Published online Feb 7, 2024. doi: 10.3748/wjg.v30.i5.485
Peer-review started: November 21, 2023
First decision: December 8, 2023
Revised: December 12, 2023
Accepted: January 11, 2024
Article in press: January 11, 2024
Published online: February 7, 2024
Processing time: 70 Days and 22.6 Hours
Gastric cancer (GC) is associated with high mortality rates. Bile acids (BAs) reflux is a well-known risk factor for GC, but the specific mechanism remains unclear. During GC development in both humans and animals, BAs serve as signaling molecules that induce metabolic reprogramming. This confers additional cancer phenotypes, including ferroptosis sensitivity. Ferroptosis is a novel mode of cell death characterized by lipid peroxidation that contributes universally to malig
To reveal the mechanism of BAs regulation in ferroptosis of GC cells.
In this study, we treated GC cells with various stimuli and evaluated the effect of BAs on the sensitivity to ferroptosis. We used gain and loss of function assays to examine the impacts of farnesoid X receptor (FXR) and BTB and CNC homology 1 (BACH1) overexpression and knockdown to obtain further insights into the molecular mechanism involved.
Our data suggested that BAs could reverse erastin-induced ferroptosis in GC cells. This effect correlated with increased glutathione (GSH) concentrations, a reduced GSH to oxidized GSH ratio, and higher GSH peroxidase 4 (GPX4) expression levels. Subsequently, we confirmed that BAs exerted these effects by activating FXR, which markedly increased the expression of GSH synthetase and GPX4. Notably, BACH1 was detected as an essential intermediate molecule in the promotion of GSH synthesis by BAs and FXR. Finally, our results suggested that FXR could significantly promote GC cell proliferation, which may be closely related to its anti-ferroptosis effect.
This study revealed for the first time that BAs could inhibit ferroptosis sensitivity through the FXR-BACH1-GSH-GPX4 axis in GC cells. This work provided new insights into the mechanism associated with BA-mediated promotion of GC and may help identify potential therapeutic targets for GC patients with BAs reflux.
Core Tip: Gastric cancer (GC) is the fifth most common cancer worldwide and the third leading cause of cancer-related deaths. Bile acids (BAs) reflux is an essential carcinogenic factor in GC, but its role has not been absolutely elaborated. BAs could serve as signaling molecules to regulate the metabolic state in cells, which is closely related to ferroptosis. In the present experiment, we explored the role of BAs in the regulation of ferroptosis in GC cells. Our data suggested that BAs could significantly inhibit the ferroptosis sensitivity of GC cells and that this effect was exerted through the activation of the farnesoid X receptor-BTB and CNC homology 1-glutathione (GSH)-GSH peroxidase 4 axis.
- Citation: Liu CX, Gao Y, Xu XF, Jin X, Zhang Y, Xu Q, Ding HX, Li BJ, Du FK, Li LC, Zhong MW, Zhu JK, Zhang GY. Bile acids inhibit ferroptosis sensitivity through activating farnesoid X receptor in gastric cancer cells. World J Gastroenterol 2024; 30(5): 485-498
- URL: https://www.wjgnet.com/1007-9327/full/v30/i5/485.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i5.485
Gastric cancer (GC) is the fifth most common cancer worldwide and the third leading cause of cancer-related deaths because of the difficulties associated with early diagnosis [1]. Along with the improvement of life conditions, there is a noticeable decrease in the prevalence of Helicobacter pylori infection, which is the major causative factor of GC[2]. Bile acids (BAs) reflux, another etiologic factor for developing GC, is receiving more attentions[3]. BAs are cholesterol-derived sterols. These small-molecule metabolites play essential roles in the human body. They are amphiphilic and can thus participate in cholesterol absorption and secretion in the intestines[4]. Previous work has shown that BAs reflux is an independent risk factor for precancerous gastric lesions and gastric carcinogenesis[5,6]. For example, gastric mucosal damage can be induced by BAs through activation of the IL-6/JAK1/STAT3 pathway[5]. However, the mechanism by which BAs can promote GC progression remains unknown.
By activating BAs receptors, BAs can modulate immune responses, gastrointestinal mucosal barrier function, gestation, metabolic diseases, and carcinogenesis[7-10]. The farnesoid X receptor (FXR, NR1H4) is a typical BA receptor that has been well investigated. Cholic acid (CA) and chenodeoxycholic acid (CDCA) are the two predominant BAs in the human body[11], the latter of which is the most potent physiologic agonist of FXR[12]. FXR activation can remodel the metabolic state of cells, including glucose metabolism and lipid metabolism, which in turn is involved in the development of a variety of metabolic diseases and cancers, such as hepatocellular carcinoma[13]. However, further research on the role of FXR in GC patients with BAs reflux is required.
An altered metabolic state, also known as metabolic reprogramming, is a vital factor in cancer progression[14]. Ferroptosis, which is closely related to metabolism, may be involved in the effects of BAs and FXR in GC[15]. Ferroptosis is a novel type of cell death characterized by intracellular phospholipid peroxidation, distinct from apoptosis, pyroptosis, necroptosis, and autophagy[16,17]. This unique mode of cell death is regulated by a variety of factors, particularly oxidative stress. Glutathione (GSH) peroxidase 4 (GPX4) specifically recognizes peroxidized lipids and scavenges them by converting reduced GSH to oxidized GSH (GSSG) for anti-ferroptosis[18,19]. Therefore, GSH, as the substrate of GPX4, also has a key role in the resistance to ferroptosis. Changes in GSH metabolism will eventually lead to alterations in cellular sensitivity to ferroptosis[20]. Although ferroptosis has been reported in GC development and treatment[21,22], few studies have described ferroptosis in GC with BAs reflux.
In the present study, we investigated the role of BAs, especially CDCA, in the regulation of ferroptosis sensitivity in GC. We subsequently identified the specific receptors for these BAs and further investigated the molecular mechanism.
CA (S3742), dehydrocholic acid (DCA, S4562), CDCA (S1843), erastin (S7242), Ferrostatin-1 (Fer-1, S7243), and GW4064 (S2782) were purchased from Selleck Chemicals (Houston, TX, United States). RSL3 (HY-100218A) was purchased from MedChemExpress (Monmouth Junction, NJ, United States). Anti-GPX4 (67763-1-Ig, 1:2500), anti-β-actin (HRP-66009, 1:5000), anti-FXR (25055-1-Ig, 1:1000), anti-GCLC (12601-1-AP, 1:4000), anti-GCLM (14241-1-AP, 1:4000), anti-GSS (67598-1-Ig, 1:4000), and anti-BTB and CNC homology 1 (BACH1, 14018-1-AP, 1:5000) antibodies were purchased from Proteintech (Wuhan, China).
