Published online Mar 7, 2024. doi: 10.3748/wjg.v30.i9.1177
Peer-review started: November 29, 2023
First decision: December 14, 2023
Revised: December 22, 2023
Accepted: January 30, 2024
Article in press: January 30, 2024
Published online: March 7, 2024
Processing time: 97 Days and 9.6 Hours
Acute decompensation (AD) of cirrhosis is associated with high short-term mortality, mainly due to the development of acute-on-chronic liver failure (ACLF). Thus, there is a need for biomarkers for early and accurate identification of AD patients with high risk of development of ACLF and mortality. Soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) is released from activated innate immune cells and correlated with various inflammatory processes.
To explore the prognostic value of sTREM-1 in patients with AD of cirrhosis.
A multicenter prospective cohort of 442 patients with cirrhosis hospitalized for AD was divided into a study cohort (n = 309) and validation cohort (n = 133). Demographic and clinical data were collected, and serum sTREM-1 was measured at admission. All enrolled patients were followed-up for at least 1 year.
In patients with AD and cirrhosis, serum sTREM-1 was an independent prognosis predictor for 1-year survival and correlated with liver, coagulation, cerebral and kidney failure. A new prognostic model of AD (P-AD) incorporating sTREM-1, blood urea nitrogen (BUN), total bilirubin (TBil), international normalized ratio (INR) and hepatic encephalopathy grades was established and performed better than the model for end-stage liver disease (MELD), MELD-sodium (MELD-Na), chronic liver failure-consortium (CLIF-C) ACLF and CLIF-C AD scores. Additionally, sTREM-1 was increased in ACLF and predicted the development of ACLF during first 28-d follow-up. The ACLF risk score incorporating serum sTREM-1, BUN, INR, TBil and aspartate aminotransferase levels was established and significantly superior to MELD, MELD-Na, CLIF-C ACLF, CLIF-C AD and P-AD in predicting risk of ACLF development.
Serum sTREM-1 is a promising prognostic biomarker for ACLF development and mortality in patients with AD of cirrhosis.
Core Tip: Acute decompensation (AD) of cirrhosis is associated with high short-term mortality, mainly due to development of acute-on-chronic liver failure (ACLF). serum Soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) is an independent risk factor for development of ACLF and mortality in patients with AD of cirrhosis. The new prognostic model of AD (P-AD) and the ACLF risk score (ACLF-R) were established and performed better than currently available prognostic models in predicting 1-year mortality and 28-d ACLF development in patients with AD of cirrhosis. Serum sTREM-1 Level, P-AD and ACLF-R score will facilitate clinical decision-making in the management of AD of cirrhosis.
- Citation: Yu SM, Li H, Deng GH, Wang XB, Zheng X, Chen JJ, Meng ZJ, Zheng YB, Gao YH, Qian ZP, Liu F, Lu XB, Shi Y, Shang J, Chen RC, Huang Y. sTREM-1 as promising prognostic biomarker for acute-on-chronic liver failure and mortality in patients with acute decompensation of cirrhosis. World J Gastroenterol 2024; 30(9): 1177-1188
- URL: https://www.wjgnet.com/1007-9327/full/v30/i9/1177.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i9.1177
Acute decompensation (AD) of cirrhosis is acute deterioration of pre-existing cirrhosis[1]. As the disease progresses, a severe syndrome hallmarked by intense systemic inflammatory response, multiorgan failure and high short-term mortality is observed and defined as acute-on-chronic liver failure (ACLF)[2]. The prognosis of AD with or without ACLF is significantly different. Hence, early and accurate biomarkers are required to identify AD patients with a high risk of development of ACLF and mortality, to improve the management and outcomes. Despite extensive research, few biomarkers can satisfactorily solve these challenges[3,4]. Therefore, further investigation is warranted to find novel biomarkers for accurate risk stratification of patients with AD of cirrhosis.
Infections and sepsis contribute to poor prognosis in AD patients with or without ACLF[1,5]. Triggering receptor expressed on myeloid cells-1 (TREM-1) is a pattern recognition receptor (PRR) and member of the immunoglobulin superfamily, which is mainly expressed on the surface of immune cells. TREM-1 is a crucial mediator of bacterial infection (BI) and septic shock that acts by synergizing with other PRRs to amplify the inflammatory responses[6,7]. Soluble TREM-1 (sTREM-1), the extracellular portion of TREM-1, is cleaved by metalloproteinases and released during inflammation[8]. sTREM-1 has been explored as a biomarker for the diagnosis and prognosis of BIs and sepsis[7,9-11].
Previous studies have demonstrated that sTREM-1 has high diagnostic value for sepsis in patients with ACLF[12]. In addition, sTREM-1 Levels perform well in identifying BI and predicting 90-d mortality for patients with cirrhosis[13]. The value of sTREM-1 as a biomarker in cirrhosis has been evaluated but not extensively. The potential value of sTREM-1 in AD patients is still unknown.
Against the above background, we hypothesized that sTREM-1 has the potential to serve as a biomarker of progression and prognosis in AD of cirrhosis, reflecting the risk of ACLF development. The aim of this study was to investigate the effectiveness of sTREM-1 for the prediction of ACLF development and mortality in patients with AD of cirrhosis. Soluble CD14 subtype (sCD14-ST), another biomarker of sepsis in ACLF found in our previous study[12], was also assessed for comparison.
This study was approved by the Renji Hospital Ethics Committee of Shanghai Jiao Tong University School of Medicine, No. (2014)148k and all of the patients signed written informed consent before the study.
Patients with AD of cirrhosis were recruited from a prospective multicenter cohort study, named CATCH-LIFE (NCT02457637), conducted by the Chinese Chronic Liver Failure Consortium, which comprised 15 hospitals from January 2015 to December 2017. All 442 patients were randomly divided into a study cohort (n = 309) and a validation cohort (n = 133) using EXCEL-generated random numbers. The sample size was determined based on the calculation results of the PASS 15.0 and by reference to high-quality literature on similar studies[4]. Exclusion criteria are listed in the Supplemental Methods.
