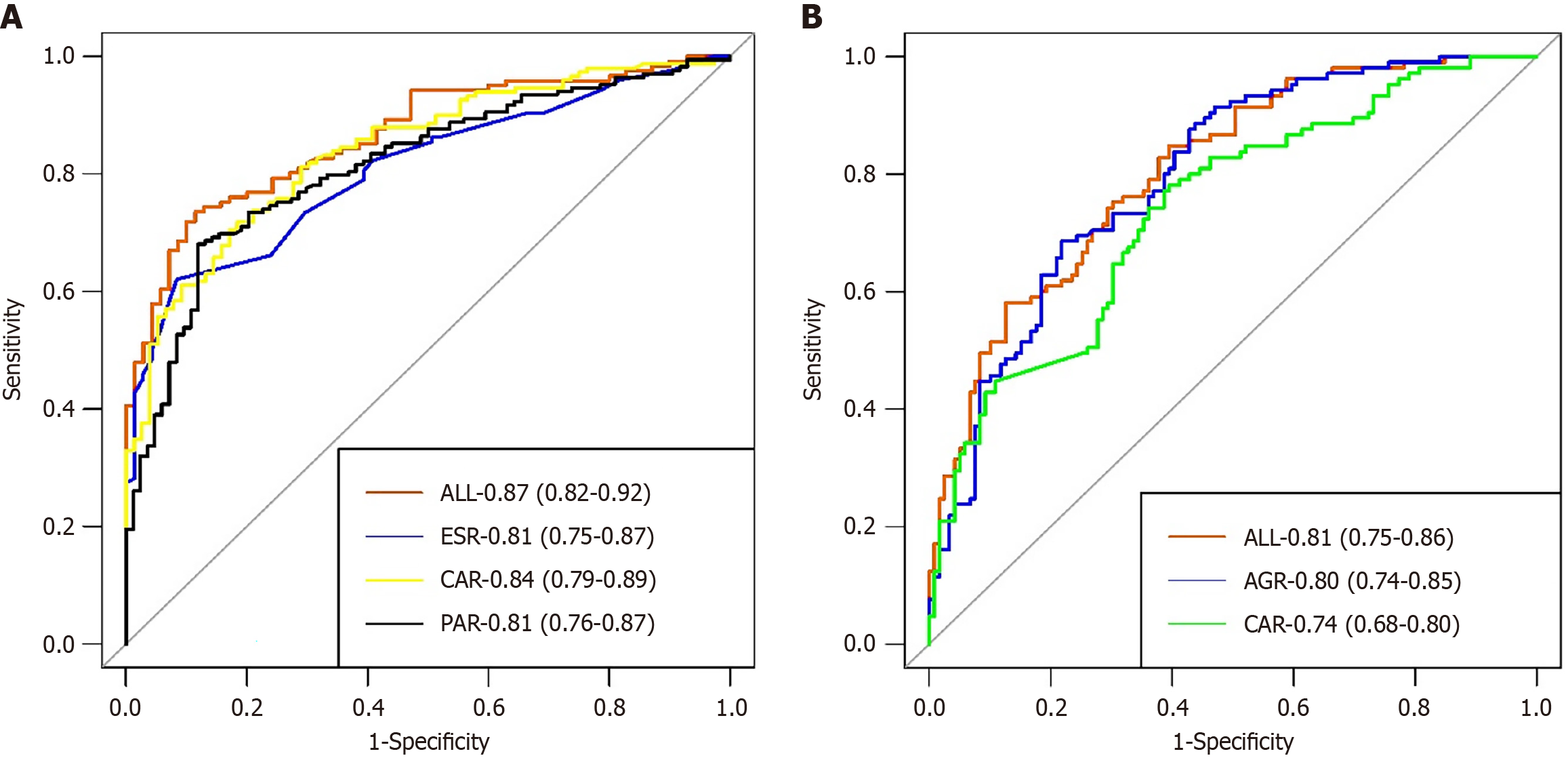
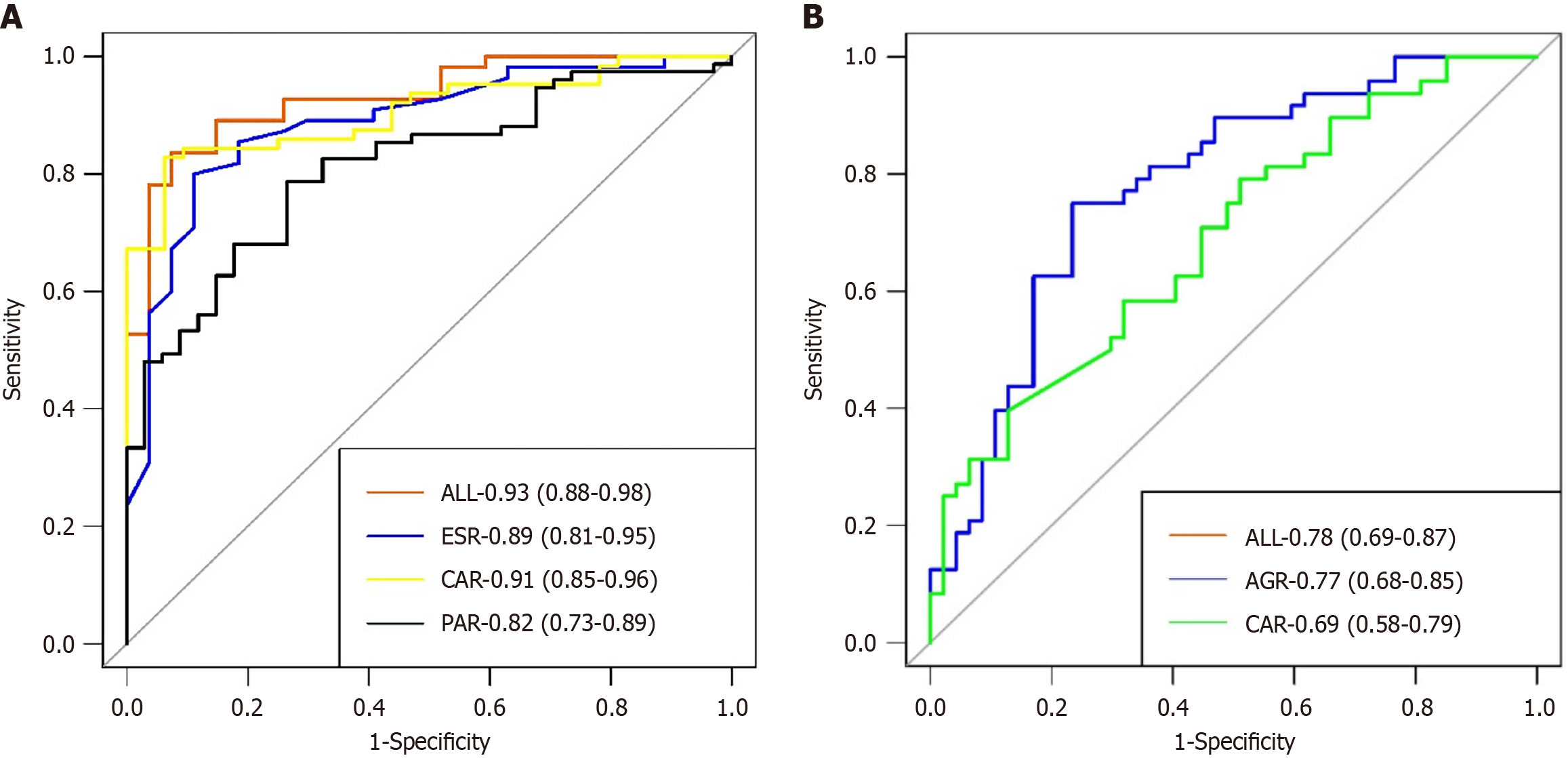
The evaluation and validation of UC predictive models: We employed the ROC curve to assess the discriminatory power of the nomogram model. The nomogram model achieved an area under the curve (AUC) of 0.87 in the training set, with a specificity of 88.6% and a sensitivity of 73.6% (Figure 2A). The AUC of the validation set was 0.93, with a specificity of 92.6% and a sensitivity of 83.6% (Figure 3A). The calibration curve indicated P = 0.938 for the training set and P = 0.554 for the validation set (both P > 0.05), demonstrating strong model consistency (Supplementary Figures 1 and 2). The DCA also indicated that it possesses favorable clinical practicability (Supplementary Figures 3 and 4).
The establishment of the predictive model for CD endoscopic activity: We randomly partitioned 319 CDs into a training set (n = 223) and a validation set (n = 96) in a 7:3 ratio. There were no significant differences in age, sex, course of disease, BMI and serum indexes between the two sets (P > 0.05). The patients were categorized into mild endoscopic activity (SES-CD score ≤ 6) and moderate to severe endoscopic activity (SES-CDs score > 7). We identified AGR (OR = 0.05, P < 0.001) and CAR (OR = 1.72, P = 0.0117) as independent risk factors for CD endoscopic activity (Table 3). The nomogram model is depicted in Figure 1B.
| Standard error | Wald-value | OR (95%CI) | P value | |
| AGR | 0.4894 | 39.6610 | 0.05 (0.02-0.12) | < 0.001 |
| CAR | 0.2161 | 6.3613 | 1.72 (1.13-2.63) | 0.0117 |
The evaluation and validation of CD predictive models: The AUC of the nomogram model in the CD training set was 0.81, with a specificity of 87.4% and a sensitivity of 58.1% (Figure 2B). The validation set exhibited an AUC of 0.78, a specificity of 76.6%, and a sensitivity of 75% (Figure 3B). The calibration curves for the training set (P = 0.869) and the verification set (P = 0.506) indicate that this model demonstrates strong concordance (Supplementary Figures 5 and 6). We additionally employed DCA to assess the clinical feasibility of the model (Supplementary Figures 7 and 8).
Biomarkers predict endoscopic responses to UC patients: Fifty UC patients in the endoscopic activity group underwent colonoscopy at week 14 post-treatment, with 29 demonstrating a response to endoscopy (MES ≥ 1 point reduction) and 21 not responding. No substantial difference in baseline parameters was observed between the endoscopic response group and the endoscopic no-response group (P > 0.05), with the exception of CRP, CAR, and CLR (P < 0.05) (Table 4).
| Endoscopic response group | Endoscopic no-response group | P value | |
| CRP | 12.77 (3.77, 43.39) | 4.47 (1.82, 9.39) | 0.037 |
| CLR | 6.69(2.56, 27.64) | 2.29 (0.63, 4.13) | 0.019 |
| CAR | 0.30(0.13, 0.86) | 0.11 (0.03, 0.27) | 0.031 |
Biomarkers predict endoscopic responses to CD patients: Fifty CD patients underwent colonoscopy 14 weeks post-treatment, of which 34 exhibited an endoscopic response (SES-CD > 50% reduction) and 14 did not respond. The results were analogous to those in the UC group; however, CRP, CAR, and CLR exhibited significant differences between the endoscopic response group and the no-response group prior to treatment (P < 0.05) (Table 5).
| Endoscopic response group | Endoscopic no-response group | P value | |
| CRP | 22.94 (4.20, 41.00) | 14.57 (6.45, 64.81) | 0.003 |
| CAR | 0.67 (0.09, 1.10) | 0.37 (0.16, 1.88) | 0.026 |
| CLR | 20.85 (2.63, 27.04) | 8.56 (4.08, 42.85) | < 0.001 |
Endoscopy is the definitive method for verifying mucosal healing in individuals with IBD[4]. However, its invasive characteristics and inconvenience highlight the need for non-invasive, convenient biomarkers to evaluate endoscopic activity. Utilizing suitable biomarkers can diminish the necessity for colonoscopies, thereby easing the financial and procedural strain on patients while providing significant clinical benefits[5].
This study assessed the efficacy of various biomarkers in evaluating endoscopic activity and created nomograms to predict endoscopic activity in patients with UC and CD. In order to avoid the interference of infection, drugs and other factors on serum biomarkers, patients with co-infection and taking hormones and immunosuppressants in the past 3 months were excluded from the study. Our findings indicated notable disparities in serological markers between IBD patients and the control group, excluding EOS and L. These modifications in IBD patients are intricately associated with intestinal inflammation, hypercoagulability, immune dysregulation, and nutritional vulnerabilities[6]. The incidence of malnutrition in IBD varies between 6.1% and 69.7%[7]. During active disease phases, patients frequently demonstrate diminished ALB levels and varying extents of anemia. Malnutrition induces alterations in the gut microbiome, disrupting homeostasis and provoking microbial imbalances that may intensify inflammation. This inflammatory response may exacerbate nutritional deficiencies, establishing a self-reinforcing cycle of malnutrition and inflammation[8]. PLT counts were elevated in IBD patients, indicative of secondary thrombocytosis, a phenomenon also observed in animal models of IBD[9]. Li and Liu[10] further highlighted the pivotal role of PLTs in IBD progression. The hypercoagulable state in IBD may arise from complex interactions among the coagulation cascade, natural clotting inhibitors, the fibrinolytic system, endothelial cells, immune responses, and PLTs[11].
Fecal calcarein, interleukin-6, tumor necrosis factor (TNF)-α and other indicators also played an important role in the evaluation of IBD. Previous studies have reported that the levels of biomarkers such as fecal calcarein, CRP and PLT are significantly correlated with endoscopic activities, and the correlation between CRP and fecal calcarein and SES-CD is the strongest[12]. TNF-α is significantly elevated in patients with IBD, and its biologic drugs are widely used to treat IBD[13]. As there is no fecal calcarein data in our hospital at present, and the data of interleukin-6 and TNF-α were incomplete these indexes were not included in this study. It is hoped that these important indicators can be included in the future.
Our study indicated no significant difference between UC and CD patients across most metrics, with the exception of EOS, L, and CRP-related measures. CRP is expressed in mesenteric cells of patients with CD, potentially explaining the elevated CRP levels in these individuals[14]. We discovered that these markers were associated with endoscopic severity in IBD, revealing significant differences between mild and severe IBD markers. Intestinal inflammation in active IBD patients may disseminate throughout the body, resulting in elevated levels of WBC, CRP, ESR, GLB, and other inflammatory markers.
Early evaluations of IBD predominantly utilized single biomarkers. However, the complexity of disease progression necessitates the use of composite biomarkers to enhance diagnostic accuracy and disease assessment[15]. Each marker possesses distinct characteristics and limitations, and their combined detection can mitigate each other’s deficiencies and enhance diagnostic accuracy[16]. Liu et al[17] integrated CRP and ALB, discovering that their ratios serve as indicators for evaluating disease activity in IBD. In our study, the six composite indicators, AGR, CAR, CLR, PLR, PAR, and NLR, are highly valuable for identifying and assessing IBD activity.
The nomogram model can amalgamate various predictors and present them as a line segment with a scale, thereby visually illustrating the relationship among the variables in the prediction model[18]. We created two nomogram models to predict endoscopic activity in UC and CD. The predictive model can enhance the treatment of IBD patients. Much of the existing literature relies on artificial intelligence to predict disease activity or prognosis of IBD. Some scholars have studied a number of artificial intelligence models of IBD, and suggested that they can achieve diagnostic performance comparable to that of pathologists[19]. Compared to artificial intelligence, our two prediction models are based on simple serum indicators and are more convenient for clinical application. Our research demonstrated that CAR, PAR, and ESR were independent risk factors for endoscopic activity in UC, while AGR and CAR were independent risk factors for CD. The AUC of the UC and CD columns, derived from these indicators in the internal verification set, were 0.93 and 0.78, respectively, signifying that the prediction models possessed substantial predictive value.
We followed up 100 IBD patients at week 14 after treatment, and alongside the enhancement of endoscopic activity, several serological indices also exhibited improvement. Previous studies have reported the predictive value of biomarkers for IBD treatment. Reinisch et al[20] reported that patients with baseline CRP < 0.7 mg/dL were more likely to be induced to remission by inflizumab. The American Gastroenterological Association guidelines also advocate for the utilization of inflammatory markers to assess IBD activity and reduce the necessity for frequent colonoscopies[21].
Our research examined the importance of 17 indicators for diagnosing IBD and assessing endoscopic activity and created two nomograms for forecasting the endoscopic activity of UC and CD. This can facilitate a more efficient evaluation of disease activity in IBD patients and inform the treatment strategy. This study possesses multiple limitations. Firstly, this is a single-center retrospective study, which may introduce potential selection bias. Secondly, certain indicators, such as fecal calprotectin, interleukin-6 and TNF-α, were excluded from the study. These indicators cannot be detected in some hospitals, so we included biomarkers with clinical universality. Thirdly, the sample size of patients monitored in week 14 post-treatment was limited. Future studies with larger sample sizes must validate this. Fourthly, this study focused on commonly used clinical indicators and did not analyze emerging biomarkers.
In conclusion, serum biomarkers are significantly correlated with the endoscopic activity of UC and CD. CRP, CAR, and CLR levels can indicate the effectiveness of treatment. We can also predict endoscopic severity of UC and CD by using the nomogram models.
First of all, I would like to express my gratitude to those who contributed to this research. My sincere appreciations go first to the distinguished Professor, Ye Zhao, whose encouragement and suggestions have given me much insight into the study. It has been a great privilege to study under her guidance. I am also extremely grateful to my companions who have provided me assistance in the course of preparing this paper. Finally, I am really grateful to all those who devote much time to reading this thesis and give me much advice, which will benefit me in my later study.
| 1. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1402] [Cited by in RCA: 1551] [Article Influence: 258.5] [Reference Citation Analysis (0)] |
| 2. | Liu D, Saikam V, Skrada KA, Merlin D, Iyer SS. Inflammatory bowel disease biomarkers. Med Res Rev. 2022;42:1856-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 3. | Sakurai T, Saruta M. Positioning and Usefulness of Biomarkers in Inflammatory Bowel Disease. Digestion. 2023;104:30-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 4. | Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, Langner C, Limdi JK, Pellino G, Zagórowicz E, Raine T, Harbord M, Rieder F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11:649-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1289] [Article Influence: 161.1] [Reference Citation Analysis (0)] |
| 5. | Elhag DA, Kumar M, Saadaoui M, Akobeng AK, Al-Mudahka F, Elawad M, Al Khodor S. Inflammatory Bowel Disease Treatments and Predictive Biomarkers of Therapeutic Response. Int J Mol Sci. 2022;23:6966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 6. | Guan Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J Immunol Res. 2019;2019:7247238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 624] [Article Influence: 104.0] [Reference Citation Analysis (1)] |
| 7. | Lin A, Micic D. Nutrition Considerations in Inflammatory Bowel Disease. Nutr Clin Pract. 2021;36:298-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Massironi S, Viganò C, Palermo A, Pirola L, Mulinacci G, Allocca M, Peyrin-Biroulet L, Danese S. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2023;8:579-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 127] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 9. | Senchenkova E, Seifert H, Granger DN. Hypercoagulability and Platelet Abnormalities in Inflammatory Bowel Disease. Semin Thromb Hemost. 2015;41:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Li HY, Liu TM. Platelet indices and inflammatory bowel disease: a Mendelian randomization study. Front Immunol. 2024;15:1377915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Cheng K, Faye AS. Venous thromboembolism in inflammatory bowel disease. World J Gastroenterol. 2020;26:1231-1241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 12. | E Penna FGC, Rosa RM, da Cunha PFS, de Souza SCS, de Abreu Ferrari ML. Faecal calprotectin is the biomarker that best distinguishes remission from different degrees of endoscopic activity in Crohn's disease. BMC Gastroenterol. 2020;20:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Billmeier U, Dieterich W, Neurath MF, Atreya R. Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J Gastroenterol. 2016;22:9300-9313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 140] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (6)] |
| 14. | Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, Rousseaux C, Dubuquoy C, Decourcelle C, Saudemont A, Tachon M, Béclin E, Odou MF, Neut C, Colombel JF, Desreumaux P. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn's disease. Gut. 2012;61:78-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 15. | Liu T, Qin Z, Yang Z, Feng X. Predictive Value of MHR and NLR for Ulcerative Colitis Disease Activity. Int J Gen Med. 2024;17:685-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Dragoni G, Innocenti T, Galli A. Biomarkers of Inflammation in Inflammatory Bowel Disease: How Long before Abandoning Single-Marker Approaches? Dig Dis. 2021;39:190-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Liu A, Lv H, Tan B, Shu H, Yang H, Li J, Qian J. Accuracy of the highly sensitive C-reactive protein/albumin ratio to determine disease activity in inflammatory bowel disease. Medicine (Baltimore). 2021;100:e25200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Zeng X, Jiang H, Dai Y, Zhang J, Zhao S, Wu Q. A radiomics nomogram based on MSCT and clinical factors can stratify fibrosis in inflammatory bowel disease. Sci Rep. 2024;14:1176. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Puga-Tejada M, Majumder S, Maeda Y, Zammarchi I, Ditonno I, Santacroce G, Capobianco I, Robles-Medranda C, Ghosh S, Iacucci M. Artificial intelligence-enabled histology exhibits comparable accuracy to pathologists in assessing histological remission in ulcerative colitis: a systematic review, meta-analysis, and meta-regression. J Crohns Colitis. 2025;19:jjae198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | Reinisch W, Wang Y, Oddens BJ, Link R. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn's disease: a post-hoc analysis from ACCENT I. Aliment Pharmacol Ther. 2012;35:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 21. | Singh S, Ananthakrishnan AN, Nguyen NH, Cohen BL, Velayos FS, Weiss JM, Sultan S, Siddique SM, Adler J, Chachu KA; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Ulcerative Colitis. Gastroenterology. 2023;164:344-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 67] [Article Influence: 33.5] [Reference Citation Analysis (0)] |