HGC-27 and MKN-45 cells were purchased from Procell (Wuhan, China) and cultured in MEM (for HGC-27) and RPMI-1640 (for MKN-45) medium (Gibco, Carlsbad, CA, United States) containing 10% fetal bovine serum (FBS; Gibco) and 1% Penicillin/Streptomycin (Gibco) at 37°C and 5% CO2. The cell lines were correctly identified by short tandem repeat (STR) analysis and periodically tested for mycoplasma.
Cells were seeded in 6-well plates (5 × 105 cells/well) and incubated for 18 h. Then, overexpression or short hairpin RNA plasmids for the indicated genes were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, United States) for 48 h according to the manufacturer’s instructions.
Cells were seeded in 96-well plates (5000 cells/well) in complete medium. After incubation for 18 h, the indicated treatments were added to the cells and incubated for certain times. Then, 100 mL complete medium containing 10 mL cell count kit-8 reagent (CK04, Dojindo Laboratories, Kumamoto, Japan) was added to each well. After incubating the cells for 2 h, the absorbance value for each well was colorimetrically measured at a wavelength of 450 nm.
The cells were collected after indicated stimuli. The GSH concentrations and GSH/GSSG ratio were quantified using the GSSG/GSH Quantification Kit (G263, Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s instructions. The results were quantified colorimetrically at a wavelength of 405 nm.
After the indicated treatments, the cells were collected and assayed using the Malondialdehyde (MDA) Assay Kit (S0131S, Beyotime, Shanghai, China) following the manufacturer’s instructions to measure the levels of MDA. The results were quantified colorimetrically at a wavelength of 532 nm.
After the indicated treatments, BODIPY-589/591 C11 (D3861, Thermo Fisher Scientific, Waltham, MA, USA) was added to each well (10 mM). After incubated at 37°C for 30 min, the cells were washed with PBS for three times. Subsequently, the nuclei were stained with DAPI (C1002, Beyotime) for 30 min at room temperature. Finally, the lipid reactive oxygen species was observed under a fluorescence microscope with 488 nm excitation.
Cell proliferation rates under different treatment conditions were assessed using 5-ethynyl-2’-deoxyuridine (EdU) assays (Beyotime) according to the manufacturer’s instructions.
Cells were seeded in 6-well plates (500 cells/well), treated with various stimuli, and incubated for 10 to 14 d. The cells were then rinsed three times with PBS and fixed with 4% paraformaldehyde at room temperature for 30 min. Subsequently, the fixed cells were treated with crystal violet at 4°C overnight.
Cells were lysed using RIPA buffer containing 1% Phenylmethanesulfonyl fluoride (PMSF, ST505, Beyotime, Shanghai, China) and 2% phosphatase inhibitor. The total protein concentration was quantified using the Bicinchoninic Acid Protein Assay Kit (ST505, Thermo Fisher Scientific, Waltham, MA, United States). Next, protein samples (30 mg) were separated using 10% SDS-PAGE (PG212, EpiZyme, Shanghai, China). Then, the proteins were transferred to PVDF membranes, followed by blocking with 5% BSA (A8020, Solarbio, Beijing, China) at room temperature for 1 h. Afterwards, the membranes were incubated with primary antibodies at 4°C overnight. Subsequently, the membranes were washed with PBST and incubated with a goat anti-mouse or goat anti-rabbit secondary antibody for 1 h at room temperature. Finally, the protein bands were visualized with ECL (Millipore) and quantified with ImageJ software (National Institutes of Health) the manufacturer’s instructions.
SPSS 22.0 software (Chicago, IL, United States) was used for data analysis. GraphPad Prism 8.0 (San Diego, CA, United States) software was used to create the images. Data are presented as mean ± SD. One-way ANOVA was used to compare the differences between groups. A P value of less than 0.05 indicated statistical significance.
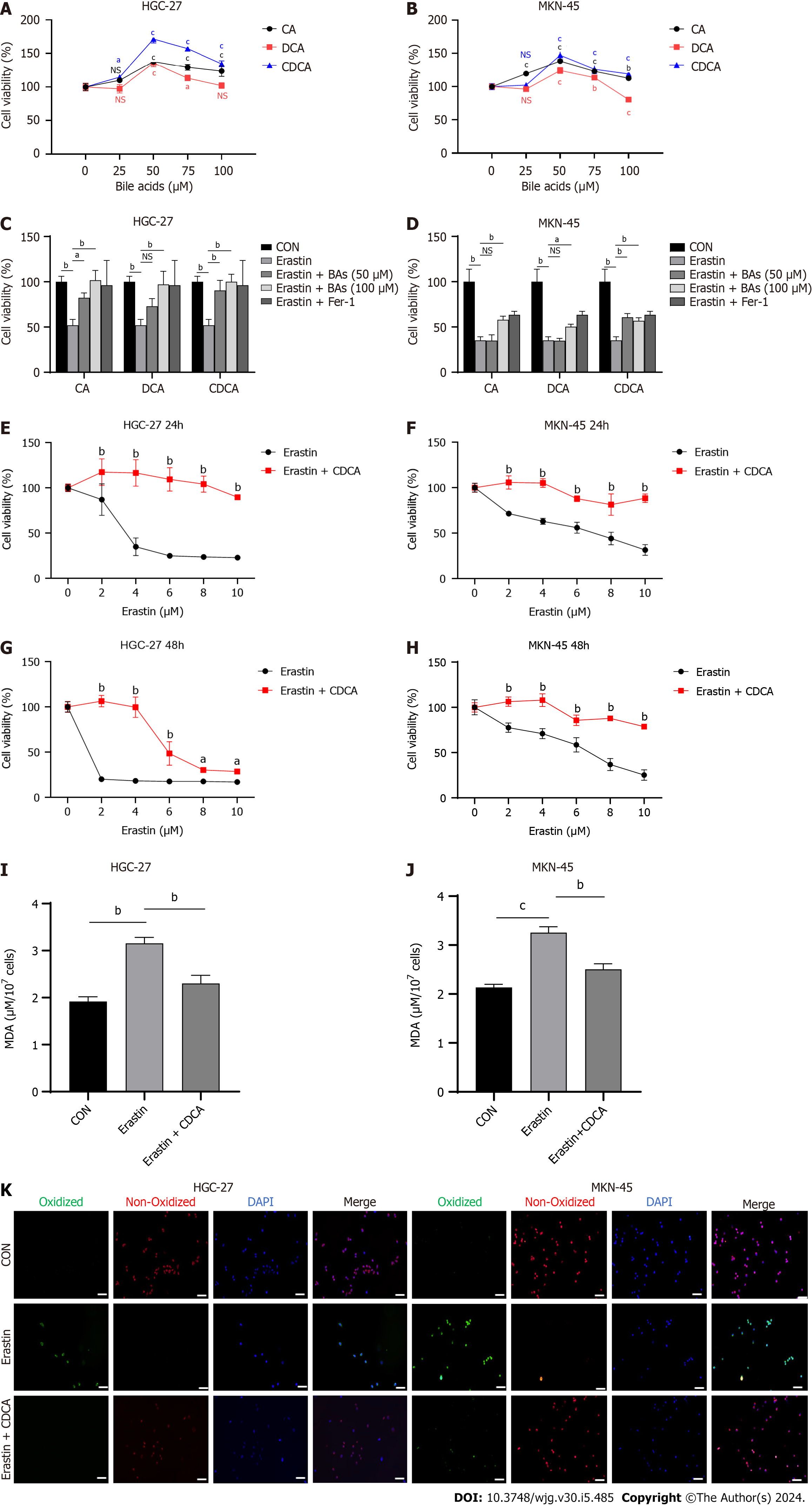
It has been shown that BAs tend to induce gastric intestinal metaplasia prior to causing GC[23]. Thus, two GC cell lines, HGC-27 and MKN-45, were chosen because they were both classified as intestinal type GC cells[24]. Three common BAs including CA, DCA, and CDCA, were chosen to stimulate GC cells in vitro. The cell viability assay results suggested that these BAs could significantly promote GC cell proliferation rates, especilly CDCA (Figure 1A and B). Subsequently, to investigate if they could modulate ferroptosis in GC cells, we examined the effects of the three BAs on HGC-27 and MKN-45 cell sensitivity to erastin, a classical inducer of ferroptosis. Interestingly, the GC cells treated with BAs exhibited higher viabilities compared with the controls, suggesting that the BAs possibly could support resistance to the ferroptosis induced by erastin (Figure 1C and D). Because it was the most effective BAs proved by above results and in previous study[23], CDCA was chosen in subsequent experiments. We then examined the effect of CDCA on the sensitivity to erastin-induced ferroptosis in GC cells at 24 and 48 h, respectively. The anti-ferroptosis effect was confirmed (Figure 1E-H). To exclude interference from other types of cell death, we performed the MDA assays (Figure 1I and J) and BODIPY-589/591 C11 staining (Figure 1K), which directly reflected ferroptosis and reconfirmed the anti-ferroptosis function of the BAs.
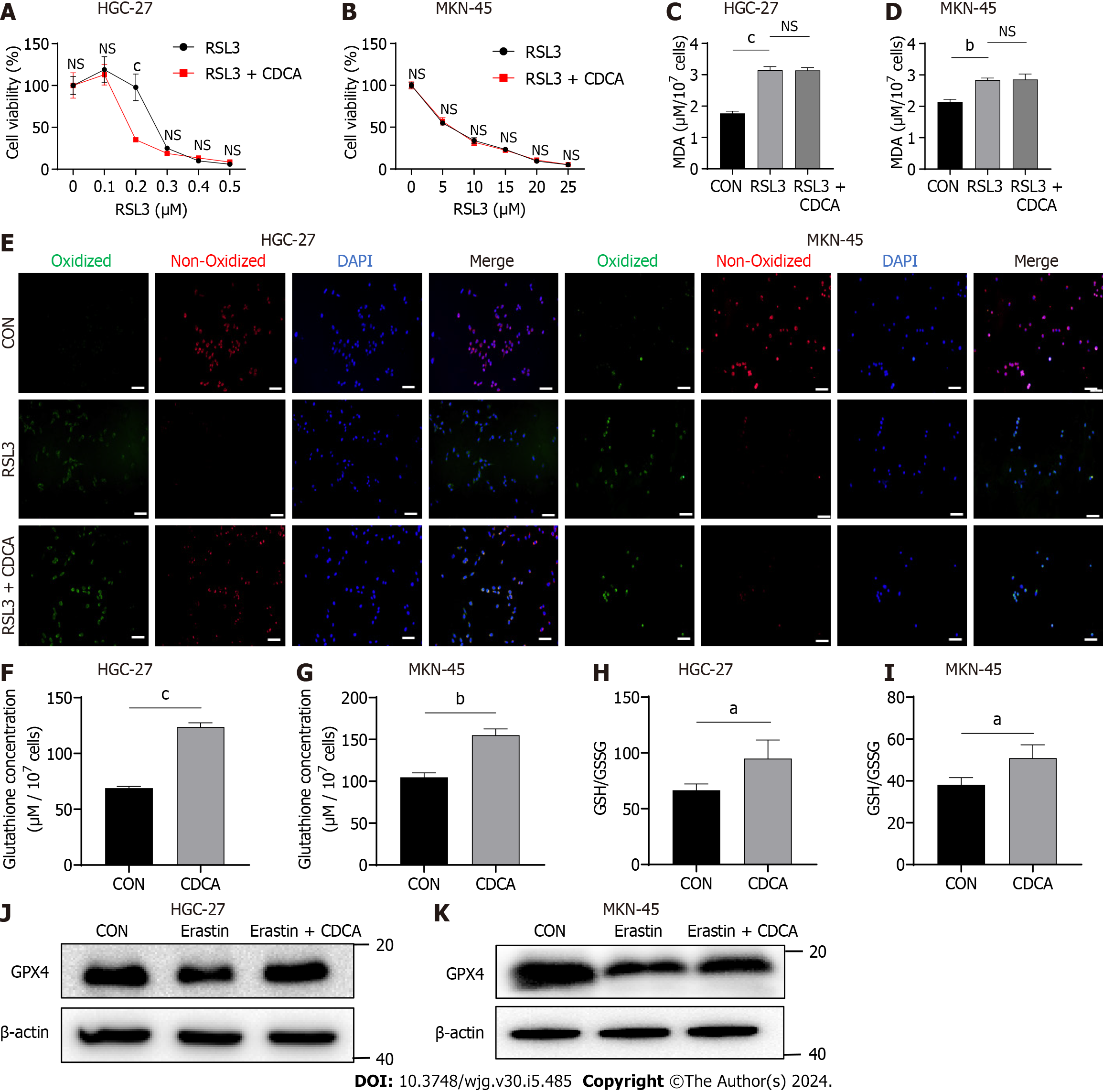
The cystine-glumate antiporter (xCT) is an essential anti-ferroptosis protein located on the cytomembrane that exchanges intracellular glutamate for extracellular cystine in a 1:1 ratio[25,26]. Mechanistically, erastin induces ferroptosis by acting on xCT and inhibiting its function. This thereby downregulates the levels of downstream GSH and GPX4, which inhibit the onset of ferroptosis[27]. Additionally, another classical ferroptosis inducer is RSL3, which targets and inactivates GPX4[28]. Therefore, to explore the anti-ferroptosis mechanism of CDCA, we examined its effect on RSL3-induced cell death using cell viability assays. Interestingly, CDCA did not ameliorate RSL3-induced GC cell death (Figure 2A and B), nor could it ameliorate the ferroptosis caused by RSL3 (Figure 2C-E). We therefore speculated that CDCA possibly exerted its anti-ferroptosis effect by upregulating GSH and GPX4 levels. To verify this hypothesis, we examined the GSH concentrations and GSH/GSSG ratio in CDCA-treated cells, finding that CDCA treatment significantly increased them compared with the control group (Figure 2F-I). Besides, CDCA also significantly attenuated the GPX4 protein expression downregulation induced by erastin, as seen with Western blot (WB) analysis (Figure 2J and K).
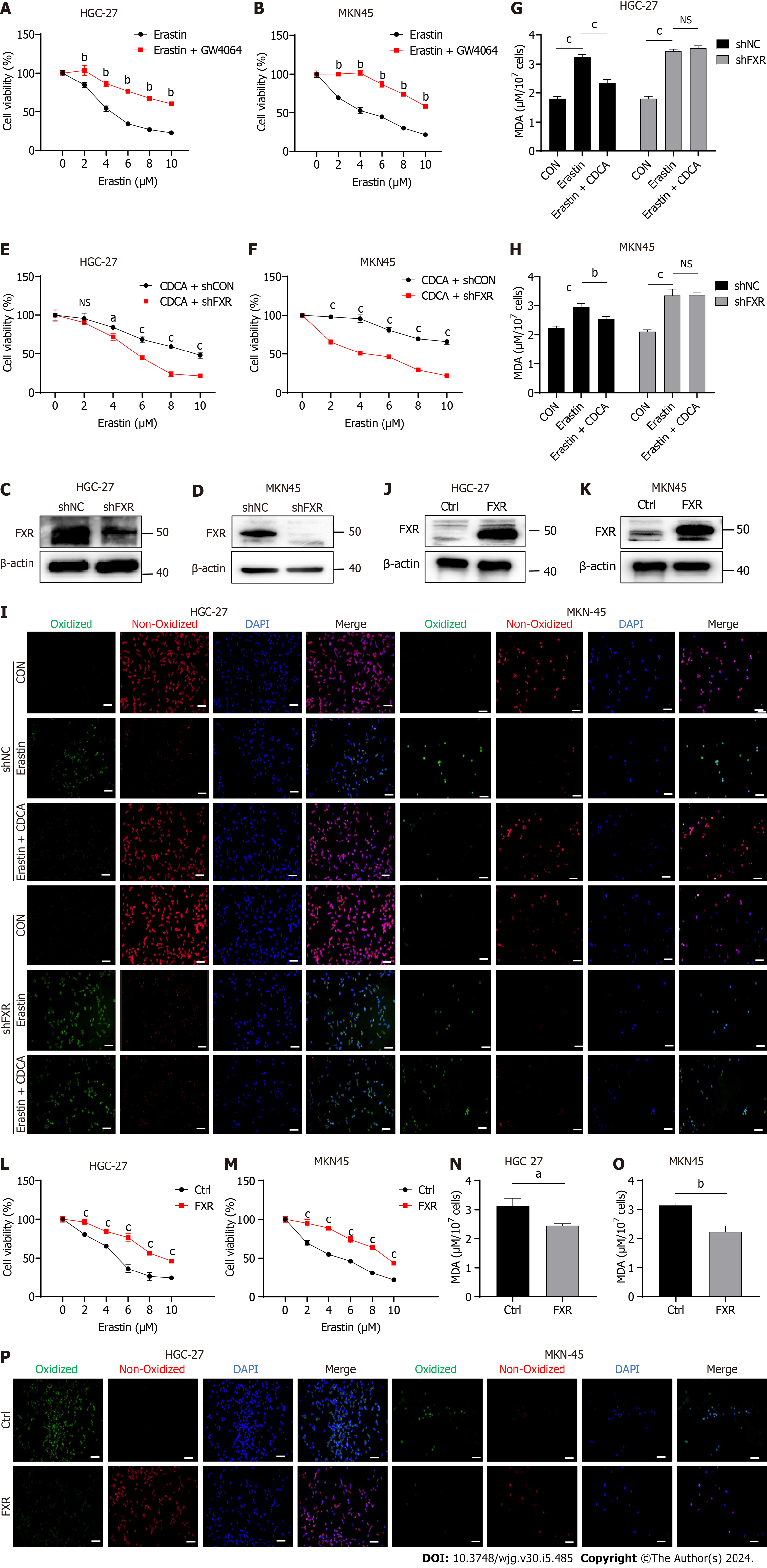
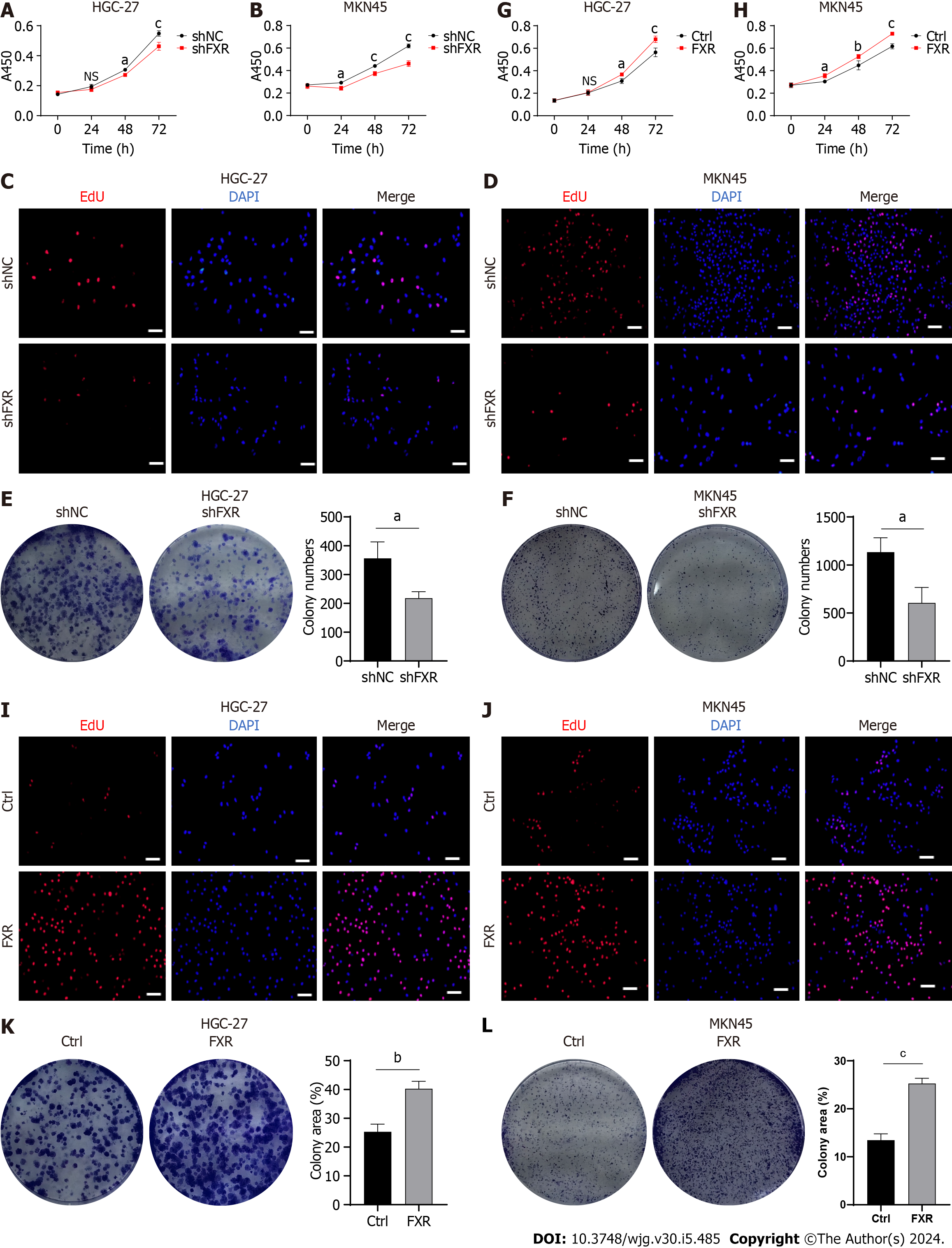
CDCA is the strongest FXR agonist in the human body[12]. Therefore, we hypothesized that CDCA acted through activating FXR to inhibit the sensitization of GC cells to ferroptosis. We firstly used GW4064, an in vitro agonist of FXR, and found that the ferroptosis sensitivity of GC cells treated with GW4064 was significantly reduced (Figure 3A and B). Subsequently, we transfected shFXR and its control plasmid in HGC-27 and MKN-45 cells, constructing a cellular knockdown model of FXR to be successfully constructed by WB analysis (Figure 3C and D). Our data showed that after knocking down FXR, CDCA-induced erastin resistance was not observed (Figure 3E and F) and it could no longer reverse the onset of erastin-induced ferroptosis (Figure 3G-I). We further constructed an overexpression model of FXR in HGC-27 and MKN-45 cells (Figure 3J and K). FXR overexpression resulted in a significant enhancement of resistance to erastin-induced cell death in HGC-27 and MKN-45 (Figure 3L and M), as well as a significant reversal of ferroptosis (Figure 3N-P).
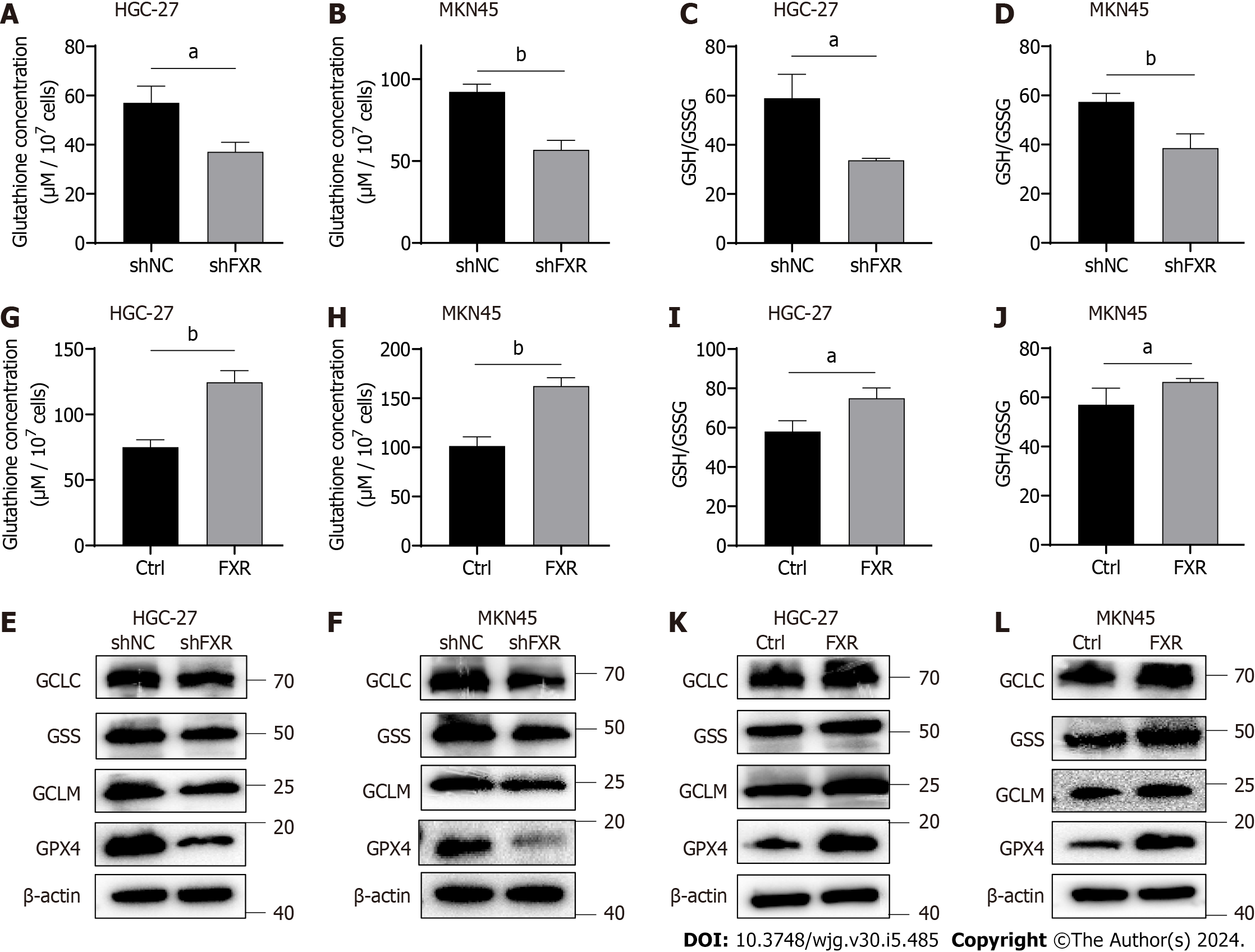
To investigate whether FXR could likewise increase intracellular GSH concentrations, we examined the effect of FXR on GSH levels. The results showed that GSH concentrations were significantly reduced after FXR knockdown in HGC-27 and MKN-45 cells (Figure 4A and B). The GSH/GSSG ratio, an indicator of cellular antioxidant capacity, was also significantly decreased after FXR knockdown (Figure 4C and D). We next examined the effect of FXR on the protein expression levels of GSH synthesis-related enzymes in GC cells using WB analysis, finding that FXR knockdown significantly reduced the expression of GSH synthases, including GCLC, GCLM and GSS. It also affected GPX4 expression levels, which used GSH as a substrate (Figure 4E and F). To further validate these observations, we repeated the above experiments using the FXR overexpression HGC-27 and MKN-45 cells. The results showed that overexpressing FXR in these GC cells led to increased GSH concentrations (Figure 4G and H), GSH/GSSG ratio (Figure 4I and J), and GSH synthase and GPX4 expression levels (Figure 4K and L).
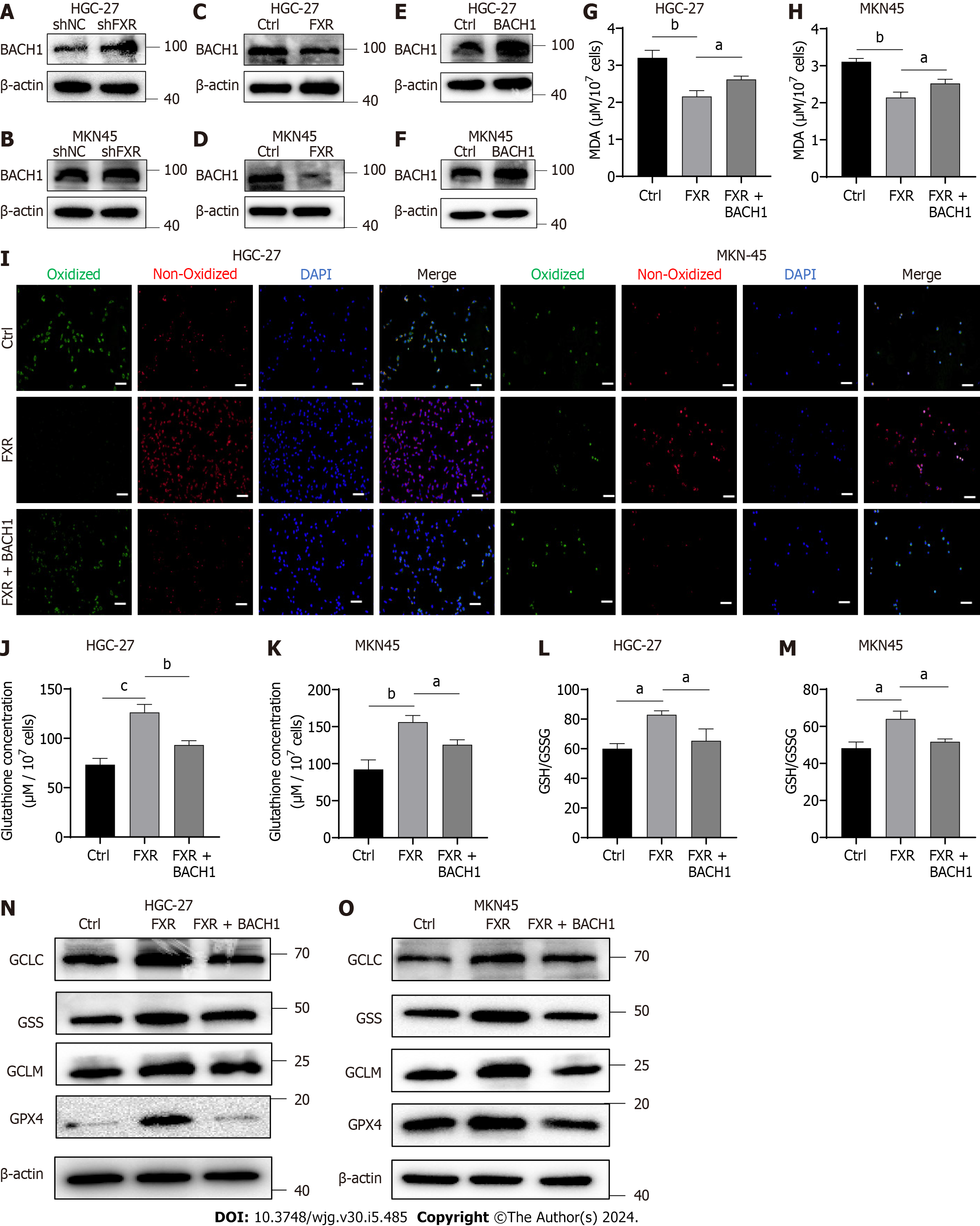
Recently, FXR was shown to inhibit heme catabolism and increase heme levels by repressing HO-1 transcription[29]. Heme in high concentrations can inhibit BACH1, which can lead to decreased expression of GSH synthases[30,31]. Therefore, BACH1 is potentially a crucial bridge through which FXR exerted its effects. We firstly detected BACH1 protein expression using WB analysis in HCG-27 and MKN-45 cells with overexpression or knockdown of FXR expression. The results showed that knocking down FXR indeed significantly elevated BACH1 protein levels (Figure 5A and B), while overexpressing FXR significantly downregulated BACH1 expression (Figure 5C and D). To further validate the role of BACH1 in this system, we constructed overexpression models of BACH1 in HGC-27 and MKN-45 cells and verified (Figure 5E and F). We then transfected cells with the FXR overexpression plasmid together with the BACH1 overexpression plasmid and erastin treatment. This rescue experiment suggested that overexpression of BACH1 Led to a significant reduction in ferroptosis resistance mediated by FXR, as seen with the MDA assay and BODIPY-589/591 C11 staining results (Figure 5G-I). Simultaneously, FXR-mediated enhancements of GSH concentrations (Figure 5J and K), GSH/GSSG ratio (Figure 5L and M), GSH synthase expression including GCLC, GCLM, GSS, and GPX4 (Figure 5N and O) were significantly reversed by overexpressed BACH1.
To further determine the role of FXR in GC progression, we analyzed its biological functions in GC cells. As described above, knockdown models of FXR in HGC-27 and MKN-45 cells were constructed (Figure 3C and D). Subsequently, cell viability assays showed that GC cell proliferation rates were significantly reduced after FXR knockdown (Figure 6A and B). This was also confirmed by EdU staining, which showed that the proportion of actively proliferating GC cells was significantly reduced with lower FXR expression levels (Figure 6C and D). Additional assays likewise revealed that the colony formation ability of GC cells was significantly decreased after knocking down FXR (Figure 6E and F). Experiments with the overexpression model showed that FXR promoted GC cell proliferation (Figure 6G and H), facilitated the capacity of DNA replication (Figure 6I and J), and enhanced the colony formation ability (Figure 6K and L).
GC is a major cause of cancer-related mortality in East Asia[32], but the molecular mechanisms and regulatory systems involved still need to be further elucidated. In the present study, we provided evidence that BAs can promote GC progression by inhibiting the ferroptosis sensitivity of GC, then explored the related mechanism in more detail.
BAs are essential small-molecule metabolites that can act as signaling molecules in the onset and progression of many diseases in humans, including various cancers[33]. For example, BAs can promote gastric carcinogenesis via the IL-6/JAK1/STAT3 axis[5]. Since the discovery of ferroptosis, numerous studies have focused on its potential use as a therapeutic target in cancer, including in GC[34]. For instance, activation of the Wnt/beta-catenin signaling pathway significantly enhanced ferroptosis resistance in GC[35]. ACTL6A inhibits the onset of ferroptosis in GC by upregulating GCLC[36]. However, only a few studies have explored whether BAs can affect GC through regulation of ferroptosis. Our data indicated that several BAs, especially CDCA, significantly inhibit erastin-induced GC cell death. Additionally, we confirmed that erastin induced cell death through ferroptosis. Subsequently, we found that the BAs did not reverse cell death induced by RSL3, a ferroptosis inducer that targeted GPX4. This suggested that BAs may exert their anti-ferroptosis activity through GSH, which is downstream of the erastin target xCT and upstream of GPX4. Indeed, both the GSH concentration and the GSH/GSSG ratio were significantly elevated following treatment of GC cells with BAs. This suggested that the BAs increased both GSH and GPX4 levels in GC cells, resulting in resistance to ferroptosis.
We hypothesized that CDCA regulates ferroptosis in GC cells by acting through FXR. FXR is a member of the nuclear hormone receptor superfamily, for which BAs are physiological ligands. Of these, CDCA has the strongest in vivo affinity for FXR[11,37]. Previous work demonstrated that FXR promotes gastric intestinal metaplasia, a precancerous lesion that can lead to GC development, via the FXR/SNAI2/miR-1 axis[38]. However, the role played by FXR in GC progression, and especially in ferroptosis, remained unknown. We found that GW4064, a classical in vitro FXR agonist, had similar effects on GC cell death as BAs. Subsequently, we performed FXR gain and loss of function assays and found that the anti-ferroptosis effect of the BAs was almost completely abolished by FXR knockdown, while FXR overexpression in the absence of BAs decreased GC cell ferroptosis. In addition, our data suggested that FXR can increase the expression of GSH synthases, including GSS, GCLC, and GCLM, as well as significantly increase the GSH concentration, GSH/GSSG ratio, and GPX4 expression in GC cells. These results suggest that BAs may inhibit ferroptosis by promoting GSH synthesis via FXR activation.
To clarify the mechanism by which FXR exerts its effects in the context of GC, we reviewed relevant studies and found that FXR suppresses the expression of HO-1, which can degrade heme, leading to inhibition of BACH1[29]. BACH1 belongs to the CNC b-Zip family of proteins and can inhibit intracellular synthesis of GSH[30,31,39]. Therefore, we considered whether BACH1 acts as a bridge between GSH synthesis and FXR. Our data indicated that FXR and BACH1 expression levels were inversely related, suggesting that FXR inhibits BACH1 expression. Subsequent functional rescue experiments revealed that BACH1 overexpression partially counteracted the pro-GSH synthesis and anti-ferroptosis effects of FXR. Finally, we investigated the effect of FXR on GC cell growth and found that FXR has marked oncogenic capacity, as it significantly increased GC cell proliferation rates, which may be closely related to the inhibition of GC cell ferroptosis by FXR.
This study had some limitations. Because of experimental restrictions, we were unable to perform in vivo experiments to validate our in vitro results. Additionally, we did not investigate the molecular mechanism by which FXR regulates BACH1; this requires further research.
Overall, our study illustrates a novel strategy by which BAs regulate ferroptosis in GC cells, and provides new insights into the molecular mechanisms underlying BA-mediated promotion of GC progression. We found that ferroptosis plays an influential role in GC progression, raising the possibility that treatments targeting FXR and BACH1 could improve the outcomes of GC patients with BAs reflux.
Gastric cancer (GC) is the fifth most common cancer worldwide and the third leading cause of cancer-related deaths because of the difficulties associated with early diagnosis. Bile acids (BAs) reflux, as an etiologic factor for GC, is receiving more attentions. BAs are engaged in the regulation of metabolism, and the latter is closely related to the ferroptosis. Thus BAs may be potentially relevant to the regulation of ferroptosis.
To elaborate the relationship between BAs and ferroptosis in GC and to providing new insights into the precise treatment of GC patients with BAs reflux.
In this study, we aimed to explore the role of BAs in regulating ferroptosis in GC and to investigate the underlying molecular mechanisms. The present study helps to further elucidate the pathophysiologic mechanisms in GC patients with BAs reflux.
The research methods are as follows: cell transfection, cell viability assay, glutathione (GSH) and Malondialdehyde assay quantification, lipid reactive oxygen species assay, 5-ethynyl-2′-deoxyuridine staining, colony formation assay, Western blot.
Firstly, we found that BAs can promote GC cell proliferation and inhibit erastin-induced ferroptosis sensitivity through upregulate GSH and GSH peroxidase 4 (GPX4). Secondly, BAs exerted its anti-ferroptosis sensitivity function in GC cells by activating farnesoid X receptor (FXR) which significantly promoted GSH synthesis. Subsequently, BTB and CNC homology 1 (BACH1) provided an essential bridging role in BAs and FXR facilitating GSH synthesis. Finally, the notable oncogenic effects of FXR were discovered.
BAs could inhibit ferroptosis sensitivity through the FXR-BACH1-GSH-GPX4 axis in GC cells.
The findings of this basic study will be validated in in vivo experiments and clinical specimens to clarify whether FXR and BACH1 can serve as therapeutic targets for GC patients with BAs reflux.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kalkum E, Germany S-Editor: Lin C L-Editor: A P-Editor: Zhao S
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55774] [Article Influence: 7967.7] [Reference Citation Analysis (132)] |
| 2. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2838] [Article Influence: 567.6] [Reference Citation Analysis (5)] |
| 3. | Oba M, Miwa K, Fujimura T, Harada S, Sasaki S, Hattori T. Chemoprevention of glandular stomach carcinogenesis through duodenogastric reflux in rats by a COX-2 inhibitor. Int J Cancer. 2008;123:1491-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Collins SL, Stine JG, Bisanz JE, Okafor CD, Patterson AD. Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat Rev Microbiol. 2023;21:236-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 465] [Article Influence: 232.5] [Reference Citation Analysis (0)] |
| 5. | Wang S, Kuang J, Zhang H, Chen W, Zheng X, Wang J, Huang F, Ge K, Li M, Zhao M, Rajani C, Zhu J, Zhao A, Jia W. Bile Acid-Microbiome Interaction Promotes Gastric Carcinogenesis. Adv Sci (Weinh). 2022;9:e2200263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 6. | Dixon MF, Mapstone NP, Neville PM, Moayyedi P, Axon AT. Bile reflux gastritis and intestinal metaplasia at the cardia. Gut. 2002;51:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, Ha S, Nelson BN, Kelly SP, Wu L, Zheng Y, Longman RS, Rastinejad F, Devlin AS, Krout MR, Fischbach MA, Littman DR, Huh JR. Bile acid metabolites control T(H)17 and T(reg) cell differentiation. Nature. 2019;576:143-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 908] [Article Influence: 151.3] [Reference Citation Analysis (0)] |
| 8. | McIlvride S, Dixon PH, Williamson C. Bile acids and gestation. Mol Aspects Med. 2017;56:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Mikó E, Kovács T, Sebő É, Tóth J, Csonka T, Ujlaki G, Sipos A, Szabó J, Méhes G, Bai P. Microbiome-Microbial Metabolome-Cancer Cell Interactions in Breast Cancer-Familiar, but Unexplored. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 10. | Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1641] [Cited by in RCA: 1692] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 11. | Režen T, Rozman D, Kovács T, Kovács P, Sipos A, Bai P, Mikó E. The role of bile acids in carcinogenesis. Cell Mol Life Sci. 2022;79:243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 176] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 12. | Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2012] [Cited by in RCA: 2090] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 13. | Sun L, Cai J, Gonzalez FJ. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat Rev Gastroenterol Hepatol. 2021;18:335-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 258] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 14. | Martínez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer. 2021;21:669-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 1013] [Article Influence: 253.3] [Reference Citation Analysis (0)] |
| 15. | Liang D, Minikes AM, Jiang X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell. 2022;82:2215-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 664] [Article Influence: 221.3] [Reference Citation Analysis (0)] |
| 16. | Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4711] [Cited by in RCA: 11650] [Article Influence: 896.2] [Reference Citation Analysis (1)] |
| 17. | Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 4181] [Article Influence: 1045.3] [Reference Citation Analysis (0)] |
| 18. | Zheng J, Conrad M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020;32:920-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 848] [Article Influence: 169.6] [Reference Citation Analysis (0)] |
| 19. | Stockwell BR, Jiang X, Gu W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol. 2020;30:478-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 776] [Article Influence: 155.2] [Reference Citation Analysis (0)] |
| 20. | Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2388] [Cited by in RCA: 5163] [Article Influence: 469.4] [Reference Citation Analysis (0)] |
| 21. | Ouyang S, Li H, Lou L, Huang Q, Zhang Z, Mo J, Li M, Lu J, Zhu K, Chu Y, Ding W, Zhu J, Lin Z, Zhong L, Wang J, Yue P, Turkson J, Liu P, Wang Y, Zhang X. Inhibition of STAT3-ferroptosis negative regulatory axis suppresses tumor growth and alleviates chemoresistance in gastric cancer. Redox Biol. 2022;52:102317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 277] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 22. | Lin Z, Song J, Gao Y, Huang S, Dou R, Zhong P, Huang G, Han L, Zheng J, Zhang X, Wang S, Xiong B. Hypoxia-induced HIF-1α/lncRNA-PMAN inhibits ferroptosis by promoting the cytoplasmic translocation of ELAVL1 in peritoneal dissemination from gastric cancer. Redox Biol. 2022;52:102312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 154] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 23. | Li T, Guo H, Li H, Jiang Y, Zhuang K, Lei C, Wu J, Zhou H, Zhu R, Zhao X, Lu Y, Shi C, Nie Y, Wu K, Yuan Z, Fan DM, Shi Y. MicroRNA-92a-1-5p increases CDX2 by targeting FOXD1 in bile acids-induced gastric intestinal metaplasia. Gut. 2019;68:1751-1763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 24. | Lee JY, Nam M, Son HY, Hyun K, Jang SY, Kim JW, Kim MW, Jung Y, Jang E, Yoon SJ, Kim J, Seo J, Min JK, Oh KJ, Han BS, Kim WK, Bae KH, Song J, Huh YM, Hwang GS, Lee EW, Lee SC. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc Natl Acad Sci U S A. 2020;117:32433-32442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 325] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 25. | Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem. 1986;261:2256-2263. [PubMed] |
| 26. | Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1891] [Cited by in RCA: 2414] [Article Influence: 603.5] [Reference Citation Analysis (0)] |
| 27. | Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17:2054-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 1257] [Article Influence: 251.4] [Reference Citation Analysis (0)] |
| 28. | Shin D, Kim EH, Lee J, Roh JL. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic Biol Med. 2018;129:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 383] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 29. | Kim DH, Choi HI, Park JS, Kim CS, Bae EH, Ma SK, Kim SW. Farnesoid X receptor protects against cisplatin-induced acute kidney injury by regulating the transcription of ferroptosis-related genes. Redox Biol. 2022;54:102382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 30. | Wiel C, Le Gal K, Ibrahim MX, Jahangir CA, Kashif M, Yao H, Ziegler DV, Xu X, Ghosh T, Mondal T, Kanduri C, Lindahl P, Sayin VI, Bergo MO. BACH1 Stabilization by Antioxidants Stimulates Lung Cancer Metastasis. Cell. 2019;178:330-345.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 399] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 31. | Nishizawa H, Yamanaka M, Igarashi K. Ferroptosis: regulation by competition between NRF2 and BACH1 and propagation of the death signal. FEBS J. 2023;290:1688-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 32. | Huang J, Lucero-Prisno DE 3rd, Zhang L, Xu W, Wong SH, Ng SC, Wong MCS. Updated epidemiology of gastrointestinal cancers in East Asia. Nat Rev Gastroenterol Hepatol. 2023;20:271-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 126] [Reference Citation Analysis (0)] |
| 33. | Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15:111-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1355] [Cited by in RCA: 1249] [Article Influence: 178.4] [Reference Citation Analysis (0)] |
| 34. | Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell. 2019;35:830-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 1616] [Article Influence: 269.3] [Reference Citation Analysis (0)] |
| 35. | Wang Y, Zheng L, Shang W, Yang Z, Li T, Liu F, Shao W, Lv L, Chai L, Qu L, Xu Q, Du J, Liang X, Zeng J, Jia J. Wnt/beta-catenin signaling confers ferroptosis resistance by targeting GPX4 in gastric cancer. Cell Death Differ. 2022;29:2190-2202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 249] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 36. | Yang Z, Zou S, Zhang Y, Zhang J, Zhang P, Xiao L, Xie Y, Meng M, Feng J, Kang L, Lee MH, Fang L. ACTL6A protects gastric cancer cells against ferroptosis through induction of glutathione synthesis. Nat Commun. 2023;14:4193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 53] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 37. | Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 925] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 38. | Wang N, Wu S, Zhao J, Chen M, Zeng J, Lu G, Wang J, Zhang J, Liu J, Shi Y. Bile acids increase intestinal marker expression via the FXR/SNAI2/miR-1 axis in the stomach. Cell Oncol (Dordr). 2021;44:1119-1131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Sun J, Brand M, Zenke Y, Tashiro S, Groudine M, Igarashi K. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc Natl Acad Sci U S A. 2004;101:1461-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 292] [Article Influence: 13.9] [Reference Citation Analysis (0)] |