Clinical characteristics, candidate indicators and outcomes were collected to develop and validate the novel ACLF risk prediction score and prognostic score for AD of cirrhosis. Serum samples were collected at admission to measure the concentrations of C-reactive protein (CRP), procalcitonin (PCT), sTREM-1 and sCD14-ST using the Luminex 200 System (Millipore). Patients’ clinical data and laboratory parameters were collected and recorded at admission (day 1) and during 28-d follow-up (on day 4, 7, 14, 21 and 28). The management of patients with AD was according to established guidelines[14]. Patients were followed-up for at least 1 year. The presence or development of ACLF was carefully evaluated at admission or during the first 28-d after enrollment.
Cirrhosis was diagnosed based on a composite of clinical signs and findings provided by imaging examination or signs of portal hypertension on endoscopy[15]. Patients with cirrhosis who had at least one AD event, including gastrointestinal bleeding, hepatic encephalopathy (HE), ascites, BI or jaundice [total bilirubin (TBil) > 5 mg/dL], within 1 month before enrollment were diagnosed with AD[2]. The diagnostic criteria of ACLF were based on the CANONIC study[16].
The model for end-stage liver disease (MELD), MELD-sodium (MELD-Na), chronic liver failure-consortium (CLIF-C) ACLF and CLIF-C AD scores were calculated as detailed in the Supplemental Methods.
Statistical analysis was performed using SPSS 26.0, MedCalc 19.0, and R 4.1.1 (https://www.r-project.org). Categorical variables, presented as numbers (percentages) in tables, were analyzed by χ2 or Fisher’s exact test. The normality assumption of continuous variables was validated using the Kolmogorov–Smirnov test. Normally distributed variables were presented as mean ± SD and compared using Student’s t test. Non-normally distributed variables were presented as median [interquartile range (IQR)] and compared using the Mann–Whitney U test. Rank correlation was analyzed by the Spearman method.
Liver transplanted patients (11 in study cohort; 6 in validation cohort) were excluded from the mortality or ACLF development analysis. Risk factors that were significantly associated with the 1-year mortality or 28-d ACLF development in univariate analysis were selected as candidate variables for the multivariate analysis. Independent prognostic factors for AD were identified by multivariate Cox regression. The proportional hazards assumption was assessed by the Schoenfeld residual test. The prognostic model of AD (P-AD) was developed utilizing multivariate Cox regression analysis according to the stepwise forward method: Likelihood ratios, with entry and removal probabilities of 0.05 and 0.10, respectively. Independent risk factors for predicting ACLF development were identified by multivariate logistic regression. The ACLF risk score (ACLF-R) was developed utilizing multivariate logistic regression analysis according to the stepwise forward method: Likelihood ratios, with entry and removal probabilities of 0.05 and 0.10, respectively.
The goodness-of-fit of the new predictive models was evaluated using the Hosmer–Lemeshow test, calibration curve and Brier score. Harrell’s C-index, area under the receiver operating curve (AUROC), and the z test (DeLong’s method) were used to assess the performance of the new models. The Kaplan–Meier method and log-rank test were used to compare the cumulative survival rates. The cut-point of the “high” and “low” group was based on Youden Index. The performance of the predictive Cox model in the validation cohort was assessed using the same statistical analysis methods applied to the derivation data. P < 0.05 was considered statistically significant.
Demographic and clinical characteristics of both study and validation cohorts are listed in Supplementary Table 1. There were no significant differences in baseline characteristics between both cohorts. Most patients were male (69%) and the most common etiology was hepatitis B virus infection (63%). About half of patients (41%) had a history of previous decompensation of cirrhosis before admission. Patients had moderate to severe hepatic impairment as reflected by a mean MELD score of 14 and CLIF-C ACLF score of 35.
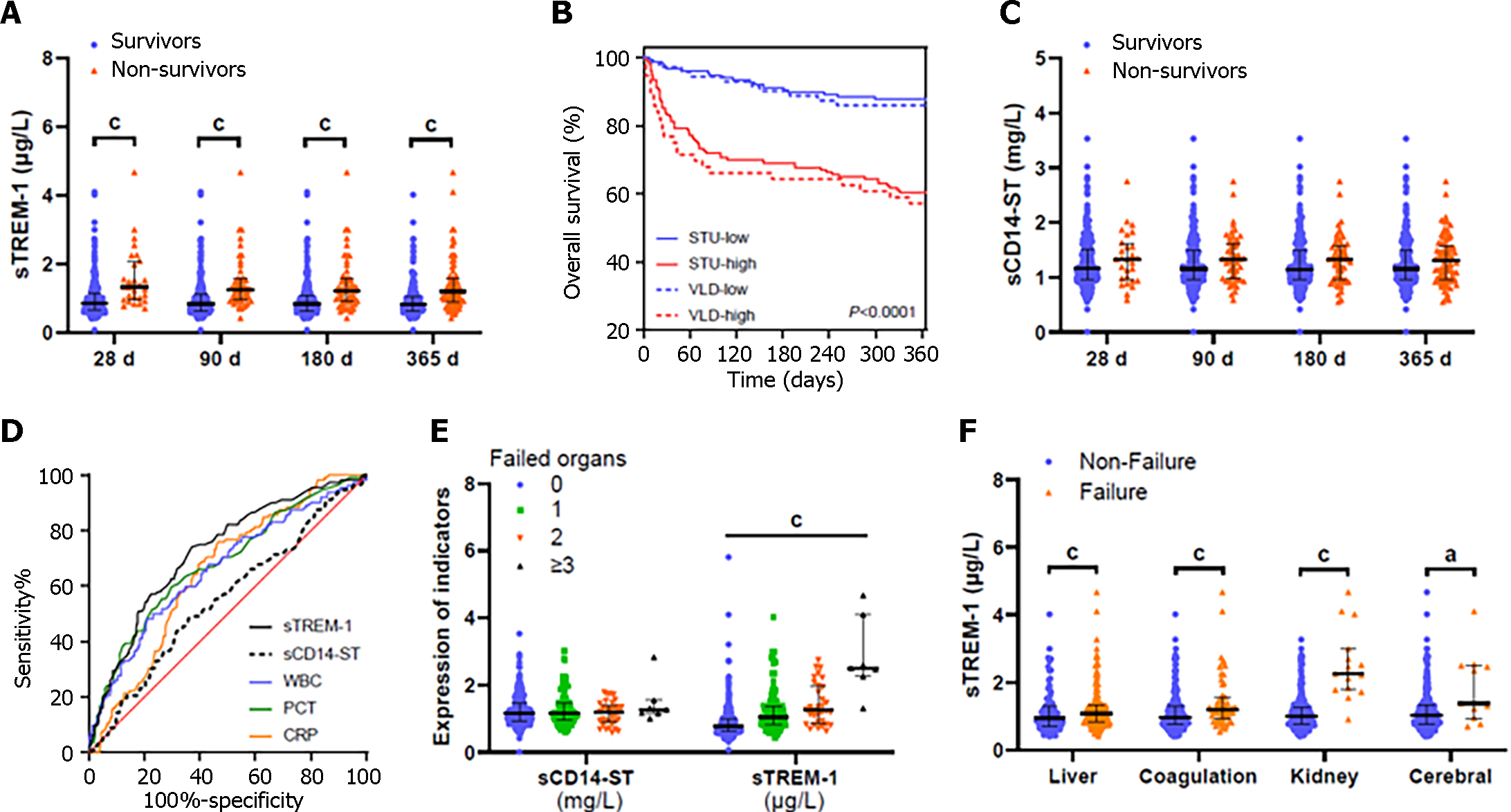
During a 1-year follow-up period, 112 (25%) patients died and 17 (4%) were transplanted. Univariate analysis of 1-year survival in the study and validation cohort are shown in Supplementary Tables 1 and 2, respectively. As expected, nonsurvivors had significantly higher levels of TBil and international normalized ratio (INR) reflecting worse liver function. The presence of organ failure (liver, kidney, coagulation and cerebral failure) was significantly more frequent in nonsurvivors. Nonsurvivors had significantly higher frequency of BIs with higher white blood cell (WBC), CRP and PCT levels. Finally, serum sTREM-1 Levels at admission were significantly higher in nonsurvivors (Figure 1A and Supplementary Figure 1A) with (Supplementary Figure 1B) or without (Supplementary Figure 1C) BI. The cumulative survival duration for patients with low sTREM-1 Levels was significantly longer than in those with high sTREM-1 Levels (Figure 1B). However, no significant difference was found between the survivors and nonsurvivors in the serum levels of sCD14-ST (Figure 1C and Supplementary Figure 1D). The AUROC of sTREM-1 for 1-year mortality was 0.724, which was significantly higher than that of sCD14-ST and CRP (AUROC = 0.551 and 0.651 respectively, both P < 0.05) and tended to be higher than those of WBC and PCT (AUROC = 0.662 and 0.679, P = 0.063 and 0.109, respectively; Figure 1D and Supplementary Table 3).
Multivariate Cox regression analyses showed that sTREM-1, TBil, INR, blood urea nitrogen (BUN) and HE grades were independent prognostic factors for AD of cirrhosis (Table 1). Therefore, we developed a prognostic model [C-index: 0.796; 95%CI: 0.745–0.846], named P-AD, based on five parameters: P-AD = 0.512 × HE + 0.288 × ln [TBil (mg/dL)] + 2.145 × ln (INR) + 0.725 × ln [BUN (mmol/L)] + 0.772 × ln [sTREM-1 (μg/L)], where HE = 0 for patients without HE; HE = 1 for patients with mild HE (grades 1 and 2); and HE = 2 for patients with severe HE (grades 3 and 4).
| Variable | Survivors1 (n = 220) | Non-survivors (n = 78) | P value | Multivariate Cox regression | |
| HR (95%CI) | P value | ||||
| Age (yr) | 51 ± 12 | 52 ± 11 | 0.692 | ||
| Sex, female | 68 (31) | 23 (30) | 0.815 | ||
| Etiology | |||||
| HBV | 149 (68) | 48 (62) | 0.321 | ||
| HCV | 17 (8) | 3 (4) | 0.239 | ||
| Alcohol | 30 (14) | 15 (19) | 0.236 | ||
| Autoimmune | 27 (12) | 10 (13) | 0.900 | ||
| Others | 15 (7) | 10 (13) | 0.100 | ||
| ACLF | 12 (6) | 28 (36) | < 0.001 | ||
| Bacterial infection | 64 (29) | 40 (51) | < 0.001 | ||
| UGIB | 40 (18) | 16 (21) | 0.651 | ||
| Ascites | 160 (73) | 64 (82) | 0.101 | ||
| HE (I-II/III-IV) | 18/3 | 12/6 | 0.003 | 1.669 (1.094-2.546) | 0.017 |
| WBC (×109/L) | 4.6 (3.1-6.0) | 6.4 (4.4-9.4) | < 0.001 | 0.627 | |
| Hb (g/L) | 111 (87-126) | 106 (80-124) | 0.132 | ||
| Platelets (×109/L) | 71 (45-113) | 60 (45-102) | 0.226 | ||
| Albumin (g/L) | 30 (27-34) | 29 (24-33) | 0.030 | 0.213 | |
| ALT (U/L) | 46 (27-120) | 77 (35-296) | 0.021 | 0.581 | |
| AST (U/L) | 64 (38-138) | 107 (62-258) | 0.001 | 0.123 | |
| TBil (mg/dL) | 3.0 (1.3-9.8) | 11.7 (4.5-25.9) | < 0.001 | 1.333 (1.053-1.688) | 0.017 |
| INR | 1.4 (1.2-1.7) | 1.9 (1.5-2.6) | < 0.001 | 8.546 (3.850-18.968) | < 0.001 |
| Creatinine (mg/dL) | 0.8 (0.6-0.9) | 0.8 (0.6-1.1) | 0.112 | ||
| BUN (mmol/L) | 4.7 (3.5-6.7) | 6.0 (3.8-10.2) | 0.003 | 2.065 (1.345-3.171) | 0.001 |
| Sodium (mmol/L) | 138 (135-140) | 135 (130-138) | < 0.001 | 0.529 | |
| CRP (mg/L) | 3.2 (1.9-4.0) | 3.9 (3.4-4.3) | < 0.001 | 0.070 | |
| PCT (μg/L) | 0.1 (0.1-0.3) | 0.3 (0.1-0.7) | < 0.001 | 0.403 | |
| sCD14-ST (mg/L) | 1.2 (1.0-1.5) | 1.3 (1.0-1.6) | 0.170 | ||
| sTREM-1 (μg/L) | 0.8 (0.6-1.1) | 1.2 (0.9-1.6) | < 0.001 | 2.163 (1.295-3.614) | 0.003 |
| Organ failure | |||||
| Liver | 43 (20) | 39 (50) | < 0.001 | ||
| Coagulation | 6 (3) | 20 (26) | < 0.001 | ||
| Cerebral | 3 (1) | 6 (8) | 0.015 | ||
| Lung | 0 (0) | 0 (0) | 1.000 | ||
| Circulation | 2 (1) | 0 (0) | 1.000 | ||
| Kidney | 3 (1) | 6 (8) | 0.015 | ||
| MELD | 11 (6-17) | 22 (11-26) | < 0.001 | ||
| MELD-Na | 11 (7-19) | 24 (17-30) | < 0.001 | ||
| CLIF-C ACLF | 33 ± 7 | 40 ± 9 | < 0.001 | ||
| CLIF-C AD | 44 ± 9 | 55 ± 12 | < 0.001 | ||
| P-AD | 2.6 (2.1-3.4) | 4.4 (3.2-5.1) | < 0.001 | ||
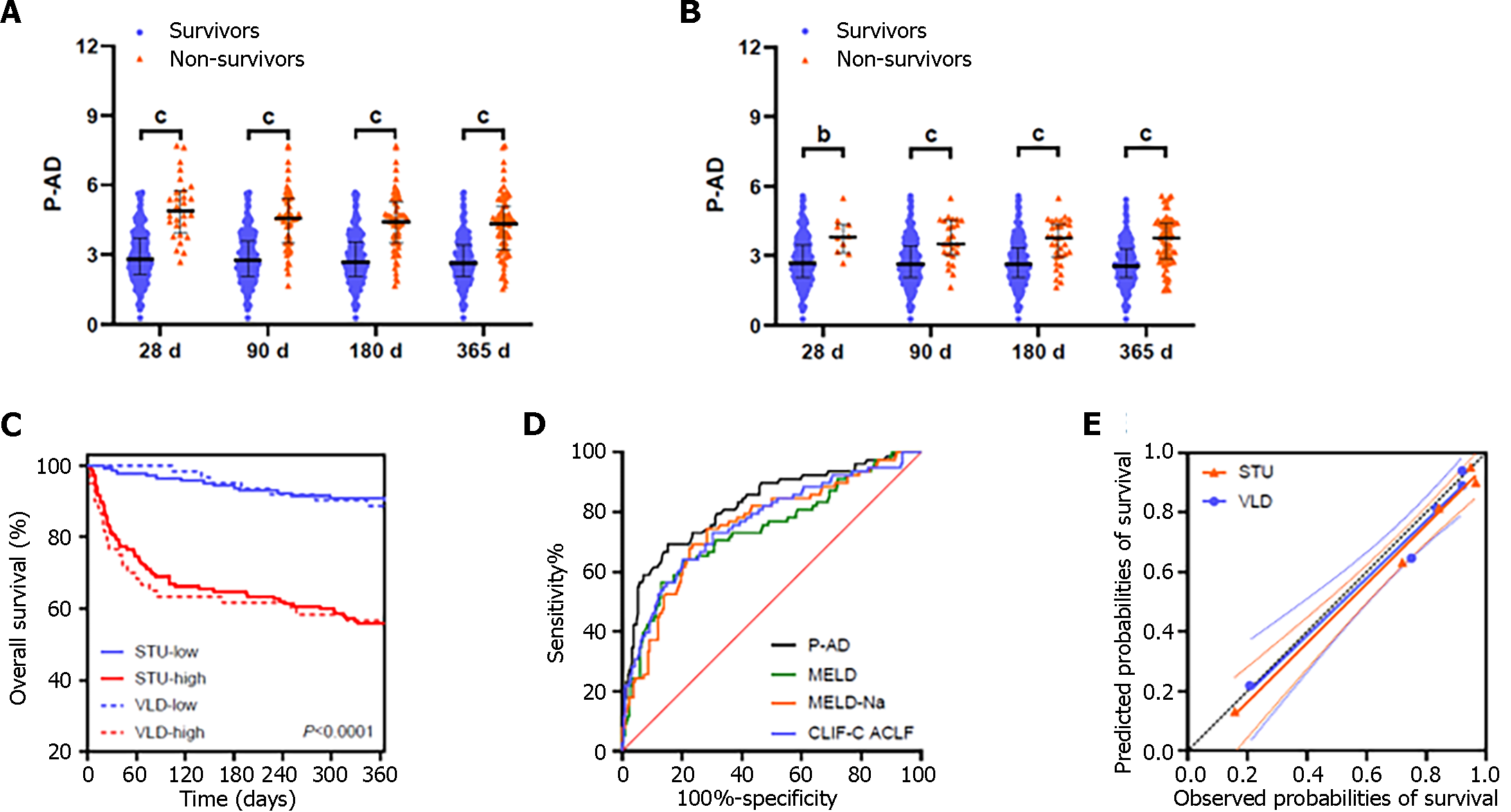
The P-AD score for the nonsurvivor group was significantly higher in the study (Figure 2A and B) and validation
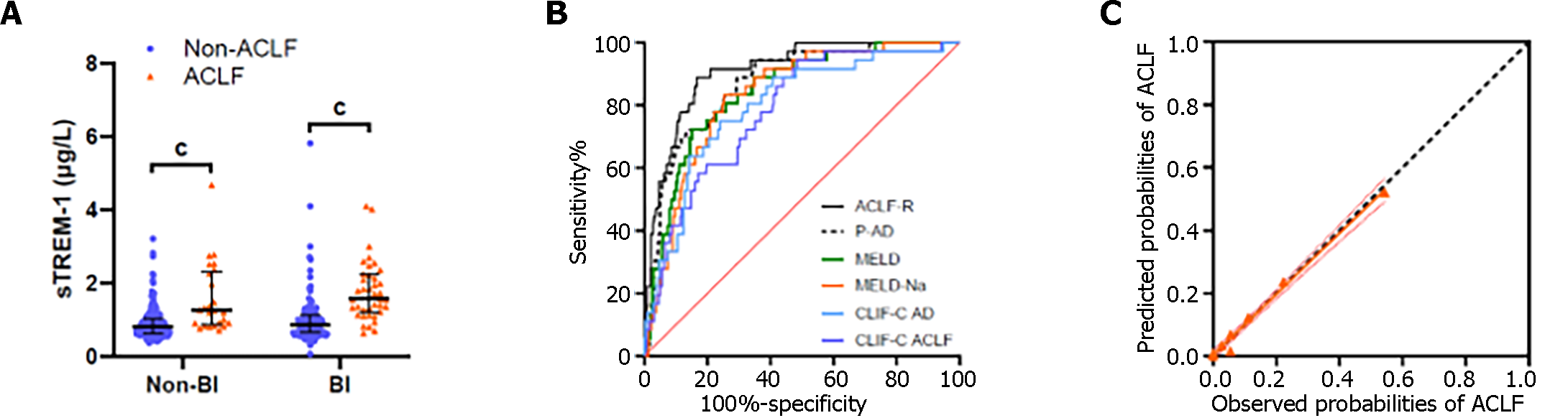
We investigated association between serum levels of sTREM-1 or sCD14-ST at admission and the presence of ACLF. Patients with ACLF had significantly higher sTREM-1 baseline levels than those without ACLF in both BI and non-BI groups (Figure 3A). sTREM-1 Levels increased with numbers of failed organs. In contrast, there was no significant difference in sCD14-ST levels whether grouped by the presence of ACLF (Supplementary Figure 3E) or by the number of failed organs (Figure 1E). Patients with liver, coagulation, kidney or cerebral failure had significantly higher sTREM-1 Levels than those without (Figure 1F). In contrast, there was no correlation between sCD14-ST and any of the aforementioned organ failures (Supplementary Figure 1E). To investigate the potential of sTREM-1 as a biomarker for ACLF development, univariate analysis of 28-d ACLF development was performed (Table 2). Patients who developed ACLF during the first 28-d follow-up had significantly higher sTREM-1 Levels at admission.
| Variable | No ACLF during1 Follow-up (n = 340) | ACLF during Follow-up (n = 36) | P value | Multivariate logistic regression | |
| RR (95%CI) | P value | ||||
| Age (yr) | 51 ± 12 | 51 ± 12 | 0.959 | ||
| Sex, female | 120 (35%) | 8 (22%) | 0.115 | ||
| Etiology | |||||
| HBV | 204 (60%) | 27 (75%) | 0.079 | ||
| HCV | 28 (8%) | 1 (3%) | 0.402 | ||
| Alcohol | 54 (16%) | 5 (14%) | 0.755 | ||
| Autoimmune | 48 (14%) | 3 (8%) | 0.479 | ||
| Others | 29 (9%) | 3 (8%) | 0.968 | ||
| Bacterial infection | 105 (31%) | 21 (58%) | 0.001 | ||
| UGIB | 71 (21%) | 4 (11%) | 0.240 | ||
| Ascites | 240 (71%) | 33 (92%) | 0.012 | 0.057 | |
| HE (I-II/III-IV) | 25/6 | 0/0 | 0.167 | ||
| WBC (×109/L) | 4.5 (3.0-6.1) | 6.3 (4.8-8.1) | < 0.001 | 0.991 | |
| Hb (g/L) | 108 (87-124) | 116 (102-126) | 0.165 | ||
| Platelets (×109/L) | 69 (47-111) | 63 (45-91) | 0.247 | ||
| Albumin (g/L) | 30 (27-34) | 30 (26-33) | 0.744 | ||
| ALT (U/L) | 46 (26-118) | 132 (67-321) | < 0.001 | 0.236 | |
| AST (U/L) | 63 (38-136) | 183 (107-326) | < 0.001 | 1.971 (1.228-3.163) | 0.005 |
| TBil (mg/dL) | 3.1 (1.4-8.9) | 16.1 (10.0-26.8) | < 0.001 | 3.151 (1.717-5.782) | < 0.001 |
| INR | 1.4 (1.2-1.7) | 1.9 (1.6-2.2) | < 0.001 | 13.841 (2.021-94.767) | 0.007 |
| Creatinine (mg/dL) | 0.7 (0.6-0.9) | 0.8 (0.6-1.0) | 0.297 | 0.929 | |
| BUN (mmol/L) | 4.6 (3.5-6.7) | 5.6 (3.5-8.2) | 0.219 | 3.302 (1.352-8.067) | 0.009 |
| Sodium (mmol/L) | 138 (135-140) | 136 (132-139) | 0.016 | 0.962 | |
| CRP (mg/L) | 3.2 (1.6-4.0) | 3.7 (3.3-4.1) | 0.006 | 0.339 | |
| PCT (μg/L) | 0.1 (0.1-0.3) | 0.4 (0.2-0.6) | < 0.001 | 0.709 | |
| sCD14-ST (mg/L) | 1.2 (1.0-1.5) | 1.2 (1.0-1.6) | 0.226 | ||
| sTREM-1 (μg/L) | 0.8 (0.6-1.0) | 1.2 (1.0-1.3) | < 0.001 | 3.023 (1.053-8.677) | 0.040 |
| Organ failure | |||||
| Liver | 58 (17%) | 21 (58%) | < 0.001 | ||
| Coagulation | 5 (2%) | 5 (14%) | < 0.001 | ||
| Cerebral | 6 (2%) | 0 (0%) | 1.000 | ||
| Lung | 0 (0%) | 0 (0%) | 1.000 | ||
| Circulation | 1 (0%) | 0 (0%) | 1.000 | ||
| Kidney | 0 (0%) | 0 (0%) | 1.000 | ||
| MELD | 11 ± 7 | 20 ± 5 | < 0.001 | ||
| MELD-Na | 12 (7-18) | 24 (20-29) | < 0.001 | ||
| CLIF-C ACLF | 33 (28-38) | 39 (35-42) | < 0.001 | ||
| CLIF-C AD | 44 ± 8 | 53 ± 8 | < 0.001 | ||
| P-AD | 2.6 (2.1-3.3) | 4.1 (3.5-4.5) | < 0.001 | ||
| ACLF-R | 6.6 (5.0-8.4) | 10.5 (9.7-11.3) | < 0.001 | ||
The multivariate analysis showed that aspartate aminotransferase (AST), TBil, INR, BUN and sTREM-1 Levels were independent factors in the occurrence of ACLF during 28-d follow-up (Table 2). Therefore, we developed an ACLF risk prediction model (Hosmer-Lemeshow test, P = 0.811), named ACLF-R, based on: ACLF-R = 1.210 × ln [TBil (mg/dL)] + 0.565 × ln [AST (U/L)] + 3.132 × ln (INR) + 1.131 × ln [BUN (mmol/L)] + 1.237 × ln [sTREM-1 (μg/L)].
The ACLF-R score for patients who developed ACLF during 28-d follow-up was significantly higher than those who did not (Table 2). The ACLF-R score showed greater predictive power for AD patients’ 28-d ACLF development than the MELD, MELD-Na, CLIF-C ACLF, CLIF-C AD and P-AD scores (Figure 3B and Table 3). The calibration curve had a good fit between the predicted and observed probabilities of ACLF development (Figure 3C). The estimated intercept (95%CI) and slope (95%CI) were 0.003 (-0.012 to 0.018) and 0.968 (0.889–1.046), and no significant difference from a perfect fit was found (P = 0.672 and 0.418 for intercept and slope, respectively). The Bier score for the ACLF-R was 0.062.
The development of simple and accurate predictive models permitting the early recognition of individuals with high risk of development of ACLF and mortality could enhance the triage, management, and prognosis of AD patients. In this study, we assessed the performance of sTREM-1 as a disease progression and prognostic biomarker in AD patients and further constructed P-AD and ACLF-R score based on sTREM-1 and four other clinical parameters. Serum sTREM-1 Levels at admission were independently associated with 1-year mortality and 28-d ACLF development in patients with AD of cirrhosis. P-AD and ACLF-R scores outperformed the MELD, MELD-Na, CLIF-C ACLF and CLIF-C AD scores in a multicenter prospective cohort.
Because the pathogenic mechanism of AD, especially ACLF, is complex and involves multiple systems[1,2], a comprehensive prognostic model with indicators reflecting multiple pathological processes is required to forecast the prognosis of AD. The MELD score is an accurate predictor of survival in patients with advanced liver disease and used in organ allocation for liver transplantation[17]. MELD-Na, a model incorporating serum sodium into MELD, has been demonstrated to provide more accurate mortality prediction than MELD[18]. Therefore, MELD-Na is the most widely used model for organ allocation in patients listed for liver transplantation. Recent studies showed that patients with cirrhosis with persistently low MELD-Na scores still experienced high rates of liver-related mortality[19]. Patients with ACLF are at a mortality disadvantage in the current MELD-Na based system because MELD-Na does not capture 90-d mortality risk in ACLF[20]. Organ failure based scores such as CLIF-C ACLF perform better than MELD based assessment in predicting waiting list mortality among ACLF patients[16,21]. The CLIF-C AD score was developed for AD patients without ACLF[1]. A recent study showed that even in the low-risk group (CLIF-C ADs ≤ 45), the 90-d mortality was as high as 10%[22]. Therefore, it is necessary to enhance prognosis stratification in patients with AD. The present study demonstrated that serum sTREM-1 Levels could act as independent predictor of 1-year mortality in patients with AD of cirrhosis.
sTREM-1 is increased in ACLF patients with sepsis[12] and cirrhotic patients with BI[13]. sTREM-1 is also increased and involved in noninfectious inflammatory diseases such as relapsing polychondritis[23] and adult-onset Still’s disease[24]. Infections and sepsis contribute to poor prognosis in AD patients with or without ACLF[1,5]. Systemic circulatory dysfunction and systemic inflammation are typical pathologies of AD and further exacerbated in ACLF[25]. Therefore, it can be speculated that there are two major theories supporting sTREM-1 as a prognostic biomarker in AD. First, sTREM-1 Levels may reflect BIs and/or sepsis in patients with AD of cirrhosis. Second, sTREM-1 Levels may reflect the severity of systemic inflammatory in AD. Moreover, we developed and validated the P-AD score, based on sTREM-1 and four other parameters (TBil, INR, BUN and HE grade). P-AD score had a better prognostic capability than MELD, MELD-Na, CLIF-C ACLF, and CLIF-C AD scores. Therefore, the P-AD score enables decision-making regarding the allocation of intensive care resources and priorities of liver transplantation for patients with AD of cirrhosis.
TREM-1 pathways contribute to the pathology of infectious diseases[6-11] and noninfectious inflammatory diseases[23,24]. sTREM-1 is a specific biomarker of TREM-1 pathway activation[26]. BI is one of the most common triggers for inflammation promoting ACLF development[2,27]. It can induce inflammation via two classes of molecules: Pathogen-associated molecular patterns and virulence factors. Both molecules are recognized by PRRs and result in the production of inflammatory molecules. The inflammatory response to bacteria can be excessive and cause tissue damage, releasing damage-associated molecular patterns (DAMPs). DAMP recognition by PRRs accentuates inflammation and leads to ACLF via a storm of inflammatory cytokines[28]. These findings suggest that systemic inflammation can drive ACLF. sTREM-1, a PRR that amplifies inflammatory responses by synergizing with other PRRs, could help define signatures to improve our understanding of inflammation in ACLF and inform preventative strategies. Our findings are consistent with these results and support the hypothesis that increased sTREM-1 Levels may be involved in and reflect the activation of inflammatory pathways occurring in ACLF.
So far, there is a paucity of commonly accepted and verified biomarkers or models to assess the probability of ACLF development. This study revealed that sTREM-1 is significantly increased in ACLF and correlated with failure of critical organs, including liver, brain and kidney, as well as coagulation. Further results revealed that for AD patients without ACLF at admission, sTREM-1 could be a useful biomarker to assess the risk of ACLF development. We developed and validated the ACLF-R score, based on sTREM-1 and four other parameters (TBil, AST, INR and BUN), which have better predictive capability in ACLF developing than MELD, MELD-Na, CLIF-C ACLF, CLIF-C AD, and P-AD scores.
There were some strengths and limitations to the present study. First, this investigation was based on a large multicenter prospective cohort of patients with cirrhosis hospitalized for AD. Second, results obtained from the study cohort were validated by the validation cohort. However, since the number of AD patients who progressed to ACLF during the 28-d follow-up was limited, our results for sTREM-1 and ACLF-R predicting ACLF development need to be validated in another independent multicenter prospective cohort. Finally, the design of this investigation did not involve a mechanistic demonstration about the role of sTREM-1 in the pathophysiology of ACLF. Therefore, the mechanistic hypothesis suggested by current results needs to be investigated in future studies.
Serum sTREM-1 is a promising biomarker to predict development of ACLF and mortality in patients with AD of cirrhosis. The P-AD and ACLF-R score were superior to currently available prognostic models in predicting mortality and development of ACLF.
Soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) is a biomarker of inflammatory diseases such as liver injury, and is released from activated innate immune cells.
Acute decompensation (AD) of cirrhosis is associated with high short-term mortality, mainly due to the development of acute-on-chronic liver failure (ACLF). Thus, accurate biomarkers are required to identify AD patients with high risk of ACLF development and mortality.
This study aimed to explore the prognostic value of sTREM-1 in patients with AD of cirrhosis, and to construct more effective prognostic models for improving patient clinical management and prognosis.
We collected data and samples from 442 hospitalized patients with AD of cirrhosis from a multicenter prospective cohort including 15 liver units in 10 provinces of China. Prognostic model of AD (P-AD) and ACLF risk score (ACLF-R) were established based on independent factors associated with mortality or ACLF development, and compared with widely used scores, such as model for end-stage liver disease (MELD), MELD-sodium (MELD-Na), chronic liver failure-consortium (CLIF-C) ACLF and CLIF-C AD.
Serum sTREM-1 Level was associated with 1-year mortality and correlated with organ failure, including liver, coagulation, kidney, and cerebral failures, in patients with AD of cirrhosis. Serum sTREM-1 was related to the development of ACLF during 28-d follow-up. P-AD and ACLF-R scores were significantly superior to MELD, MELD-Na, CLIF-C ACLF and CLIF-C AD.
Serum sTREM-1 Level, P-AD and ACLF-R score will facilitate clinical decision-making in the management of patients with AD of cirrhosis.
A new approach to early diagnosis and treatment of patients with AD of cirrhosis.
We thank the faculty and nurses contributed to this study, the patients who participated in the study and their families.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Shabrawi MHF, Egypt S-Editor: Li L L-Editor: A P-Editor: Chen YX
| 1. | Jalan R, Pavesi M, Saliba F, Amorós A, Fernandez J, Holland-Fischer P, Sawhney R, Mookerjee R, Caraceni P, Moreau R, Ginès P, Durand F, Angeli P, Alessandria C, Laleman W, Trebicka J, Samuel D, Zeuzem S, Gustot T, Gerbes AL, Wendon J, Bernardi M, Arroyo V; CANONIC Study Investigators; EASL-CLIF Consortium. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol. 2015;62:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 256] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 2. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2167] [Article Influence: 180.6] [Reference Citation Analysis (5)] |
| 3. | Macdonald S, Andreola F, Bachtiger P, Amoros A, Pavesi M, Mookerjee R, Zheng YB, Gronbaek H, Gerbes AL, Sola E, Caraceni P, Moreau R, Gines P, Arroyo V, Jalan R. Cell death markers in patients with cirrhosis and acute decompensation. Hepatology. 2018;67:989-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Juanola A, Graupera I, Elia C, Piano S, Solé C, Carol M, Pérez-Guasch M, Bassegoda O, Escudé L, Rubio AB, Cervera M, Napoleone L, Avitabile E, Ma AT, Fabrellas N, Pose E, Morales-Ruiz M, Jiménez W, Torres F, Crespo G, Solà E, Ginès P. Urinary L-FABP is a promising prognostic biomarker of ACLF and mortality in patients with decompensated cirrhosis. J Hepatol. 2022;76:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Fernández J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, Reverter E, Martínez J, Saliba F, Jalan R, Welzel T, Pavesi M, Hernández-Tejero M, Ginès P, Arroyo V; European Foundation for the Study of Chronic Liver Failure. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67:1870-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 396] [Article Influence: 56.6] [Reference Citation Analysis (2)] |
| 6. | Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 811] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 7. | Matos AO, Dantas PHDS, Silva-Sales M, Sales-Campos H. TREM-1 isoforms in bacterial infections: to immune modulation and beyond. Crit Rev Microbiol. 2021;47:290-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Weiss G, Lai C, Fife ME, Grabiec AM, Tildy B, Snelgrove RJ, Xin G, Lloyd CM, Hussell T. Reversal of TREM-1 ectodomain shedding and improved bacterial clearance by intranasal metalloproteinase inhibitors. Mucosal Immunol. 2017;10:1021-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Wu Y, Wang F, Fan X, Bao R, Bo L, Li J, Deng X. Accuracy of plasma sTREM-1 for sepsis diagnosis in systemic inflammatory patients: a systematic review and meta-analysis. Crit Care. 2012;16:R229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Phua J, Koay ES, Zhang D, Tai LK, Boo XL, Lim KC, Lim TK. Soluble triggering receptor expressed on myeloid cells-1 in acute respiratory infections. Eur Respir J. 2006;28:695-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Al-Asy HM, Gamal RM, Albaset AMA, Elsanosy MG, Mabrouk MM. New diagnostic biomarker in acute diarrhea due to bacterial infection in children. Int J Pediatr Adolesc Med. 2017;4:75-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Chen J, Huang ZB, Li H, Zheng X, Chen JJ, Wang XB, Qian ZP, Liu XX, Fan XG, Hu XW, Liao CJ, Long LY, Huang Y. Early Diagnostic Biomarkers of Sepsis for Patients with Acute-on-Chronic Liver Failure: A Multicenter Study. Infect Dis Ther. 2021;10:281-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Tornai D, Vitalis Z, Jonas A, Janka T, Foldi I, Tornai T, Sipeki N, Csillag A, Balogh B, Sumegi A, Foldesi R, Papp M, Antal-Szalmas P. Increased sTREM-1 Levels identify cirrhotic patients with bacterial infection and predict their 90-day mortality. Clin Res Hepatol Gastroenterol. 2021;45:101579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1812] [Article Influence: 258.9] [Reference Citation Analysis (2)] |
| 15. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 846] [Article Influence: 211.5] [Reference Citation Analysis (1)] |
| 16. | Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P, Hopf C, Alessandria C, Rodriguez E, Solis-Muñoz P, Laleman W, Trebicka J, Zeuzem S, Gustot T, Mookerjee R, Elkrief L, Soriano G, Cordoba J, Morando F, Gerbes A, Agarwal B, Samuel D, Bernardi M, Arroyo V; CANONIC study investigators of the EASL-CLIF Consortium. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 733] [Article Influence: 66.6] [Reference Citation Analysis (1)] |
| 17. | Kamath PS, Kim WR; Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1225] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 18. | Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, Benson J, Therneau T, Kremers W, Wiesner R, Kamath P, Klintmalm G. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 547] [Article Influence: 28.8] [Reference Citation Analysis (1)] |
| 19. | Mazumder NR, Atiemo K, Daud A, Kho A, Abecassis M, Levitsky J, Ladner DP. Patients With Persistently Low MELD-Na Scores Continue to Be at Risk of Liver-related Death. Transplantation. 2020;104:1413-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Hernaez R, Liu Y, Kramer JR, Rana A, El-Serag HB, Kanwal F. Model for end-stage liver disease-sodium underestimates 90-day mortality risk in patients with acute-on-chronic liver failure. J Hepatol. 2020;73:1425-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 21. | Abdallah MA, Kuo YF, Asrani S, Wong RJ, Ahmed A, Kwo P, Terrault N, Kamath PS, Jalan R, Singal AK. Validating a novel score based on interaction between ACLF grade and MELD score to predict waitlist mortality. J Hepatol. 2021;74:1355-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Baldin C, Piedade J, Guimarães L, Victor L, Duarte J, Veiga Z, Alcântara C, Fernandes F, Pereira JL, Pereira G. CLIF-C AD Score Predicts Development of Acute Decompensations and Survival in Hospitalized Cirrhotic Patients. Dig Dis Sci. 2021;66:4525-4535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Arnaud L, Mathian A, Haroche J, Gorochov G, Amoura Z. Pathogenesis of relapsing polychondritis: a 2013 update. Autoimmun Rev. 2014;13:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Wang Z, Chi H, Sun Y, Teng J, Feng T, Liu H, Cheng X, Ye J, Shi H, Hu Q, Jia J, Liu T, Wan L, Zhou Z, Qiao X, Yang C, Su Y. Serum sTREM-1 in adult-onset Still's disease: a novel biomarker of disease activity and a potential predictor of the chronic course. Rheumatology (Oxford). 2020;59:3293-3302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, Morales-Ruiz M, Angeli P, Domenicali M, Alessandria C, Gerbes A, Wendon J, Nevens F, Trebicka J, Laleman W, Saliba F, Welzel TM, Albillos A, Gustot T, Benten D, Durand F, Ginès P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 555] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 26. | Jolly L, Carrasco K, Salcedo-Magguilli M, Garaud JJ, Lambden S, van der Poll T, Mebazaa A, Laterre PF, Gibot S, Boufenzer A, Derive M. sTREM-1 is a specific biomarker of TREM-1 pathway activation. Cell Mol Immunol. 2021;18:2054-2056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Arroyo V, Moreau R, Kamath PS, Jalan R, Ginès P, Nevens F, Fernández J, To U, García-Tsao G, Schnabl B. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers. 2016;2:16041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 310] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 28. | Engelmann C, Zhang IW, Clària J. Mechanisms of immunity in acutely decompensated cirrhosis and acute-on-chronic liver failure. Liver Int. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |