Published online Mar 7, 2024. doi: 10.3748/wjg.v30.i9.1224
Peer-review started: November 11, 2023
First decision: December 7, 2023
Revised: January 3, 2024
Accepted: February 3, 2024
Article in press: February 3, 2024
Published online: March 7, 2024
Processing time: 116 Days and 3.7 Hours
As a critical early event in hepatocellular carcinogenesis, telomerase activation might be a promising and critical biomarker for hepatocellular carcinoma (HCC) patients, and its function in the genesis and treatment of HCC has gained much attention over the past two decades.
To perform a bibliometric analysis to systematically assess the current state of research on HCC-related telomerase.
The Web of Science Core Collection and PubMed were systematically searched to retrieve publications pertaining to HCC/telomerase limited to “articles” and “reviews” published in English. A total of 873 relevant publications related to HCC and telomerase were identified. We employed the Bibliometrix package in R to extract and analyze the fundamental information of the publications, such as the trends in the publications, citation counts, most prolific or influential writers, and most popular journals; to screen for keywords occurring at high frequency; and to draw collaboration and cluster analysis charts on the basis of coauthorship and co-occurrences. VOSviewer was utilized to compile and visualize the bibliometric data.
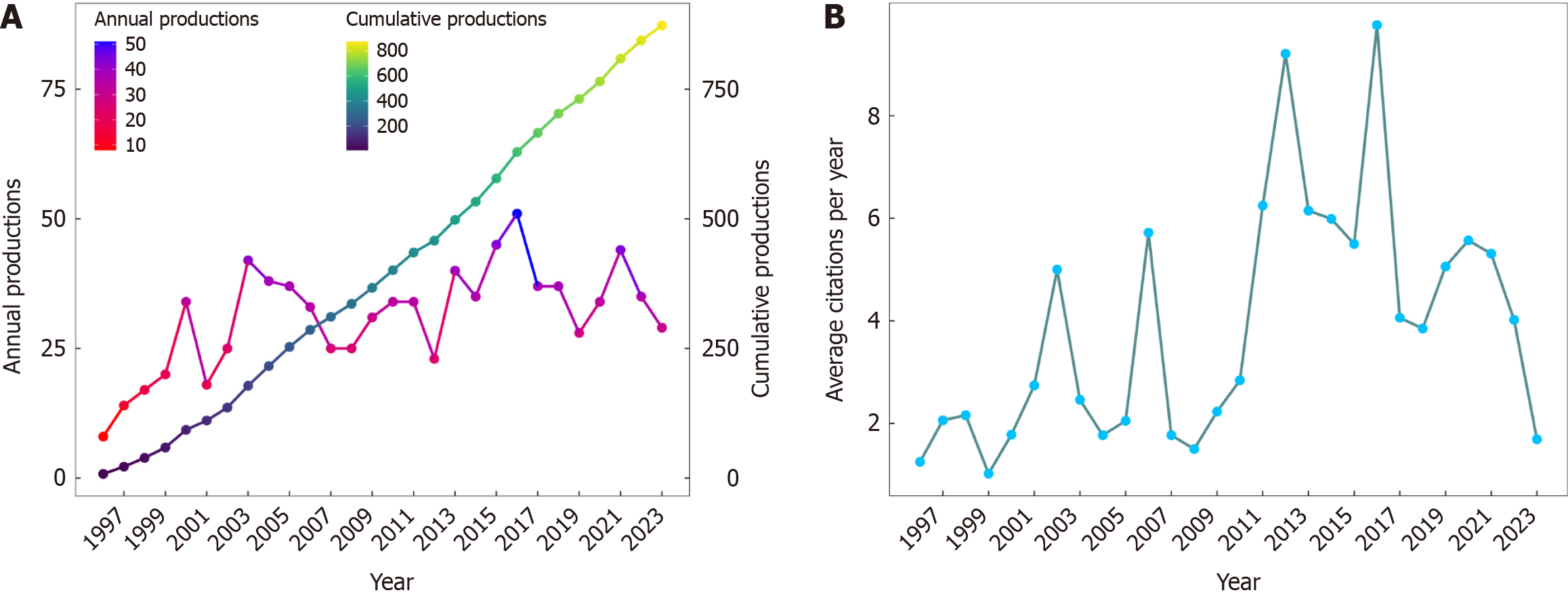
A surge of 51 publications on HCC/telomerase research occurred in 2016, the most productive year from 1996 to 2023, accompanied by the peak citation count recorded in 2016. Up to December 2023, 35226 citations were made to all publications, an average of 46.6 citations to each paper. The United States received the most citations (n = 13531), followed by China (n = 7427) and Japan (n = 5754). In terms of national cooperation, China presented the highest centrality, its strongest bonds being to the United States and Japan. Among the 20 academic institutions with the most publications, ten came from China and the rest of Asia, though the University of Paris Cité, Public Assistance-Hospitals of Paris, and the National Institute of Health and Medical Research (INSERM) were the most prolific. As for individual contributions, Hisatomi H, Kaneko S, and Ide T were the three most prolific authors. Kaneko S ranked first by H-index, G-index, and overall publication count, while Zucman-Rossi J ranked first in citation count. The five most popular journals were the World Journal of Gastroenterology, Hepatology, Journal of Hepatology, Oncotarget, and Oncogene, while Nature Genetics, Hepatology, and Nature Reviews Disease Primers had the most citations. We extracted 2293 keywords from the publications, 120 of which appeared more than ten times. The most frequent were HCC, telomerase and human telomerase reverse transcriptase (hTERT). Keywords such as mutational landscape, TERT promoter mutations, landscape, risk, and prognosis were among the most common issues in this field in the last three years and may be topics for research in the coming years.
Our bibliometric analysis provides a comprehensive overview of HCC/telomerase research and insights into promising upcoming research.
Core Tip: As a common event and promising biomarker in the early stage of hepatocellular carcinoma (HCC), telomerase activation is tightly connected to the survival rate and clinical prognosis of HCC patients. In this vein, the progress of immunotherapy and relevant studies on telomerase reverse transcriptase vaccination are strongly valuable. This study presents the first bibliometric analysis of telomerase-related research on HCC, offering a comprehensive overview of HCC-related telomerase studies. Keywords such as mutational landscape, telomerase reverse transcriptase promoter mutations, landscape, risk, and prognosis will be hot topics in the near future.
- Citation: Li HY, Zheng LL, Hu N, Wang ZH, Tao CC, Wang YR, Liu Y, Aizimuaji Z, Wang HW, Zheng RQ, Xiao T, Rong WQ. Telomerase-related advances in hepatocellular carcinoma: A bibliometric and visual analysis. World J Gastroenterol 2024; 30(9): 1224-1236
- URL: https://www.wjgnet.com/1007-9327/full/v30/i9/1224.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i9.1224
Primary liver cancer (PLC) is the sixth most common cancer and the third leading cause of cancer-related mortality worldwide[1]. PLC encompasses three major histological types, hepatocellular carcinoma (HCC), cholangiocarcinoma, and mixed-type PLC, with HCC accounting for about 90% of all cases[2]. At present, the major risk factors for HCC include viral hepatitis, alcohol consumption, exposure to aflatoxin B1, metabolic diseases, and genetics[3,4]. The treatment of HCC relies primarily on surgical resection, liver transplantation, local ablation, chemoembolization, and molecular targeted therapy[5-8]. Unfortunately, only about 20% of patients are amenable to surgery, and they face high rates of recurrence and metastasis after surgery[9,10]. Therefore, finding biomarkers with high sensitivity and specificity for the early screening and diagnosis of HCC is particularly important.
Telomeres, which are repetitive DNA sequences (TTAGGG) at the ends of chromosomes, are essential for maintaining genomic integrity. The enzyme telomerase consists of telomerase reverse transcriptase (TERT), telomerase RNA component (TERC), and telomerase-associated proteins, which act to prolong and protect telomeres[11,12]. Telomerase activation is an early event in the process of hepatocellular carcinogenesis, mainly through somatic TERT promoter mutations and TERT gene amplification[13,14]. Telomerase can serve as a promising biomarker and potential therapeutic target for HCC[15].
Over the past few decades, research on HCC/telomerase has become more popular. No bibliometric analysis has systematically reviewed and analyzed the literature in this field. Bibliometric analysis uses statistical methods and information visualization technology to quantitatively analyze the studies in a research field and reveal any tendencies in the field[16,17]. Currently, CiteSpace[18,19], VOSviewer[20], and R software are used for scientometric analysis of the literature. Many researchers in biomedicine and mechanical engineering use this strategy to evaluate their respective research areas[21,22]. This study evaluates the literature on HCC and telomerase from year to year, describes the research progress and hotspots in this field, and reveals future research directions in this field.
On December 20, 2023, a comprehensive search was conducted for publications in the field of HCC/telomerase research from 1996 to 2023. The search encompassed the Science Citation Index Extended (SCIE) within the Web of Science Core Collection (WoSCC) and the PubMed database. The literature types were limited to “article” and “review”, and only English-language publications were considered. The search strategy is illustrated in Supplementary Figure 1. Two independent authors systematically queried both the WoSCC and PubMed databases, downloaded pertinent information (title, keywords, author details, abstracts, etc.), and meticulously excluded duplicate or irrelevant papers. Their results showed substantial accordance. The most pertinent publications on HCC/telomerase were meticulously gathered from the combined results of the WoSCC and PubMed searches.
Bibliometrix is an automated data analysis and visualization tool[23]. The Bibliometrix package for R 4.3.2 was used to extract and analyze the fundamental information of the publications. This package enables users to obtain insights such as the number of annual publications, institutional and author analysis (including H-index, G-index, and M-index, journal analysis, and national cooperation networks. With Bibliometrix, researchers can comprehensively explore bibliometric data to better understand the scientific landscape and trends. To visualize and construct bibliometric data, researchers commonly use VOSviewer, a software tool that enables keyword cluster analysis and visualization[24]. We used VOSviewer (R1.6.19) to extract all keywords that appeared more than 10 times in the analyzed publications. Ultimately, 120 keywords were extracted and divided into 4 clusters. The collaboration and cluster analysis charts generated from these software tools are based on coauthorship and co-occurrences[25,26].
In total, 873 publications, comprising 725 primary articles and 148 reviews, were obtained (Figure 1A). Before 2001, the annual publication output was relatively sparse, with only 8 to 34 articles being published each year. Since 2001, the number of papers has remained stable at 23 or more articles per year. Remarkably, there was a surge in publications in 2016, when 51 studies were published, the most in the study period. Figure 1B depicts the average citation count to these publications recorded annually, the peak citation count recorded in 2016. This peak indicated that there may have been groundbreaking publications in 2016, which could explain the small peak in the number of publications from 2019 to 2021.
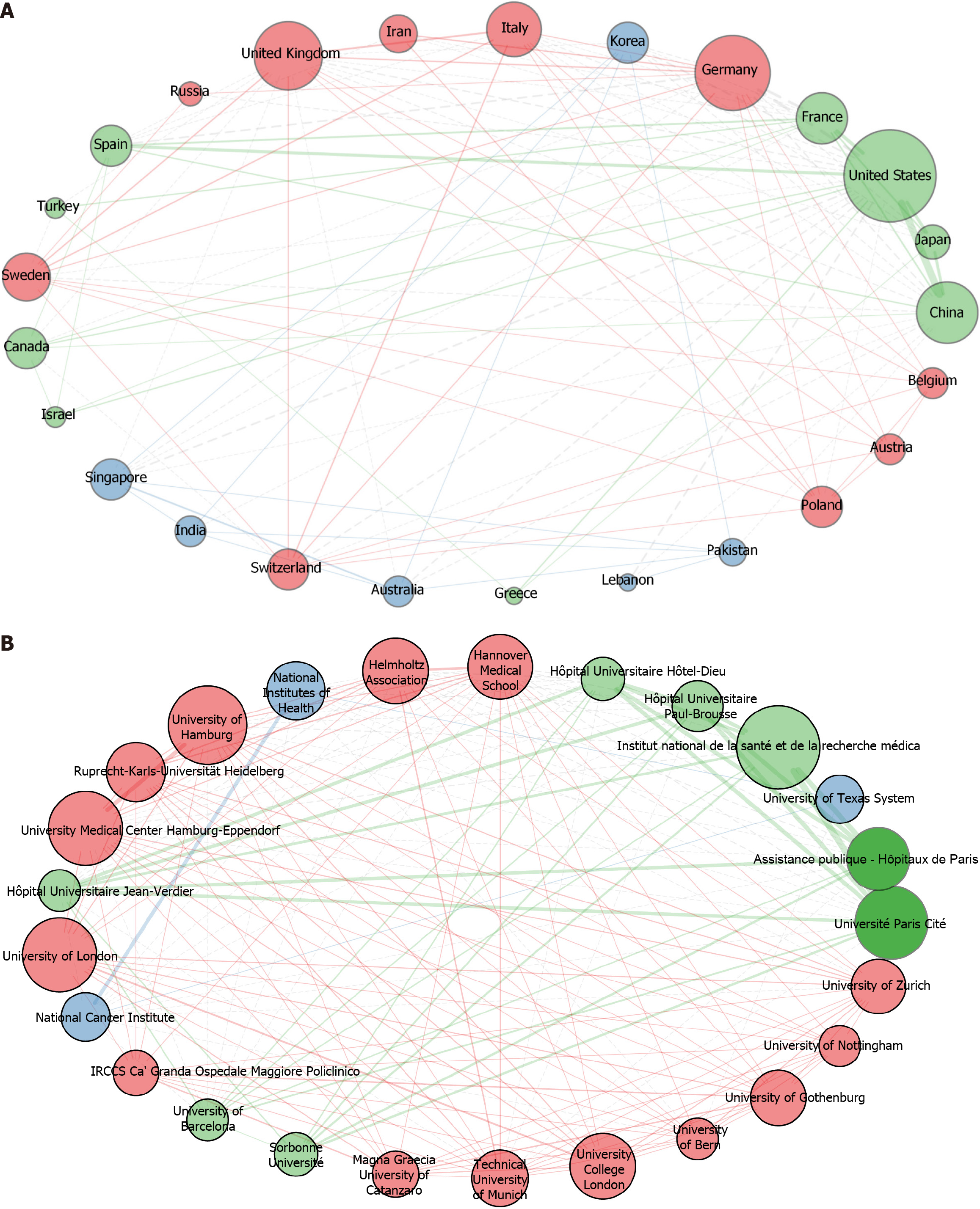
Among the 41 nations whose articles were recognized, China had the most publications (n = 292), followed by Japan (n = 151), the United States (n = 118), and Korea (n = 50) (Table 1). Overall, the publications had received 35,226 citations as of December 2022, an average of 44.8 citations per paper. The United States (n = 13531) ranked first in total citations, followed by China (n = 7427) and Japan (n = 5754) (Table 1). The cooperation network of various nations is shown in Figure 2A; the thickness of the lines between nodes indicates the level of collaboration between countries. China was the node with the highest centrality. Its strongest ties were to the United States and Japan, demonstrating a high degree of cooperation between these nations. Table 2 highlights the 20 academic institutions with the most publications in this discipline. The University of Paris Cité, Public Assistance-Hospitals of Paris, and the National Institute of Health and Medical Research (INSERM) were the most prolific, releasing 64, 45, and 34 papers, respectively. Five of the top 20 institutions were from China, and five more were from elsewhere in Asia. Figure 2B shows the network of institutional collaboration, with various hues denoting the degree of such connection. Interestingly, French institutions collaborated with one another most frequently.
| Rank | Country | Articles | SCP | MCP | Total citations | Average article citations |
| 1 | China | 292 | 262 | 30 | 7427 | 25.4 |
| 2 | Japan | 151 | 139 | 12 | 5754 | 38.1 |
| 3 | United States | 118 | 91 | 27 | 13531 | 114.7 |
| 4 | Korea | 50 | 43 | 7 | 1140 | 22.8 |
| 5 | Germany | 48 | 31 | 17 | 4073 | 84.9 |
| 6 | Italy | 30 | 25 | 5 | 741 | 24.7 |
| 7 | France | 29 | 22 | 7 | 2135 | 73.6 |
| 8 | Iran | 16 | 8 | 8 | 300 | 18.8 |
| 9 | United Kingdom | 14 | 8 | 6 | 704 | 50.3 |
| 10 | Egypt | 11 | 10 | 1 | 135 | 12.3 |
| Rank | Affiliations | Country | Articles |
| 1 | Université Paris Cité | France | 64 |
| 2 | Assistance Publique-Hôpitaux de Paris | France | 45 |
| 3 | Institut National de la Santé et de la Recherche Médicale | France | 34 |
| 4 | University of Texas System | United States | 34 |
| 5 | Hôpital Universitaire Paul-Brousse | France | 31 |
| 6 | Hiroshima University | Japan | 30 |
| 7 | Hôpital Universitaire Hôtel-Dieu | France | 29 |
| 8 | National Taiwan University | China | 29 |
| 9 | Yonsei University Health System | Korea | 29 |
| 10 | Université de Bordeaux | France | 26 |
| 11 | Tottori University | Japan | 25 |
| 12 | Johns Hopkins University | United States | 24 |
| 13 | Sun Yat-sen University | China | 24 |
| 14 | Chinese Academy of Medical Sciences- Peking Union Medical College | China | 22 |
| 15 | Yonsei University | Korea | 22 |
| 16 | MD Anderson Cancer Center | United States | 21 |
| 17 | Helmholtz Association | Germany | 20 |
| 18 | Shandong University | China | 20 |
| 19 | University of Tokyo | Japan | 20 |
| 20 | The Chinese University of Hong Kong | China | 19 |
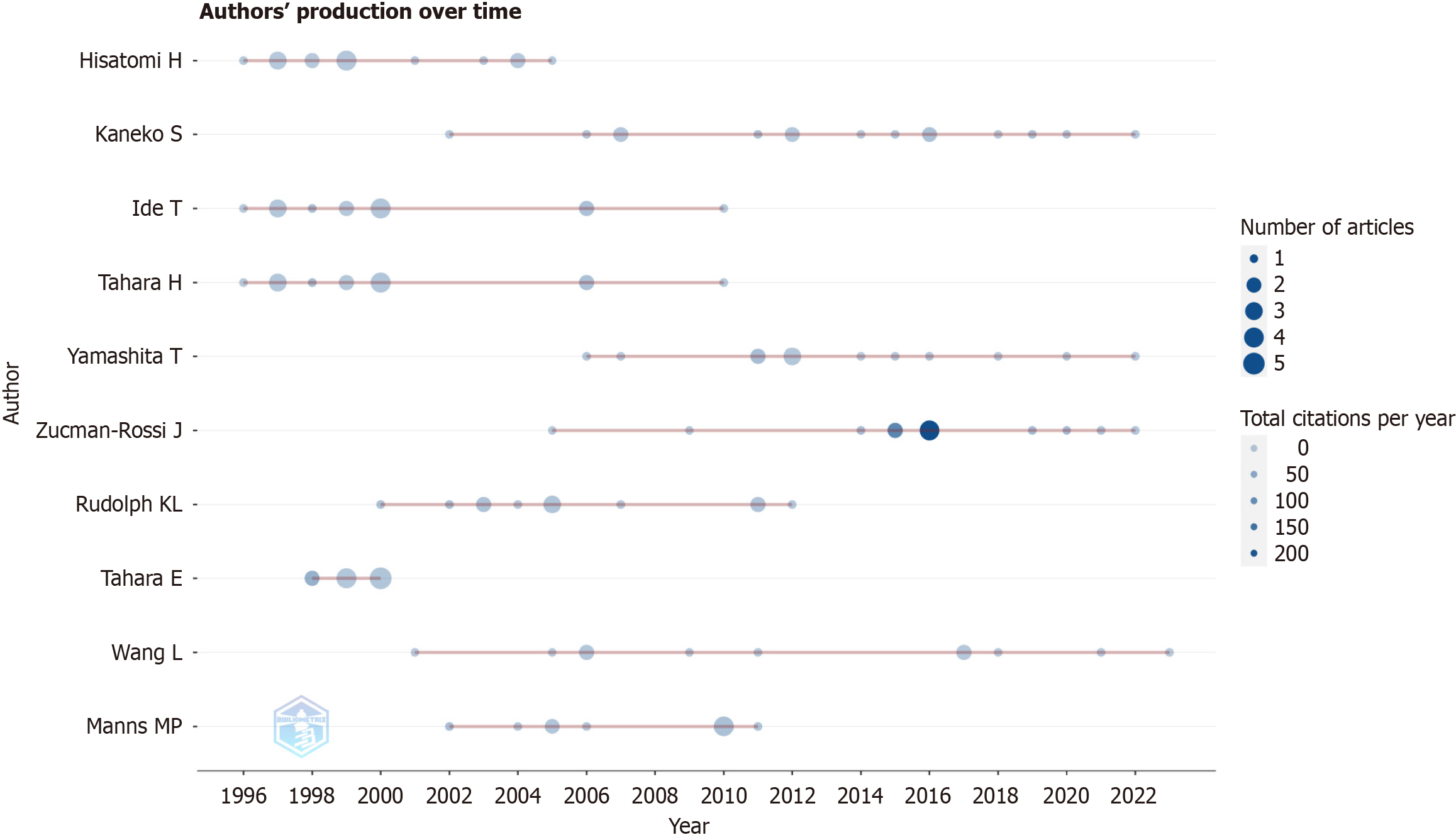
In this analysis, 873 studies by 4895 authors were examined. The ten most prolific writers are shown in Figure 3. Kaneko S, Hisatomi H, and Ide T placing first, second, and third, respectively. The nodes indicate how many works an author released in a certain year. The number of articles each author is credited with is shown by the size of the node, and the number of citations each author received is indicated by the color of the node. Important publications by Zucman-Rossi J were published in 2015 and 2016, in each of which years this author received more than 130 citations. Indicators of academic influence, such as the H-index, G-index, and M-index, are shown in Table 3 for the top 10 most prolific authors. The H-index, G-index, and overall publication count were highest for Kaneko S. Zucman-Rossi J, who has made substantial contributions to the field, ranked first in terms of citations and fifth in terms of the H-index and G-index. The included studies were published in 365 journals, the five most popular being the World Journal of Gastroenterology, Hepatology, Journal of Hepatology, Oncotarget, and Oncogene (Table 4). Nature Genetics, Hepatology, and Nature Reviews Disease Primers garnered the most citations.
| Rank | Author | H-index | G-index | M-index | Total citations | Number of publications |
| 1 | Kaneko S | 12 | 15 | 0.522 | 637 | 15 |
| 2 | Hisatomi H | 10 | 15 | 0.345 | 333 | 15 |
| 3 | Ide T | 12 | 14 | 0.414 | 1261 | 14 |
| 4 | Tahara H | 12 | 14 | 0.414 | 1271 | 14 |
| 5 | Zucman-Rossi J | 11 | 13 | 0.55 | 3701 | 13 |
| 6 | Yamashita T | 10 | 13 | 0.526 | 604 | 13 |
| 7 | Rudolph KL | 12 | 12 | 0.48 | 1423 | 12 |
| 8 | Tahara E | 11 | 11 | 0.407 | 1486 | 11 |
| 9 | Wang L | 7 | 11 | 0.292 | 216 | 11 |
| 10 | Manns MP | 10 | 10 | 0.435 | 977 | 10 |
| Rank | Journal | H-index | G-index | M-index | Total citations | Number of publications |
| 1 | World Journal of Gastroenterology | 16 | 36 | 0.615 | 1344 | 40 |
| 2 | Hepatology | 21 | 24 | 0.778 | 1920 | 24 |
| 3 | Journal of Hepatology | 13 | 19 | 0.5 | 909 | 19 |
| 4 | Oncotarget | 12 | 19 | 0.857 | 557 | 19 |
| 5 | Oncogene | 16 | 17 | 0.571 | 1225 | 17 |
| 6 | Oncology Reports | 10 | 14 | 0.37 | 213 | 17 |
| 7 | PLoS One | 12 | 16 | 0.75 | 407 | 16 |
| 8 | Cancer Letters | 11 | 15 | 0.407 | 694 | 15 |
| 9 | International Journal of Molecular Sciences | 9 | 13 | 1 | 226 | 13 |
| 10 | International Journal of Cancer | 10 | 12 | 0.357 | 344 | 12 |
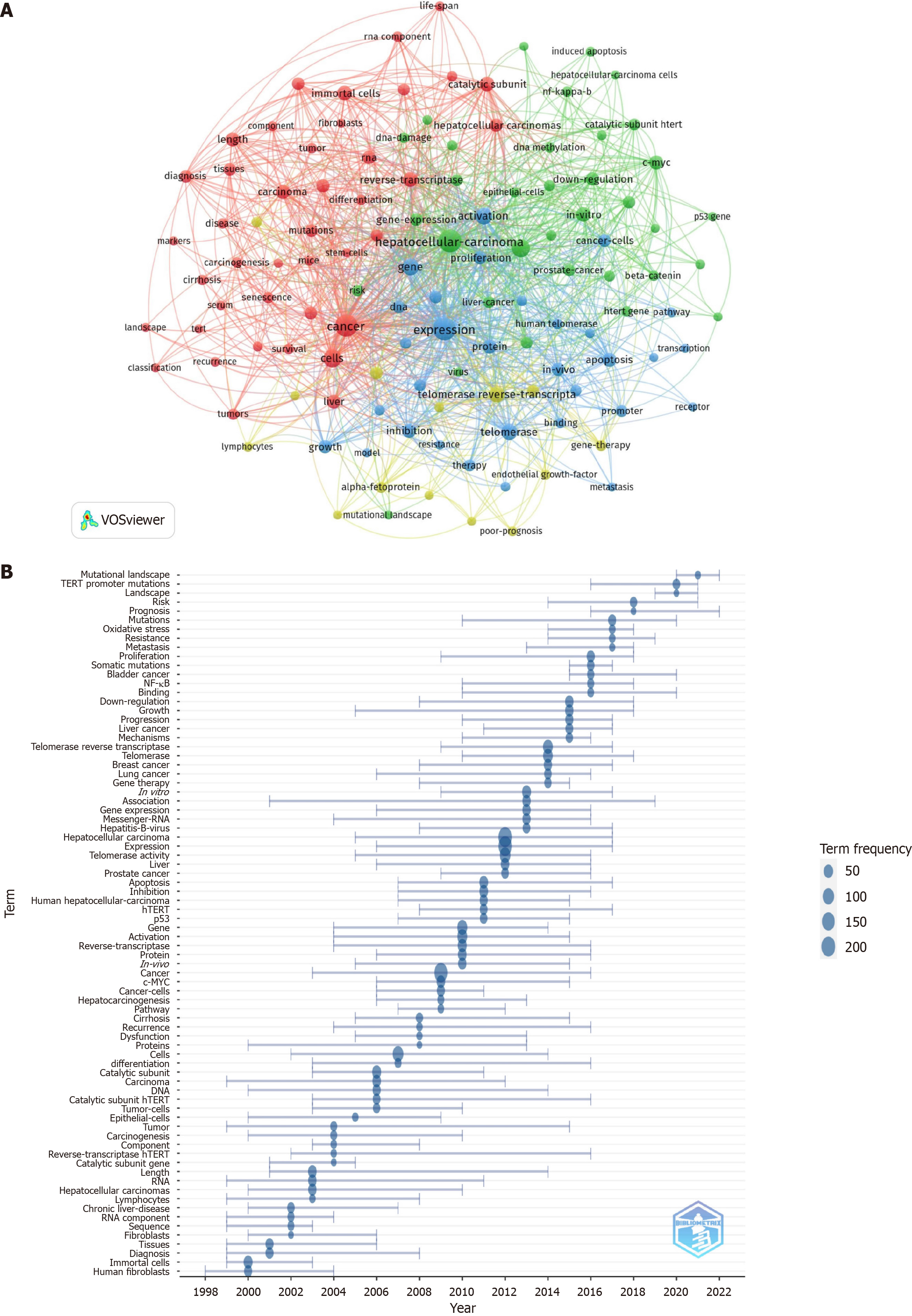
Keyword co-occurrence analysis revealed patterns of co-occurrence as well as potential research hotspots. VOSviewer software was used to extract 2293 keywords for this research, 120 of which appeared more than ten times. HCC was the most commonly used term, followed by expression, cancer, cells and telomerase activity (Table 5). Four clusters were formed after clustering using the VOSviewer program (Figure 4A). The size of each node denotes the occurrence of the relevant term, and each color represents a cluster. Among the 41 terms that co-occurred in the largest cluster (red), which was associated with telomerase structure and carcinogenesis, were cancer, cell, p53, catalytic subunit, reverse transcriptase, cellular senescence, and carcinogenesis. Thirty-four co-occurring terms in Cluster 2 (green), including beta-catenin, c-MYC, DNA damage, DNA methylation, oxidative stress, and NF-kappa-B, were associated with telomerase-related signaling pathways. Thirty-one terms from Cluster 3 (blue), including activation, apoptosis, growth, hTERT, proliferation, and promoter, co-occurred in this cluster, and were associated with the regulation of telomerase. The majority of terms in Cluster 4 (yellow) focused on the role of telomerase and its associated structural elements in the treatment of HCC. Four terms that were among the 14 others in this cluster were TERT, gene therapy, immunotherapy, and poor prognosis. In Figure 4B, the temporal evolution of keywords is shown, with blue nodes indicating the frequency of term usage in a particular year. Mutational landscape, TERT promoter mutations, landscape, risk, and prognosis are just a few of the major topics that have emerged as significant research fields in the last three years and are expected to continue to be important for a while.
| Rank | Keywords | Occurrences | Total link strength |
| 1 | Hepatocellular carcinoma | 217 | 211 |
| 2 | Expression | 208 | 205 |
| 3 | Cancer | 201 | 197 |
| 4 | Cells | 95 | 93 |
| 5 | Telomerase activity | 95 | 95 |
| 6 | Gene | 78 | 77 |
| 7 | Activation | 73 | 73 |
| 8 | Telomerase reverse-transcriptase | 67 | 64 |
| 9 | Telomerase | 62 | 62 |
| 10 | Catalytic subunit | 50 | 50 |
| 11 | Reverse-transcriptase | 50 | 49 |
| 12 | Immortal cells | 48 | 47 |
| 13 | In vitro | 47 | 47 |
| 14 | Apoptosis | 43 | 42 |
| 15 | Inhibition | 42 | 42 |
| 16 | Protein | 41 | 41 |
| 17 | C-MYC | 40 | 39 |
| 18 | Carcinoma | 40 | 38 |
| 19 | Length | 40 | 39 |
| 20 | Liver | 40 | 40 |
| 21 | Down-regulation | 36 | 36 |
| 22 | Growth | 35 | 35 |
| 23 | Hepatocellular carcinomas | 35 | 34 |
| 24 | RNA | 35 | 35 |
| 25 | In vivo | 34 | 34 |
In this era of big data, researchers find it relevant to understand the most recent developments in their field of study. To comprehensively assess and visually depict the current literature, bibliometric analysis uses a variety of bibliometric software programs[18,20,23]. Numerous investigations have looked into the role of telomerase and its components in the development and treatment of HCC during the past 20 years. A correlation between telomerase and HCC was first proposed by the Japanese scholar Tahara et al[27]. They observed that telomerase activity was present in HCC tissues from hepatitis B virus-positive individuals, but not in their normal liver tissues and not in patients with chronic liver disease. Telomerase expression may play a significant role in the development of HCC[27]. Since then, numerous studies on this topic have been conducted[28-31].
Over the past two decades, research on telomerase in HCC has shown steady growth, the annual number of published papers remaining stable at more than 30. The number of studies has fluctuated somewhat, showing peaks in 2003 and 2016, indicating a continued increase in research achievements in this field. These findings suggest that the study of telomerase action in HCC has great potential. Along with France, the United States, and Japan, China has made notable contributions to telomerase and HCC-related research. More telomerase and HCC investigations have been published in Asian nations than in European nations, likely owing to the high incidence of viral liver diseases and HCC on the Asian continent[32-34]. Despite its later start, China has emerged as one of the leading contributors to this subject, possibly as a result of increasing financing for academic research and its fast economic growth in recent years.
The average number of citations per article from China, at 25.4, was still less than the global average of 46.6. Six of the 10 institutions contributing the most to this field are in France, making it the most powerful and technologically sophisticated nation. According to the findings of institutional collaboration studies, there is often no cooperation between international institutions on telomerase- and HCC-related research. We think that more international collaboration might boost research in this field and advance telomerase- and HCC-related studies.
With high citation indices, Kaneko S and Zucman-Rossi J have made substantial contributions to the rapidly emerging field of telomerase in HCC. Among the articles they have authored, “Comparative analysis of various tumor-associated antigen (TAA)-specific T-cell responses in patients with hepatocellular carcinoma” and “Genetic Landscape and Biomarkers of Hepatocellular Carcinoma” were the most prominent. When employed as an immunogenic target for HCC immunotherapy, hTERT was shown by Mizukoshi et al[35] to be a potential TAA. These authors suggested that antibodies targeting cytotoxic T-lymphocyte antigen-4 (anti-CTLA-4) antibodies may improve antitumor immunity and that hTERT or peptides containing its epitopes may be useful for vaccination. The development of HCC immunotherapy and associated research on TERT vaccination have both been strongly supported by this study. TERT promoter mutations, which are often seen in HCC, were demonstrated by Zucman-Rossi et al[36] to be related to enhanced telomerase expression, representing the first recurring somatic genetic alteration, and are associated with malignant development and a poor prognosis in patients with HCC.
The World Journal of Gastroenterology published the most papers by far. Although fewer in number, those published in Nature Genetics were cited the most. Other journals with a significant number of publications include Hepatology, Nature Reviews Disease Primers, and Nature Reviews Cancer. In bibliometrics, the co-occurrence of terms can identify academic hotspots. Four key areas of telomerase-related research in HCC, namely, telomerase structure, treatment, regulation of telomerase, and telomerase-related signaling pathways, were revealed through cluster analysis.
In terms of the structure of telomerase, tumorigenesis, senescence, and p53 have been noted. p53 is an important tumor suppressor gene that is activated in response to DNA damage[37] or telomere shortening[38,39], thereby promoting apoptosis or cell cycle arrest and preventing cells from turning into cancer cells. When telomeres are become short enough to cause senescence, p53 is activated in normal cells[39]. One of the most prevalent p53 mutations in tumors is often seen in HCC[2], which can activate telomerase and prolong telomeres through the cascade of the p53-CUDR-PKM2-Pim1-TERT pathway, hence promoting HCC[40]. Notably, early in the development of HCC, both p53 and TERT have driver mutations[13,14]. Further study is needed to determine the molecular pathways involved in the formation and progression of HCC, as well as their interactions. Interestingly, in recent years, the role of TERT mutation in the prognosis of HCC patients and its therapeutic mechanism have attracted increasing attention.
Alpha-fetoprotein, metastasis, poor prognosis, recurrence, survival, immunotherapy, TERT promoter mutation, somatic mutation, mutant landscape, and HCC are the main themes associated with clinical prognosis and with telomerase-related mutations in HCC. Patients with HCC who have elevated telomerase activity have a poor prognosis and a considerably shorter survival time[41,42]. These findings highlight the importance of telomerase activity as a key marker of poor prognosis of HCC. In addition, the TERT gene, which encodes TERT, is crucial. TERT promoter mutations are often found in HCC and are linked to a poor prognosis and low survival rate, suggesting that they might be important biomarkers indicating how HCC patients will fare[43,44].
The current research hotspots in the regulation of telomerase activity and related pathways include the c-MYC, NF-kappa-B, cell cycle, and -catenin pathways; DNA methylation; and other pathways. As for the treatment of HCC, telomerase has become a key target in anticancer therapy. hTERT is a TAA that stimulates the production of CD8+ cytotoxic T lymphocytes (CTLs) in a variety of tumor types[45]. Peptide-derived vaccines directly activate the immune response of telomerase-positive cancer cells In vivo. GV1001, a 16-amino-acid peptide derived from hTERT, has been developed as an anticancer peptide vaccine to induce CD4+ and CD8+ T-cell immune responses[46]. Several phase I/II clinical trials have demonstrated that GV1001 induces a response in non-small-cell lung cancer (NSCLC), HCC, pancreatic cancer, and malignant melanoma without any serious adverse effects[47-49]. In a phase I/II NSCLC study (CTN-2000 trial), a GV1001-specific immune response was observed in 13/24 evaluable patients, and immune responders had longer survival than nonresponders (median 19.0 months vs 3.5 months, P < 0.001)[50]. A phase III clinical study of GV1001 in combination with chemoradiotherapy for stage III NSCLC is ongoing. Vx-001 is composed of two peptides: The native cryptic peptide ARG-Vx001 (TERT572) and the optimized variant TYR-Vx001 (TERT572Y)[51]. The Vx-001 vaccine has been found to be clinically safe and well tolerated, with only local skin reactions reported in clinical trials[52]. In summary, immunotherapy directly targets TERT epitopes that have shown potent anticancer activity.
This study has several limitations. First, the data were retrieved only from the WoSCC and PubMed databases. Second, this study assessed only English-language articles or reviews, which might have led us to overlook some studies. In the future, we may use a wider variety of assessment methods to gain a deeper understanding of this research field.
This study provides the first systematic analysis of HCC/telomerase research over the past two decades via bibliometric analysis, covering trends in publications, overall citation counts, most prolific or influential writers, popular journals, keywords occurring at high frequency, and international cooperation. In this field, along with Japan, the United States, and France, China has made notable contributions. Moreover, Asia appeared to account for the bulk corresponding to the high incidence of viral liver diseases and HCC on the Asian continent. The results of the present study strongly support the development of HCC immunotherapy and associated research on TERT vaccination. Moreover, keywords such as mutational landscape, TERT promoter mutations, landscape, risk, and prognosis are promising emerging topics, which offers insights into upcoming research.
Telomerase activation is common in hepatocellular carcinogenesis and might become a key biomarker for hepatocellular carcinoma (HCC) patients. Its influence on the genesis and treatment of hepatocellular carcinoma has gained much attention over the past two decades.
To Evaluate the present state of research and the research hotspots in the field of HCC and telomerase will help bridge the information gap and reveal promising research insights.
To provide a comprehensive overview of the current research on HCC/telomerase via bibliometric analysis.
The Web of Science Core Collection and PubMed were systematically searched to retrieve publications pertaining to HCC/telomerase. VOSviewer and R software were utilized to conduct the analysis.
A total of 873 publications on HCC/telomerase were identified. A surge of 51 studies happened in 2016, the peak yearly citation counts in this field. China emerged as a leading contributor, and Asia represented the bulk of research done in this field, consistent with the high incidence of regional liver diseases. Four major clusters were generated using keywords co-occurrence analysis.
Our study provides the first general analysis of HCC-related telomerase gene expression over the past two decades via bibliometric analysis. Advances in telomerase reverse transcriptase (TERT) treatment, such as vaccination or immunotherapy, are highly valuable and warrant further study.
Keywords such as mutational landscape, TERT promoter mutations, landscape, risk, and prognosis have been topics of interest in this field in recent years and likely suggest the upcoming research directions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maida M, Italy S-Editor: Chen YL L-Editor: A P-Editor: Zheng XM
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55814] [Article Influence: 7973.4] [Reference Citation Analysis (132)] |
| 2. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3165] [Article Influence: 527.5] [Reference Citation Analysis (37)] |
| 3. | Mazzanti R, Gramantieri L, Bolondi L. Hepatocellular carcinoma: epidemiology and clinical aspects. Mol Aspects Med. 2008;29:130-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1341] [Article Influence: 335.3] [Reference Citation Analysis (1)] |
| 5. | Agnello F, Salvaggio G, Cabibbo G, Maida M, Lagalla R, Midiri M, Brancatelli G. Imaging appearance of treated hepatocellular carcinoma. World J Hepatol. 2013;5:417-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Cabibbo G, Maida M, Genco C, Alessi N, Peralta M, Butera G, Galia M, Brancatelli G, Genova C, Raineri M, Orlando E, Attardo S, Giarratano A, Midiri M, Di Marco V, Craxì A, Cammà C. Survival of patients with hepatocellular carcinoma (HCC) treated by percutaneous radio-frequency ablation (RFA) is affected by complete radiological response. PLoS One. 2013;8:e70016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Rong W, Xia H, Zhang K, Zhang Y, Tao C, Wu F, Wang L, Zhang H, Sun G, Wu J. Serum metabolic effects of corn oligopeptides with 7-day supplementation on early post-surgery primary liver cancer patients: a double-blind randomized controlled trial. Hepatobiliary Surg Nutr. 2022;11:834-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer. 2018;7:235-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 444] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 9. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15307] [Article Influence: 3061.4] [Reference Citation Analysis (4)] |
| 10. | Roayaie S, Jibara G, Tabrizian P, Park JW, Yang J, Yan L, Schwartz M, Han G, Izzo F, Chen M, Blanc JF, Johnson P, Kudo M, Roberts LR, Sherman M. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62:440-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 322] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 11. | Smith EM, Pendlebury DF, Nandakumar J. Structural biology of telomeres and telomerase. Cell Mol Life Sci. 2020;77:61-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 12. | Zhao Y, Sfeir AJ, Zou Y, Buseman CM, Chow TT, Shay JW, Wright WE. Telomere extension occurs at most chromosome ends and is uncoupled from fill-in in human cancer cells. Cell. 2009;138:463-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Nault JC, Mallet M, Pilati C, Calderaro J, Bioulac-Sage P, Laurent C, Laurent A, Cherqui D, Balabaud C, Zucman-Rossi J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat Commun. 2013;4:2218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 414] [Cited by in RCA: 511] [Article Influence: 46.5] [Reference Citation Analysis (1)] |
| 14. | Nault JC, Calderaro J, Di Tommaso L, Balabaud C, Zafrani ES, Bioulac-Sage P, Roncalli M, Zucman-Rossi J. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology. 2014;60:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 258] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 15. | Jafri MA, Ansari SA, Alqahtani MH, Shay JW. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016;8:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 463] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 16. | Shen Z, Wu H, Chen Z, Hu J, Pan J, Kong J, Lin T. The Global Research of Artificial Intelligence on Prostate Cancer: A 22-Year Bibliometric Analysis. Front Oncol. 2022;12:843735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 17. | Kumar R, Singh S, Sidhu AS, Pruncu CI. Bibliometric Analysis of Specific Energy Consumption (SEC) in Machining Operations: A Sustainable Response. Sustainability. 2021;13:5617. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Chen C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol. 2006;57:359-377. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2009] [Cited by in RCA: 2057] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 19. | Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. 2004;101 Suppl 1:5303-5310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1311] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 20. | van Eck NJ, Waltman L. Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics. 2017;111:1053-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 507] [Cited by in RCA: 567] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 21. | Yang DW, Wang XP, Wang ZC, Yang ZH, Bian XF. A scientometric analysis on hepatocellular carcinoma magnetic resonance imaging research from 2008 to 2017. Quant Imaging Med Surg. 2019;9:465-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Zhong D, Luo S, Zheng L, Zhang Y, Jin R. Epilepsy Occurrence and Circadian Rhythm: A Bibliometrics Study and Visualization Analysis via CiteSpace. Front Neurol. 2020;11:984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Aria M, Cuccurullo C. Bibliometrix: An R-tool for comprehensive science mapping analysis. J Informetr. 2017;11:959-975. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1736] [Cited by in RCA: 2270] [Article Influence: 283.8] [Reference Citation Analysis (0)] |
| 24. | van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4505] [Cited by in RCA: 5141] [Article Influence: 321.3] [Reference Citation Analysis (0)] |
| 25. | Higaki A, Uetani T, Ikeda S, Yamaguchi O. Co-authorship network analysis in cardiovascular research utilizing machine learning (2009-2019). Int J Med Inform. 2020;143:104274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Trujillo CM, Long TM. Document co-citation analysis to enhance transdisciplinary research. Sci Adv. 2018;4:e1701130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 27. | Tahara H, Nakanishi T, Kitamoto M, Nakashio R, Shay JW, Tahara E, Kajiyama G, Ide T. Telomerase activity in human liver tissues: comparison between chronic liver disease and hepatocellular carcinomas. Cancer Res. 1995;55:2734-2736. [PubMed] |
| 28. | Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science. 2000;287:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 303] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 29. | Ferber MJ, Montoya DP, Yu C, Aderca I, McGee A, Thorland EC, Nagorney DM, Gostout BS, Burgart LJ, Boix L, Bruix J, McMahon BJ, Cheung TH, Chung TK, Wong YF, Smith DI, Roberts LR. Integrations of the hepatitis B virus (HBV) and human papillomavirus (HPV) into the human telomerase reverse transcriptase (hTERT) gene in liver and cervical cancers. Oncogene. 2003;22:3813-3820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 163] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 30. | Chen J, Zhang B, Wong N, Lo AW, To KF, Chan AW, Ng MH, Ho CY, Cheng SH, Lai PB, Yu J, Ng HK, Ling MT, Huang AL, Cai XF, Ko BC. Sirtuin 1 is upregulated in a subset of hepatocellular carcinomas where it is essential for telomere maintenance and tumor cell growth. Cancer Res. 2011;71:4138-4149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Totoki Y, Tatsuno K, Covington KR, Ueda H, Creighton CJ, Kato M, Tsuji S, Donehower LA, Slagle BL, Nakamura H, Yamamoto S, Shinbrot E, Hama N, Lehmkuhl M, Hosoda F, Arai Y, Walker K, Dahdouli M, Gotoh K, Nagae G, Gingras MC, Muzny DM, Ojima H, Shimada K, Midorikawa Y, Goss JA, Cotton R, Hayashi A, Shibahara J, Ishikawa S, Guiteau J, Tanaka M, Urushidate T, Ohashi S, Okada N, Doddapaneni H, Wang M, Zhu Y, Dinh H, Okusaka T, Kokudo N, Kosuge T, Takayama T, Fukayama M, Gibbs RA, Wheeler DA, Aburatani H, Shibata T. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet. 2014;46:1267-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 610] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 32. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4265] [Article Influence: 236.9] [Reference Citation Analysis (2)] |
| 33. | Gonzalez SA, Keeffe EB. Chronic viral hepatitis: epidemiology, molecular biology, and antiviral therapy. Front Biosci (Landmark Ed). 2011;16:225-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 781] [Article Influence: 156.2] [Reference Citation Analysis (0)] |
| 35. | Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Sakai A, Sakai Y, Kagaya T, Honda M, Kaneko S. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology. 2011;53:1206-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 36. | Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology. 2015;149:1226-1239.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 951] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 37. | Ozaki T, Nakagawara A. Role of p53 in Cell Death and Human Cancers. Cancers (Basel). 2011;3:994-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 479] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 38. | Saretzki G, Sitte N, Merkel U, Wurm RE, von Zglinicki T. Telomere shortening triggers a p53-dependent cell cycle arrest via accumulation of G-rich single stranded DNA fragments. Oncogene. 1999;18:5148-5158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 39. | Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 570] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 40. | Wu M, An J, Zheng Q, Xin X, Lin Z, Li X, Li H, Lu D. Double mutant P53 (N340Q/L344R) promotes hepatocarcinogenesis through upregulation of Pim1 mediated by PKM2 and LncRNA CUDR. Oncotarget. 2016;7:66525-66539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Oh BK, Kim H, Park YN, Yoo JE, Choi J, Kim KS, Lee JJ, Park C. High telomerase activity and long telomeres in advanced hepatocellular carcinomas with poor prognosis. Lab Invest. 2008;88:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Ko E, Seo HW, Jung G. Telomere length and reactive oxygen species levels are positively associated with a high risk of mortality and recurrence in hepatocellular carcinoma. Hepatology. 2018;67:1378-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 43. | Kawai-Kitahata F, Asahina Y, Tanaka S, Kakinuma S, Murakawa M, Nitta S, Watanabe T, Otani S, Taniguchi M, Goto F, Nagata H, Kaneko S, Tasaka-Fujita M, Nishimura-Sakurai Y, Azuma S, Itsui Y, Nakagawa M, Tanabe M, Takano S, Fukasawa M, Sakamoto M, Maekawa S, Enomoto N, Watanabe M. Comprehensive analyses of mutations and hepatitis B virus integration in hepatocellular carcinoma with clinicopathological features. J Gastroenterol. 2016;51:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 44. | Ningarhari M, Caruso S, Hirsch TZ, Bayard Q, Franconi A, Védie AL, Noblet B, Blanc JF, Amaddeo G, Ganne N, Ziol M, Paradis V, Guettier C, Calderaro J, Morcrette G, Kim Y, MacLeod AR, Nault JC, Rebouissou S, Zucman-Rossi J. Telomere length is key to hepatocellular carcinoma diversity and telomerase addiction is an actionable therapeutic target. J Hepatol. 2021;74:1155-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 45. | Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 415] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 46. | Kim H, Seo EH, Lee SH, Kim BJ. The Telomerase-Derived Anticancer Peptide Vaccine GV1001 as an Extracellular Heat Shock Protein-Mediated Cell-Penetrating Peptide. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Brunsvig PF, Aamdal S, Gjertsen MK, Kvalheim G, Markowski-Grimsrud CJ, Sve I, Dyrhaug M, Trachsel S, Møller M, Eriksen JA, Gaudernack G. Telomerase peptide vaccination: a phase I/II study in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2006;55:1553-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 48. | Greten TF, Forner A, Korangy F, N'Kontchou G, Barget N, Ayuso C, Ormandy LA, Manns MP, Beaugrand M, Bruix J. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer. 2010;10:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 49. | Inderberg-Suso EM, Trachsel S, Lislerud K, Rasmussen AM, Gaudernack G. Widespread CD4+ T-cell reactivity to novel hTERT epitopes following vaccination of cancer patients with a single hTERT peptide GV1001. Oncoimmunology. 2012;1:670-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 50. | Brunsvig PF, Kyte JA, Kersten C, Sundstrøm S, Møller M, Nyakas M, Hansen GL, Gaudernack G, Aamdal S. Telomerase peptide vaccination in NSCLC: a phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin Cancer Res. 2011;17:6847-6857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 51. | Vetsika EK, Papadimitraki E, Aggouraki D, Konsolakis G, Mela ME, Kotsakis A, Christou S, Patramani S, Alefantinou M, Kaskara A, Christophyllakis C, Kosmatopoulos K, Georgoulias V, Mavroudis D. Sequential administration of the native TERT572 cryptic peptide enhances the immune response initiated by its optimized variant TERT(572Y) in cancer patients. J Immunother. 2011;34:641-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Mavroudis D, Bolonakis I, Cornet S, Myllaki G, Kanellou P, Kotsakis A, Galanis A, Nikoloudi I, Spyropoulou M, Menez J, Miconnet I, Niniraki M, Cordopatis P, Kosmatopoulos K, Georgoulias V. A phase I study of the optimized cryptic peptide TERT(572y) in patients with advanced malignancies. Oncology. 2006;70:306-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |