Published online Jan 28, 2024. doi: 10.3748/wjg.v30.i4.318
Peer-review started: November 1, 2023
First decision: December 4, 2023
Revised: December 11, 2023
Accepted: January 8, 2024
Article in press: January 8, 2024
Published online: January 28, 2024
Processing time: 85 Days and 21.1 Hours
Hepatic arterial infusion chemotherapy (HAIC) has been proven to be an ideal choice for treating unresectable hepatocellular carcinoma (uHCC). HAIC-based treatment showed great potential for treating uHCC. However, large-scale studies on HAIC-based treatments and meta-analyses of first-line treatments for uHCC are lacking.
To investigate better first-line treatment options for uHCC and to assess the safety and efficacy of HAIC combined with angiogenesis inhibitors, programmed cell death of protein 1 (PD-1) and its ligand (PD-L1) blockers (triple therapy) under real-world conditions.
Several electronic databases were searched to identify eligible randomized controlled trials for this meta-analysis. Study-level pooled analyses of hazard ratios (HRs) and odds ratios (ORs) were performed. This was a retrospective single-center study involving 442 patients with uHCC who received triple therapy or angiogenesis inhibitors plus PD-1/PD-L1 blockades (AIPB) at Sun Yat-sen University Cancer Center from January 2018 to April 2023. Propensity score matching (PSM) was performed to balance the bias between the groups. The Kaplan-Meier method and cox regression were used to analyse the survival data, and the log-rank test was used to compare the suvival time between the groups.
A total of 13 randomized controlled trials were included. HAIC alone and in combination with sorafenib were found to be effective treatments (P values for ORs: HAIC, 0.95; for HRs: HAIC + sorafenib, 0.04). After PSM, 176 HCC patients were included in the analysis. The triple therapy group (n = 88) had a longer median overall survival than the AIPB group (n = 88) (31.6 months vs 14.6 months, P < 0.001) and a greater incidence of adverse events (94.3% vs 75.4%, P < 0.001).
This meta-analysis suggests that HAIC-based treatments are likely to be the best choice for uHCC. Our findings confirm that triple therapy is more effective for uHCC patients than AIPB.
Core Tip: The network meta-analysis showed the treatment based on hepatic arterial infusion chemotherapy (HAIC) had the best efficacy on unresectable hepatocellular carcinoma (uHCC). The retrospective, relatively large-scale study suggested HAIC combined with angiogenesis inhibitors and programmed cell death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) blockers could improve the uHCC patients’ prognosis. After propensity score matching, it demonstrated that triple therapy was able to prolong the uHCC patients’ survival than angiogenesis inhibitors and PD-1/PD-L1 blockers.
- Citation: Cao YZ, Zheng GL, Zhang TQ, Shao HY, Pan JY, Huang ZL, Zuo MX. Hepatic arterial infusion chemotherapy with anti-angiogenesis agents and immune checkpoint inhibitors for unresectable hepatocellular carcinoma and meta-analysis. World J Gastroenterol 2024; 30(4): 318-331
- URL: https://www.wjgnet.com/1007-9327/full/v30/i4/318.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i4.318
Primary liver cancer is a malignant tumor of the digestive system that is common worldwide. China has a particularly high incidence of liver cancer, with approximately 410000 new cases and 391000 deaths annually; liver cancer is the second largest cause of cancer-related death in the country[1]. Among primary liver cancers, hepatocellular carcinoma (HCC) is the main pathological type. In the subclinical phase, patients tend to be asymptomatic. Therefore, at the time of diagnosis, most patients have already reached advanced stages of the disease. Therefore, fewer than 30% of patients are candidates for surgical resection[2,3].
According to several clinical trials, sorafenib and lenvatinib have been recommended by multiple authoritative guidelines as first-line treatment options for advanced HCC for some time[4-6]. The results of the IMbrave150 trial ushered in a new era in HCC therapy, in which angiogenesis inhibitors were combined with programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) blockees, which is now becoming the new standard first-line therapy[7]. Researchers have conducted several clinical studies on various immune-related drugs, angiogenesis inhibitors, and various combination regimens for unresectable HCC (uHCC). The FOHAIC study also revealed that hepatic arterial perfusion chemotherapy (HAIC) using the FOLFOX regimen was more effective than sorafenib in patients with uHCC[8]. Several studies have suggested that triple therapy has the potential to further improve the prognosis in patients with uHCC[9-12]. As the results of multiple clinical studies have been published, several questions remain surrounding this type of therapy, such as which treatment approach has the best therapeutic effect on uHCC? How effective is triple therapy when used in large-scale real-world clinical applications?
In this study, we attempted to identify the optimal treatment for uHCC based on data from phase III randomized controlled trials (RCTs) through a network meta-analysis. We also investigated the safety and efficacy of triple therapy in patients with HCC from a Chinese population under real-world conditions. We then performed propensity score matching (PSM) to compare triple therapy to angiogenesis inhibitors plus PD1/PDL1 blockers (AIPB), which has been recommended as a first-line treatment for uHCC by some guidelines[13,14]. This study also confirmed the safety of triple therapy under real-world conditions.
We performed an extensive literature search of the PubMed, Embase, and Cochrane Library databases for RCTs published from January 1, 2018, to January 1, 2023. The Supplementary material details the search strategy and inclusion criteria. Two authors independently screened the trials for eligibility and extracted information from each one. The included RCTs were then assessed for risk of bias using the Cochrane risk of bias 2 tool, which showed low risk levels for all the included studies (Supplementary Figure 1).
Patients who were treated with triple therapy or AIPB as a first-line treatment for advanced HCC between January 2018 and April 2023 at the Department of Minimally Invasive Interventional Therapy, Sun Yat-sen University Cancer Center in Guangzhou, China, were screened for eligibility. HCC was diagnosed histologically or radiologically in accordance with the latest international guidelines[15]. The inclusion criteria were as follows: (1) Stage B (not applicable for surgery or progressed on locoregional therapy) or stage C HCC according to the Barcelona Clinic Liver Cancer (BCLC) staging system; (2) Child–Pugh score of A–C; (3) Eastern cooperative oncology group performance status (ECOG PS) of 0–2; (4) Age ≥ 18 years; and (5) At least one available follow-up data point. The exclusion criteria were as follows: (1) History of receiving any systemic chemotherapy, angiogenesis inhibitors, or immunotherapy; (2) Lack of medical imaging data; and (3) History of a second primary malignant tumor. The details are shown in the Supplementary materials. This study was reviewed and approved by the Sun Yat-sen University Cancer Center Ethics Committee. Informed consent was waived due to the retrospective nature of the analysis.
Treatment regimens: Small-molecule tyrosine kinase inhibitors such as sorafenib, a type of angiogenesis inhibitor, were administered orally, and the doses were determined based on the manufacturers’ instructions. Bevacizumab, another type of angiogenesis inhibitor, was administered intravenously at a dose of 15 mg/kg body weight every 3 wk. Atezolizumab, a type of programmed cell death ligand 1 blocker, was administered intravenously at a dose of 1200 mg every 3 wk. Programmed cell death protein 1 (PD-1) blockers, including pembrolizumab, camrelizumab, tislelizumab, and sintilimab, were administered intravenously at 200 mg every 3 wk. Toripalimab, another PD-1 blocker, was injected through an intravenous drip of 240 mg every 3 wk following the instructions. The HAIC regimen was based on FOLFOX and consisted of 85 mg/m2 oxaliplatin, 200 mg/m2 calcium folinate, and 2.5 g/m2 5-fluorouracil every 3 wk. HAIC was performed under the guidance of digital subtraction angiography by interventional radiologists. The celiac axis, superior mesenteric artery, inferior phrenic artery and right renal artery were selectively catheterized for angiography. If angiography revealed that the HCC blood supply originated from different vessels, the main feeding artery was reserved for super selective catheterization, and an indwelling microcatheter was inserted into the HAIC while the other feeding vessels were embolized. During the study period, dose modifications and treatment interruptions were sometimes initiated according to drug-related toxicity grades, as recommended relative to the physiological condition of each patient. HAIC was performed for 4–6 rounds in the absence of disease progression. Patients received angiogenesis inhibitors and PD-1/PD-L1 blockers during and after HAIC treatment to consolidate the therapeutic effects in the long-term.
Assessment of clinical outcomes: The patients involved in the study were followed up every 6–12 wk to assess treatment response. Radiological response was assessed according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria based on liver dynamic computed tomography or magnetic resonance imaging data. The primary endpoint that was assessed was overall survival (OS), which was defined as the time from the start date of systemic chemotherapy or HAIC to death. progression-free survival (PFS) (the time from the start date of systemic chemotherapy or HAIC to the date of disease progression or death from any cause). The secondary endpoints that were determined included PFS and 6-, 12- and 24-mo OS rates; objective response rate (ORR); and adverse events (AEs). The ORR was defined as the proportion of patients who achieved a complete response (CR) or partial response (PR), and the disease control rate was defined as the proportion of patients who achieved CR, PR, or stable disease. AEs during treatment were identified using patient-reported symptom data, examination-based findings, and clinical laboratory test results. The National Cancer Institute Common Terminology Criteria for AEs, version 5.0, was used to classify AEs from any cause according to type and severity.
Unstratified hazard ratios (HRs) with 95%CI and odds ratios (ORs) with the number of responders and sample sizes that compared the different treatment regimens for treating uHCC were retrieved and synthesized to determine the overall treatment effects. Potential heterogeneity among the studies was assessed using I2 statistics. Random effects models were used to calculate pooled ORR or HR in the presence of significant heterogeneity (I2 > 50%); otherwise, the fixed effects model was used.
To account for the different distributions of covariates between the two groups, we performed PSM. Then, 1:1 mat-ching was performed using nearest-neighbor matching based on the performance status data. In this study, the caliper of the match was 0.03. OS, PFS and survival rates were calculated using the Kaplan–Meier method and were compared between the groups using the log-rank test. Cox regression was used to explore the potential risk factors associated with survival time. All real-world clinical data are expressed as the mean ± SD, median (range), or number (%), as appropriate. Continuous variables were compared using Student’s t test (or the Mann–Whitney U test, if appropriate), and categorical variables were compared using the chi-square test (or Fisher’s exact test, if appropriate).
Statistical analyses were conducted using R v4.2.2 (R Core Team, Vienna, Austria). https://www.R-project.org/). Two-sided P values < 0.05 were considered to indicate statistical significance.
Literature search and screening results: Our initial literature search resulted 1735 articles. After deleting duplicate publications, 1079 articles remained. After screening the titles and abstracts, 68 articles were excluded. Our full-text review resulted in the removal of an additional seven articles. Ultimately, 13 studies involving 7817 patients were included in this network meta-analysis[6,8,16-25]. The literature selection process is described in Supplementary Figure 2, and the characteristics of the included patient population are shown in Supplementary Table 1.
Results of network-meta-analysis: ORRs per Response Evaluation Criteria in Solid Tumors 1.1 and HRs were reported in all 13 studies and included 15 different interventions. There was no significant heterogeneity between the studies (ORR: I2 = 5%; HR: I2 = 7%), so the fixed-effects model was adopted. The P scores for ORR showed that the best ORR outcomes were obtained with HAIC compared to sorafenib (OR: 35.66; 95%CI: 9.94–249.91; P: 0.952; Table 1, Supplemen
| Intervention | OR | Intervention | HR |
| HAIC | 0.9520107 | HAIC + Sorafenib | 0.03757857 |
| HAIC + Sorafenib | 0.9420071 | HAIC | 0.09333929 |
| SIRT | 0.8131607 | TACE + Lenvatinib | 0.098075 |
| TACE + Lenvatinib | 0.8014429 | Sintilimab + Bevacizumabbiosimila | 0.24973929 |
| Sintilimab + Bevacizumabbiosimila | 0.6559643 | Camrelizumab + Rivoceranib | 0.30909286 |
| Lenvatinib + Pembrolizumab | 0.5922857 | Atezolizumab + Bevacizumab | 0.35838929 |
| Camrelizumab + Rivoceranib | 0.5853643 | Lenvatinib + Pembrolizumab | 0.51200714 |
| Durvalumab + Tremelimumab | 0.523425 | Durvalumab + Tremelimumab | 0.52634286 |
| Durvalumab | 0.3891893 | Donafenib | 0.62278571 |
| Atezolizumab + Bevacizumab | 0.3369036 | Tislelizumab | 0.66164643 |
| Lenvatinib | 0.3254286 | Nivolumab | 0.67260357 |
| Tislelizumab | 0.2732107 | Durvalumab | 0.68975714 |
| Nivolumab | 0.1859964 | Lenvatinib | 0.78121071 |
| Donafenib | 0.1165357 | Sorafenib | 0.9135 |
| Sorafenib | 0.007075 | SIRT | 0.97393214 |
Baseline characteristics of the patients: A total of 442 patients with uHCC were enrolled in the study; 324 patients underwent triple therapy, and 118 patients underwent AIPB. The median follow-up times were 14.6 months and 16.8 ± 10.3 months in the triple therapy group and 8.25 months and 11.7 ± 10.2 months in the AIPB group. The algorithm used for case enrollment is shown in Supplementary Figure 5. The average number of patients who received 5.08 ± 1.61 rounds of HAIC in the triple therapy group. Based on our multivariable logistic regression model, baseline characteristics, including age, ECOG PS, Child–Pugh class, maximum tumor diameter, AFP level, tumor number, and vascular invasion and extrahepatic metastasis conditions, which were significantly different between the groups, were matched (Table 2). After PSM, 88 patients in the triple therapy group were matched to 88 patients in the AIPB group (Table 2). The median age in both groups was 55.0 years, and all the patients were evaluated as having an ECOG PS ranging from 0-1. Notably, some patients in the AIPB group were diagnosed at an earlier stage. In other words, the proportion of BCLC C patients in the AIPB cohort was lower (87.5% vs 92%).
| Variable | Prior to PSM | Following PSM | ||||
| Triple therapy (n = 324) | AIPB (n = 118) | P value | Triple therapy (n = 88) | AIPB (n = 88) | P value | |
| Age | ||||||
| Mean (SD) | 50.2 (11.2) | 55.4 (11.6) | < 0.001 | 54.2 (10.8) | 53.9 (11.2) | 0.859 |
| Median [Min, Max] | 51.0 [23.0, 80.0] | 56.5 [23.0, 82.0] | 55.0 [26.0, 78.0] | 55.0 [23.0, 74.0] | ||
| Sex | ||||||
| Female | 36 (11.1) | 8 (6.8) | 0.244 | 11 (12.5) | 6 (6.8) | 0.307 |
| Male | 288 (88.9) | 110 (93.2) | 77 (87.5) | 82 (93.2) | ||
| ECOG PS | ||||||
| 0 | 318 (98.1) | 106 (89.8) | < 0.001 | 85 (96.6) | 85 (96.6) | 1 |
| 1 | 6 (1.9) | 11 (9.3) | 3 (3.4) | 3 (3.4) | ||
| 2 | 0 (0) | 1 (0.8) | 0 (0) | 0 (0) | ||
| Hepatitis virus | ||||||
| Negative | 27 (8.3) | 15 (12.7) | 0.228 | 11 (12.5) | 12 (13.6) | 1 |
| Positive | 297 (91.7) | 103 (87.3) | 77 (87.5) | 76 (86.4) | ||
| ALT | ||||||
| mean (SD) | 62.4 (72.8) | 53.6 (34.9) | 0.0894 | 58.4 (60.5) | 53.2 (36.1) | 0.489 |
| Median [Min, Max] | 44.6 [8.90, 930] | 45.4 [6.10, 196] | 41.1 [8.90, 448] | 44.6 [6.10, 196] | ||
| AST | ||||||
| mean (SD) | 92.5 (83.1) | 88.3 (78.5) | 0.627 | 82.0 (65.3) | 87.1 (84.9) | 0.657 |
| Median [Min, Max] | 66.2 [13.5, 702] | 66.8 [11.1, 470] | 58.7 [13.5, 327] | 63.7 [11.1, 470] | ||
| Child-Pugh class | ||||||
| A | 296 (91.4) | 94 (79.7) | 0.00133 | 75 (85.2) | 77 (87.5) | 0.826 |
| B | 28 (8.6) | 24 (20.3) | 13 (14.8) | 11 (12.5) | ||
| AFP > 400 ng/mL | ||||||
| No | 142 (43.8) | 60 (50.8) | 0.229 | 45 (51.1) | 42 (47.7) | 0.763 |
| Yes | 182 (56.2) | 58 (49.2) | 43 (48.9) | 46 (52.3) | ||
| Maximum diameter of tumor/cm | ||||||
| mean (SD) | 10.5 (4.25) | 9.38 (4.75) | 0.0291 | 9.86 (4.07) | 9.57 (4.83) | 0.668 |
| Median [Min, Max] | 10.3 [1.90, 23.5] | 9.25 [1.10, 21.0] | 10.0 [2.10, 19.2] | 9.10 [1.10, 20.5] | ||
| Tumor number | ||||||
| Single | 122 (37.7) | 19 (16.1) | < 0.001 | 21 (23.9) | 19 (21.6) | 0.857 |
| Multiple | 202 (62.3) | 99 (83.9) | 67 (76.1) | 69 (78.4) | ||
| Vascular invasion | ||||||
| No | 78 (24.1) | 42 (35.6) | 0.0221 | 25 (28.4) | 30 (34.1) | 0.515 |
| Yes | 246 (75.9) | 76 (64.4) | 63 (71.6) | 58 (65.9) | ||
| Extrahepatic metastasis | ||||||
| No | 171 (52.8) | 45 (38.1) | 0.00888 | 34 (38.6) | 38 (43.2) | 0.646 |
| Yes | 153 (47.2) | 73 (61.9) | 54 (61.4) | 50 (56.8) | ||
| BCLC | ||||||
| A | 0 (0) | 2 (1.7) | 0.0632 | 0 (0) | 2 (2.3) | 0.309 |
| B | 30 (9.3) | 11 (9.3) | 7 (8.0) | 9 (10.2) | ||
| C | 294 (90.7) | 105 (89.0) | 81 (92.0) | 77 (87.5) | ||
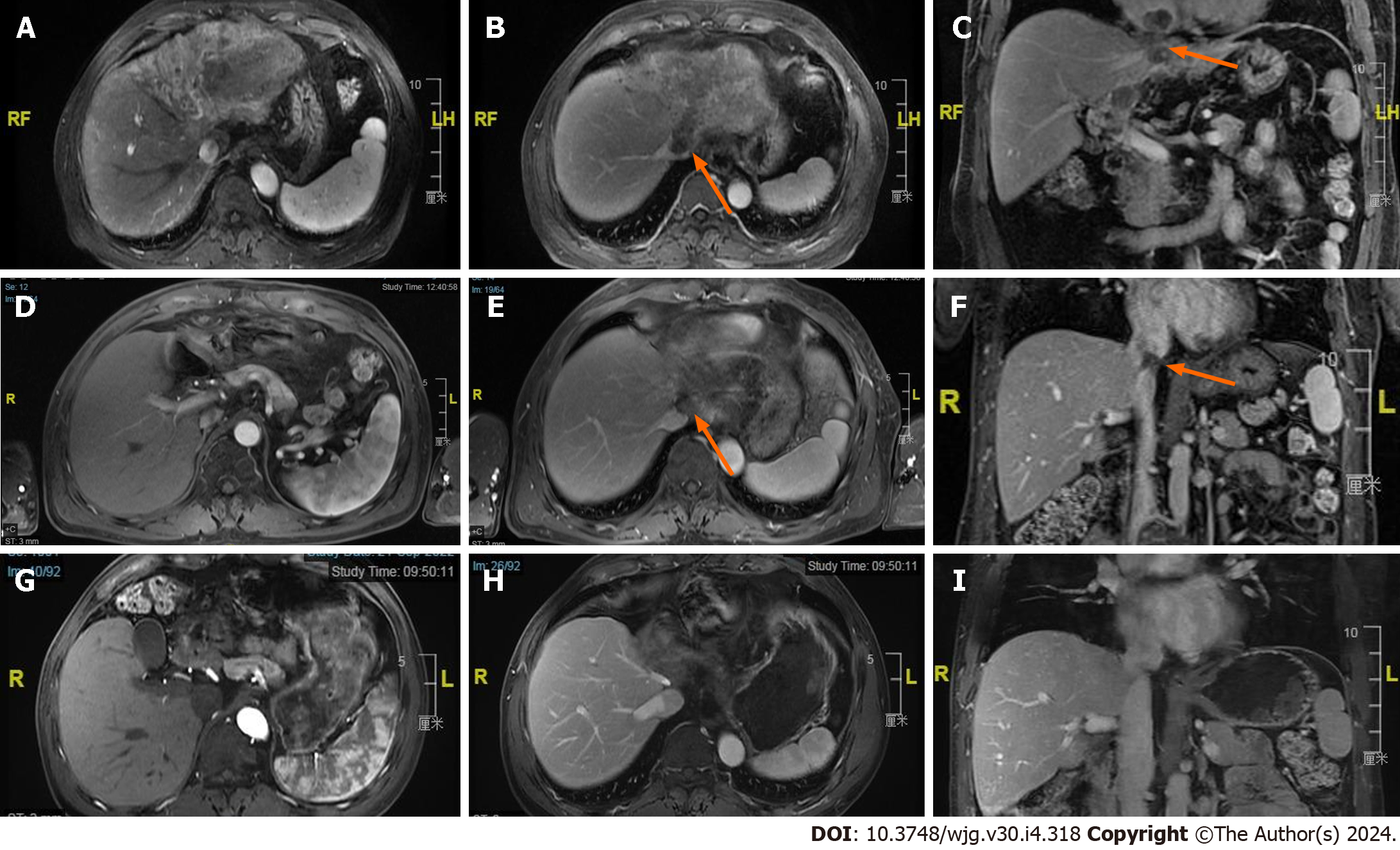
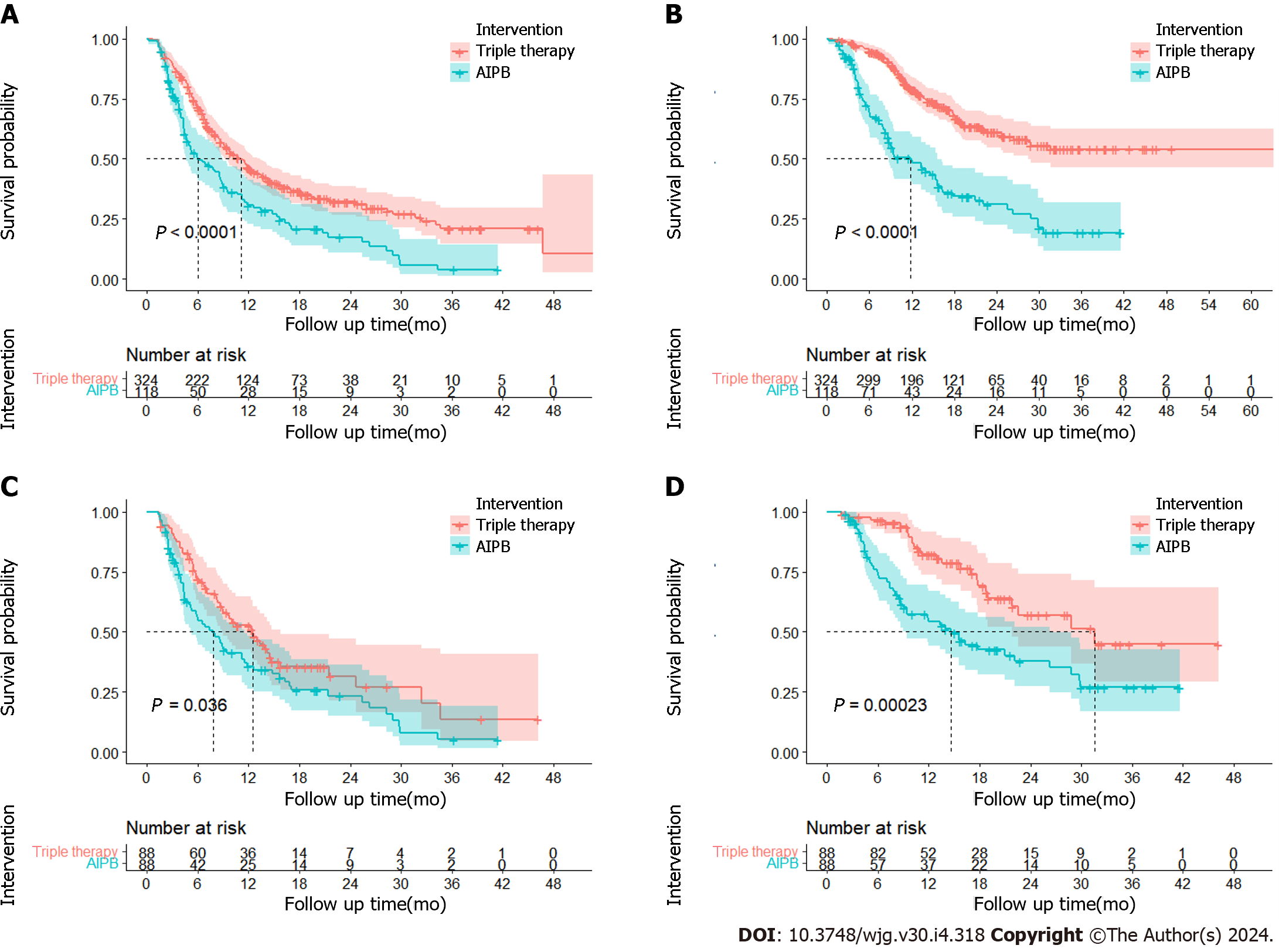
Efficacy of different treatments: The ORR was 62.9% (n = 204) in the triple group and 29.7% (n = 35) in the AIPB group in the primary database. After PSM, the ORR of the triple therapy group was still greater (55.7% vs 35.2%, P = 0.032) (Supplementary Table 4). CR was observed in 21 patients prior to PSM and in 6 patients following PSM in the triple therapy group (an example can be seen in Figure 1). According to the Kaplan–Meier analysis, the primary data showed longer median PFS (11.1 months vs 6.0 months, P < 0.001) and median OS (not reached vs 11.8 months, P < 0.001) with triple therapy. After PSM, the median PFSs were estimated to be 12.5 months and 7.8 months (P = 0.036), and the median OS were 31.6 months and 14.6 months (HR = 2.42, 95%CI = 1.49-3.92, P < 0.001) in the triple therapy group and AIPB group, respectively (Figure 2). The 6-months, 12-months, and 24-months survival rates of the patients receiving triple therapy were 96.5%, 82.2% and 57.0%, respectively, while they were 73.5%, 54.3% and 37.7%, respectively, in the AIPB group.
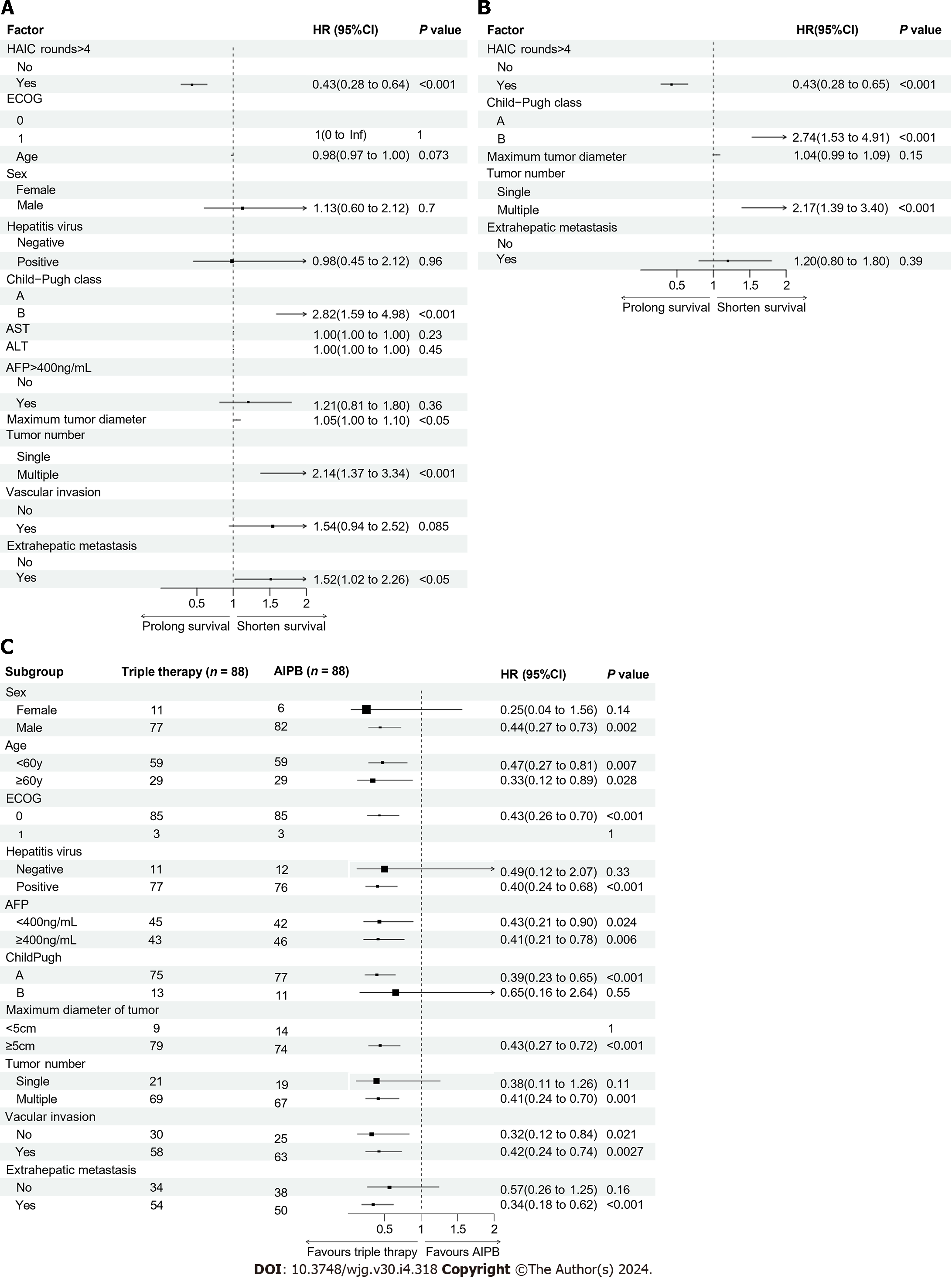
Univariate analysis revealed that four factors had effects on OS in the triple therapy group: Larger tumor diameter, multiple foci, extrahepatic metastasis, Child–Pugh grade B and number of rounds of HAIC (Figure 3A). Cox multivariate regression analysis revealed that Child–Pugh grade B (HR: 1.74, P < 0.001; Figure 3B) and multiple foci (HR: 2.17, P < 0.001; Figure 3B) were risk factors for poor long-term survival, and > 4 rounds of HAIC was a protective factor for survival (HR: 0.43, P < 0.001; Figure 3B). Survival analysis also revealed that patients who received > 4 rounds of HAIC (not reached vs 18.2 months; P < 0.001; Supplementary Figure 6A) or who were diagnosed with a single disease focus (not reached vs 24.6 months; P < 0.001; Supplementary Figure 6B) had longer OS. Subgroup analysis of OS using forest plots revealed that triple therapy was more effective in most patients, especially for males, Child-Pugh A patients, patients aged < 60 years, and patients diagnosed with multiple tumors or extrahepatic metastasis (Figure 3C).
Safety of different treatments: After PSM, the incidence of AEs in the triple therapy group was greater than that in the AIPB group (94.3% vs 75.4%, P < 0.001). Although more Grade 3-4 AEs occurred in the triple therapy group, there was no significant difference in the incidence of Grade 3-4 AEs (56.8% vs 43.2%, P = 0.097), and there were no Grade 5 AEs (Table 3). The most common AE was abdominal pain in the triple therapy group, for which the incidence was 79.8% (259/324). When they started HAIC treatment, many patients had varying degrees of abdominal pain during the infusion of oxaliplatin. This was typically managed by slowing the speed of infusion or temporarily stopping it. In some cases of particularly severe and acute abdominal pain, anisodamine or lidocaine was administered through intravenous injection or an arterial catheter to relieve the pain. Two patients developed hepatic comas following HAIC but fully recovered during treatment. In addition, liver dysfunction, including increases in aminotransferases and/or bilirubin, was relatively common in both groups, not only because of drug-related side effects but also because of their own background of liver cirrhosis.
| Before PSM | After PSM | |||||||
| Triple therapy group (n = 324) | AIPB group (n = 118) | Triple therapy group (n = 88) | AIPB group (n = 88) | |||||
| Grade 1-2 | Grade 3-4 | Grade 1-2 | Grade 3-4 | Grade 1-2 | Grade 3-4 | Grade 1-2 | Grade 3-4 | |
| Fever | 67 (20.7) | 23 (7.1) | 4 (3.4) | 2 (1.7) | 20 (22.7) | 8 (9.1) | 4 (4.5) | 0 (0) |
| Nausea | 100 (30.9) | 0 (0) | 6 (5.1) | 0 (0) | 24 (27.3) | 0 (0) | 5 (5.7) | 0 (0) |
| Vomit | 32 (9.9) | 15 (4.6) | 7 (5.9) | 6 (5.1) | 7 (8.0) | 3 (3.4) | 6 (6.8) | 4 (4.5) |
| Abdominal pain | 97 (29.9) | 162 (50.0) | 18 (15.3) | 18 (15.3) | 24 (27.3) | 44 (50.0) | 15 (17.0) | 13 (14.8) |
| ALT increased | 79 (24.4) | 125 (38.6) | 32 (27.1) | 39 (33.1) | 27 (30.4) | 33 (37.5) | 22 (25.0) | 29 (33.0) |
| AST increased | 130 (40.1) | 161 (49.7) | 36 (30.5) | 44 (37.3) | 40 (45.5) | 49 (55.7) | 26 (29.5) | 34 (38.6) |
| Hyperbilirubinemia | 37 (11.4) | 7 (2.2) | 7 (5.9) | 11 (9.3) | 10 (11.4) | 0 (0) | 4 (4.5) | 8 (9.1) |
| Anemia | 37 (11.4) | 8 (2.5) | 7 (5.9) | 4 (3.4) | 11 (12.5) | 1 (1.1) | 5 (5.7) | 3 (3.4) |
| Neutropenia | 86 (26.5) | 20 (6.2) | 9 (7.6) | 12 (10.2) | 29 (33.0) | 5 (5.7) | 5 (5.7) | 9 (10.2) |
| Thrombocytopenia | 103 (31.8) | 90 (27.8) | 27 (22.9) | 20 (16.9) | 24 (27.3) | 16 (18.2) | 20 (22.7) | 13 (14.8) |
| Bleeding | 48 (14.8) | 3 (0.9) | 1 (0.8) | 1 (0.8) | 12 (13.6) | 1 (1.1) | 1 (1.1) | 1 (1.1) |
| Diarrhea | 62 (19.1) | 60 (18.5) | 22 (18.6) | 3 (2.5) | 17 (19.3) | 5 (5.7) | 17 (19.3) | 3 (3.4) |
| Hoarseness | 80 (24.7) | 0 (0) | 13 (11.0) | 0 (0) | 21 (23.9) | 0 (0) | 10 (11.4) | 0 (0) |
| Rash | 92 (28.4) | 4 (1.2) | 28 (23.7) | 4 (3.4) | 24 (27.3) | 0 (0) | 20 (22.7) | 3 (3.4) |
| HFS | 69 (21.3) | 9 (2.8) | 19 (16.1) | 18 (15.3) | 18 (20.5) | 1 (1.1) | 14 (15.9) | 11 (12.5) |
| Hypertension | 76 (23.5) | 11 (3.4) | 31 (26.3) | 34 (28.8) | 22 (25.0) | 3 (3.4) | 23 (26.1) | 27 (30.7) |
| RCCEP | 42 (13.0) | 14 (4.3) | 16 (13.6) | 6 (5.1) | 11 (12.5) | 5 (5.7) | 13 (14.8) | 6 (6.8) |
| Hypothyroidism | 64 (19.8) | 8 (2.5) | 2 (1.7) | 0 (0) | 18 (20.5) | 2 (2.3) | 0 (0) | 0 (0) |
| Fatigue | 32 (9.9) | 31 (9.6) | 4 (3.4) | 4 (3.4) | 6 (6.8) | 8 (9.1) | 3 (3.4) | 2 (2.3) |
| Hepatitis | 10 (3.1) | 1 (0.3) | 0 (0) | 0 (0) | 5 (5.7) | 1 (1.1) | 0 (0) | 0 (0) |
| Coma | 0 (0) | 2 (0.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Although the first-line treatment recommended by authoritative clinical guidelines for uHCC is AIPB, such as atezolizumab plus bevacizumab, these treatments have a number of limitations in clinical applications. The default anti-inflammatory or immunotolerant immune status of the liver may interfere with the drugs that act on it[26], which may lead to a relatively low ORR. The main cause of death among patients with uHCC is liver tumor progression[27,28]. Although there are a number of different protocols for administering AIPB, the median patient survival time using this approach has remained less than 24 months[17,19,20,29]. In addition, the IMbrave150 studies suggested that the effects of AIPB treatment are likely to be severely diminished if patients are diagnosed with high-risk factors, such as tumor invasion of the main trunk of the portal vein (Vp4), bile duct invasion, or/or tumor occupancy of ≥ 50% of the liver[17,30].
Many uHCC patients in some areas, especially in China, are diagnosed with vascular invasion or/or a high tumor burden. The most effective way to prolong survival is to control liver lesions. In terms of local hepatic treatment for uHCC, the most popular choice for most physicians is transcatheter arterial chemoembolization (TACE). Nonetheless, if the tumor burden is high, there is a very high probability of “TACE failure/refractoriness”[31-33]. If patients are diagnosed with reduced or absent portal vein blood supplies caused by portal vein tumor thrombi or severe cirrhosis, the use of TACE will be limited. Several studies have revealed that HAIC is more effective than TACE for large HCCs[34]. The FOHAIC study suggested that FOLFOX-HAIC had a significant effect on patients with uHCC and that HAIC could be used as an additional local hepatic treatment for uHCC[8]. According to our meta-analysis, HAIC plus sorafenib or HAIC alone was able to prolong the survival time of patients with uHCC more than AIPB regimens. Unfortunately, there is a scarcity of prospective or retrospective studies with large sample sizes on triple therapy.
Our retrospective data revealed that triple therapy was effective and safe. The ORR, PFS, and OS of patients receiving triple therapy (ORR: 33.2% per mRECIST; PFS: 6.9 months; OS: 19.2 months) outperformed those of patients receiving most AIPB regimens. For example, this was true for atezolizumab plus bevacizumab (ORR: 33.2% per mRECIST; PFS: 6.9 months; OS: 19.2 months) in the IMbrave150 trial; pembrolizumab plus lenvatinib (ORR: 40.8% per mRECIST; PFS: 8.2 months; OS: 21.2 months) in the LEAP002 trial; and camrelizumab plus rivoceranib (ORR: 33.1% per mRECIST; PFS: 5.6 months; OS: 22.1 months) in the CARES 310 study[7,17,19,29]. The survival benefit observed in this study may be due to the synergistic antitumor effects of these chemical agents. Transarterial chemotherapy can induce immunogenic cell death by releasing tumor-related antigens and supporting the evolution of tumor-specific CD8+ T cells, which may synergize with angiogenesis inhibitors to enhance the effect of PD-1/PD-L1 blockers[35-37]. Increased concentrations of drugs in the liver can cause liver lesions to shrink directly and slow the deterioration of liver function caused by disease progression. According to our survival analysis, the patients in the AIPB subgroup had shorter survival times than those in the other trials on AIPB regimens, likely due to their poor baseline conditions prior to treatment. More than half of the patients (n = 58) in the AIBP group were diagnosed with major vein tumor thrombus, and the mean maximum tumor diameter was more than 9 cm, suggesting that the patients in this group had high tumor burdens. The median OS of similar patients in the IMbrave150 study was only 7.8 months, which is consistent with the results of our study[30].
We also attempted to identify which factors could influence the effect of triple therapy and found that > 4 rounds of HAIC were a protective factor. Four rounds of HAIC represent a regimen similar to the median number of HAIC rounds reported in several other studies[8,38-40]. The number of HAIC rounds performed was strongly affected by each patient’s response to triple therapy because if tumors progress after the first few HAIC cycles, the HAIC cycles will be discontinued. Multiple liver lesions have also been recognized as risk factors because the presence of multiple lesions often implies the presence of multiple feeding vessels. Therefore, even if attempts are made to embolize other arteries until there is only a single blood supply, there is a high probability that some small arteries may be missed. To ensure that all lesions can be treated by HAIC, a microcatheter should be placed in a larger blood vessel branch, which implies that more normal liver tissue is likely to be damaged by the administered drugs, potentially harming liver function.
Overall, the incidence of adverse reactions to triple therapy was greater than that reported in the AIPB group. The combination of HAIC and systematic treatments was able to increase the incidence of AEs; another reasonable explanation is that most AIBP patients were treated and followed up as outpatients so that some AEs, especially some slight AEs, were ignored. Notably, abnormal liver function was the common AE in the triple therapy group. However, unlike many other local treatments, the effects of HAIC on liver function appear to be largely short-term, with few apparent adverse effects on long-term survival. However, we believe that the limitation of triple therapy in the Child-Pugh B population with poor hepatic functional reserve may result from irreversible liver injury secondary to chemotherapy. Another common AE, thrombocytopenia, is caused not only by myelosuppression due to chemotherapy but also by hypersplenism secondary to cirrhosis. A substantial proportion of the patients recovered from thrombocytopenia following splenic embolization.
A substantial amount of information was lost due to the limitations of this retrospective study. Our sample included only patients from China, so the study was inevitably affected by some degree of sampling bias. It remains unclear exactly which biomarkers can be used to judge patients’ prognoses. Studies at the cellular or molecular level could not be carried out due to a lack of tumor biopsy samples. To prove the efficacy and safety of triple therapy, additional large-scale prospective RCTs on this topic are warranted.
This meta-analysis suggested that HAIC-based treatment regimens were able to effectively improve the prognosis in patients with uHCC. Our findings confirmed that even though the triple therapy protocol increased the incidence and severity of AEs, it yielded a higher ORR and longer PFS and OS than AIPB.
Unresectable hepatocellular carcinoma had been difficult to be treated in the past, hepatic arterial chemotherapy infusion chemotherapy (HAIC) as well as angiogenesis inhibitors plus programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) blockers were proved to prolong the unresectable hepatocellular carcinoma (uHCC) patients' survival, respectively. Meanwhile, some phase II single arm suggested that the combination of HAIC and angiogenesis inhibitors and PD-1/PD-L1 blockers (AIPB) (triple therapy) was effective in treating uHCC. But which treatment is the best choice was still confused. The study was designed to answer the question.
The best first-line treatment for uHCC was unclear and there was lack of studies to compare the efficacy and safety between triple therapy and AIPB. There were so many choices that clinical staff may be confused when they need to treat uHCC patients. If we can find the relatively better regimen, it is helpful for the standardization of the uHCC treatment to improve the patients' prognosis.
The study aimed to identified the HAIC and HAIC-based treatments was the best choice for uHCC. Based on the result, we explored the efficacy and safety of one of HAIC-based treatment, triple therapy in the real-world condition compared to AIPB. The results of the study could be the evidence to guide clinical reasonable treatment and prospective clinical study.
We have tried to perform a network meta-analysis to find the first choice to uHCC and identified the efficacy and safety of triple therapy compared to AIPB through a retrospective cohort study.
The network meta-analysis including 13 phase randomized controlled trials (RCTs) showed HAIC and HAIC-based treatments were likely to be the first choice to treat uHCC. HAIC plus camrelizumab plus AIPB (triple therapy) had better progression-free survival and overall survival than AIPB without HAIC for uHCC. Even though the incidence of adverse events in the triple therapy group was higher than the AIPB group, the safety of triple therapy was still acceptable.
HAIC-based treatments were better than other regimens for treating uHCC. Triple therapy was more effective than AIPB in the Chinese uHCC population. All of the above results proved the significance of local treatments in the uHCC treating.
There is absolutely a need for studies at the cellular or molecular level and additional large-scale prospective RCTs on this topic.
We are thankful for the support of the patients in this study. We are grateful to Dr. Fei Cao and Ms. Xiao-Ting Bei for their assistance with the clinical management of the patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Saadi S, United States S-Editor: Li L L-Editor: A P-Editor: Chen YX
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64681] [Article Influence: 16170.3] [Reference Citation Analysis (177)] |
| 2. | Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, Gores G, Kerlan R, Merle P, O'Neil B, Poon R, Schwartz L, Tepper J, Yao F, Haller D, Mooney M, Venook A. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994-4005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 323] [Article Influence: 21.5] [Reference Citation Analysis (1)] |
| 3. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3176] [Article Influence: 529.3] [Reference Citation Analysis (37)] |
| 4. | Kudo M, Arizumi T. Transarterial Chemoembolization in Combination with a Molecular Targeted Agent: Lessons Learned from Negative Trials (Post-TACE, BRISK-TA, SPACE, ORIENTAL, and TACE-2). Oncology. 2017;93:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10271] [Article Influence: 604.2] [Reference Citation Analysis (2)] |
| 6. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib vs sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3831] [Article Influence: 547.3] [Reference Citation Analysis (1)] |
| 7. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4704] [Article Influence: 940.8] [Reference Citation Analysis (2)] |
| 8. | Lyu N, Wang X, Li JB, Lai JF, Chen QF, Li SL, Deng HJ, He M, Mu LW, Zhao M. Arterial Chemotherapy of Oxaliplatin Plus Fluorouracil Versus Sorafenib in Advanced Hepatocellular Carcinoma: A Biomolecular Exploratory, Randomized, Phase III Trial (FOHAIC-1). J Clin Oncol. 2022;40:468-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 185] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 9. | Gu YK, Zhang TQ, Zuo MX, Geng ZJ, Li JB, Huang ZL, Wu PH. Hepatic artery infusion chemotherapy (HAIC) combined with apatinib and camrelizumab for hepatocellular carcinoma (HCC) in BCLC stage c: A prospective, single-arm, phase II trial (TRIPLET study). Conference Abstract. Journal of Clinical Oncology. 40. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Lai Z, He M, Bu X, Xu Y, Huang Y, Wen D, Li Q, Xu L, Zhang Y, Wei W, Chen M, Kan A, Shi M. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: A biomolecular exploratory, phase II trial. Eur J Cancer. 2022;174:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 110] [Reference Citation Analysis (0)] |
| 11. | Liu BJ, Gao S, Zhu X, Guo JH, Kou FX, Liu SX, Zhang X, Wang XD, Cao G, Chen H, Liu P, Xu HF, Gao QZ, Yang RJ. Real-world study of hepatic artery infusion chemotherapy combined with anti-PD-1 immunotherapy and tyrosine kinase inhibitors for advanced hepatocellular carcinoma. Immunotherapy. 2021;13:1395-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 12. | Xu Y, Fu S, Mao Y, Huang S, Li D, Wu J. Efficacy and safety of hepatic arterial infusion chemotherapy combined with programmed cell death protein-1 antibody and lenvatinib for advanced hepatocellular carcinoma. Front Med (Lausanne). 2022;9:919069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Chen LT, Martinelli E, Cheng AL, Pentheroudakis G, Qin S, Bhattacharyya GS, Ikeda M, Lim HY, Ho GF, Choo SP, Ren Z, Malhotra H, Ueno M, Ryoo BY, Kiang TC, Tai D, Vogel A, Cervantes A, Lu SN, Yen CJ, Huang YH, Chen SC, Hsu C, Shen YC, Tabernero J, Yen Y, Hsu CH, Yoshino T, Douillard JY. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol. 2020;31:334-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 164] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 14. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2615] [Article Influence: 871.7] [Reference Citation Analysis (59)] |
| 15. | Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE, Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 1172] [Article Influence: 586.0] [Reference Citation Analysis (1)] |
| 16. | He M, Li Q, Zou R, Shen J, Fang W, Tan G, Zhou Y, Wu X, Xu L, Wei W, Le Y, Zhou Z, Zhao M, Guo Y, Guo R, Chen M, Shi M. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma With Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol. 2019;5:953-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 381] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 17. | Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 962] [Article Influence: 320.7] [Reference Citation Analysis (0)] |
| 18. | Qin S, Chan LS, Gu S, Bai Y, Ren Z, Lin X, Chen Z, Jia W, Jin Y, Guo Y, Sultanbaev AV, Pazgan-Simon M, Pisetska M, Liang X, Chen C, Nie Z, Wang L, Cheng AL, Kaseb A, Vogel A. LBA35 Camrelizumab (C) plus rivoceranib (R) vs sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): A randomized, phase III trial. Conference Abstract. Annals of Oncology. 2022;33:S1401-S1402. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 19. | Finn RS, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, Xu R, Edeline J, Ryoo BY, Ren Z, Cheng AL, Galle PR, Kaneko S, Kumada H, Wang A, Mody K, Dubrovsky L, Siegel AB, Llovet J. LBA34 Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab vs lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Conference Abstract. Annals of Oncology. 2022;33:S1401. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 146] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 20. | Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, Li Q, Lu Y, Chen Y, Guo Y, Chen Z, Liu B, Jia W, Wu J, Wang J, Shao G, Zhang B, Shan Y, Meng Z, Gu S, Yang W, Liu C, Shi X, Gao Z, Yin T, Cui J, Huang M, Xing B, Mao Y, Teng G, Qin Y, Xia F, Yin G, Yang Y, Chen M, Wang Y, Zhou H, Fan J; ORIENT-32 study group. Sintilimab plus a bevacizumab biosimilar (IBI305) vs sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22:977-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 672] [Article Influence: 168.0] [Reference Citation Analysis (1)] |
| 21. | Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Sieghart W, Assenat E, Zaucha R, Furuse J, Abou-Alfa GK, El-Khoueiry AB, Melero I, Begic D, Chen G, Neely J, Wisniewski T, Tschaika M, Sangro B. Nivolumab vs sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 745] [Article Influence: 186.3] [Reference Citation Analysis (0)] |
| 22. | Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, Choo SP, Cheow PC, Chotipanich C, Lim K, Lesmana LA, Manuaba TW, Yoong BK, Raj A, Law CS, Cua IHY, Lobo RR, Teh CSC, Kim YH, Jong YW, Han HS, Bae SH, Yoon HK, Lee RC, Hung CF, Peng CY, Liang PC, Bartlett A, Kok KYY, Thng CH, Low AS, Goh ASW, Tay KH, Lo RHG, Goh BKP, Ng DCE, Lekurwale G, Liew WM, Gebski V, Mak KSW, Soo KC; Asia-Pacific Hepatocellular Carcinoma Trials Group. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J Clin Oncol. 2018;36:1913-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 473] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 23. | Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, Huang F, Tang R, Cheng Y, Huang Z, Liang Y, Fan H, Qiao L, Li F, Zhuang W, Peng B, Wang J, Li J, Kuang M. Lenvatinib Combined With Transarterial Chemoembolization as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Phase III, Randomized Clinical Trial (LAUNCH). J Clin Oncol. 2023;41:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 251] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 24. | Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, Ying J, Lu Y, Meng Z, Pan H, Yang P, Zhang H, Chen X, Xu A, Cui C, Zhu B, Wu J, Xin X, Wang J, Shan J, Chen J, Zheng Z, Xu L, Wen X, You Z, Ren Z, Liu X, Qiu M, Wu L, Chen F. Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial. J Clin Oncol. 2021;39:3002-3011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 258] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 25. | Qin S, Kudo M, Meyer T, Finn RS, Vogel A, Bai Y, Guo Y, Meng Z, Zhang T, Satoh T, Hiraoka A, Marino D, Assenat E, Wyrwicz L, Campos MC, Hsing-Tao K, Boisserie F, Li S, Chen Y, Zhu AX. LBA36 Final analysis of RATIONALE-301: Randomized, phase III study of tislelizumab vs sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Conference Abstract. Annals of Oncology. 33:S1402-S1403. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 26. | Kubes P, Jenne C. Immune Responses in the Liver. Annu Rev Immunol. 2018;36:247-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 601] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 27. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1900] [Article Influence: 271.4] [Reference Citation Analysis (0)] |
| 28. | Gauci ML, Lanoy E, Champiat S, Caramella C, Ammari S, Aspeslagh S, Varga A, Baldini C, Bahleda R, Gazzah A, Michot JM, Postel-Vinay S, Angevin E, Ribrag V, Hollebecque A, Soria JC, Robert C, Massard C, Marabelle A. Long-Term Survival in Patients Responding to Anti-PD-1/PD-L1 Therapy and Disease Outcome upon Treatment Discontinuation. Clin Cancer Res. 2019;25:946-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 29. | Qin S, Chan SL, Gu S, Bai Y, Ren Z, Lin X, Chen Z, Jia W, Jin Y, Guo Y, Hu X, Meng Z, Liang J, Cheng Y, Xiong J, Ren H, Yang F, Li W, Chen Y, Zeng Y, Sultanbaev A, Pazgan-Simon M, Pisetska M, Melisi D, Ponomarenko D, Osypchuk Y, Sinielnikov I, Yang TS, Liang X, Chen C, Wang L, Cheng AL, Kaseb A, Vogel A; CARES-310 Study Group. Camrelizumab plus rivoceranib vs sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. 2023;402:1133-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 342] [Article Influence: 171.0] [Reference Citation Analysis (2)] |
| 30. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Lim HY, Breder V, Merle P, Kaseb A, Li D, Feng YH, Verret W, Nicholas A, Li L, Ma N, Zhu AX, Cheng AL. IMbrave150: Updated efficacy and safety by risk status in patients (pts) receiving atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first-line treatment for unresectable hepatocellular carcinoma (HCC). Conference Abstract. Cancer Research. 2021;81. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1574] [Article Influence: 92.6] [Reference Citation Analysis (1)] |
| 32. | Yasui Y, Tsuchiya K, Kurosaki M, Takeguchi T, Takeguchi Y, Okada M, Wang W, Kubota Y, Goto T, Komiyama Y, Higuchi M, Takaura K, Hayashi T, Takada H, Tamaki N, Nakanishi H, Itakura J, Takahashi Y, Asahina Y, Enomoto N, Himeno Y, Izumi N. Up-to-seven criteria as a useful predictor for tumor downstaging to within Milan criteria and Child-Pugh grade deterioration after initial conventional transarterial chemoembolization. Hepatol Res. 2018;48:442-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Wang Q, Xia D, Bai W, Wang E, Sun J, Huang M, Mu W, Yin G, Li H, Zhao H, Li J, Zhang C, Zhu X, Wu J, Gong W, Li Z, Lin Z, Pan X, Shi H, Shao G, Liu J, Yang S, Zheng Y, Xu J, Song J, Wang W, Wang Z, Zhang Y, Ding R, Zhang H, Yu H, Zheng L, Gu W, You N, Wang G, Zhang S, Feng L, Liu L, Zhang P, Li X, Chen J, Xu T, Zhou W, Zeng H, Huang W, Jiang W, Zhang W, Shao W, Li L, Niu J, Yuan J, Lv Y, Li K, Yin Z, Xia J, Fan D, Han G; China HCC-TACE Study Group. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: A multicentre observational study. J Hepatol. 2019;70:893-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 34. | Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, Wei W, Zhang YJ, Guo Y, Guo RP, Chen MS, Shi M. Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Transarterial Chemoembolization for Large Hepatocellular Carcinoma: A Randomized Phase III Trial. J Clin Oncol. 2022;40:150-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 242] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 35. | Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 438] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 36. | Zhu H, Shan Y, Ge K, Lu J, Kong W, Jia C. Oxaliplatin induces immunogenic cell death in hepatocellular carcinoma cells and synergizes with immune checkpoint blockade therapy. Cell Oncol (Dordr). 2020;43:1203-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 37. | Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, Mendiboure J, Pignon JP, Jooste V, van Endert P, Ducreux M, Zitvogel L, Piard F, Kroemer G. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 919] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 38. | Lyu N, Kong Y, Mu L, Lin Y, Li J, Liu Y, Zhang Z, Zheng L, Deng H, Li S, Xie Q, Guo R, Shi M, Xu L, Cai X, Wu P, Zhao M. Hepatic arterial infusion of oxaliplatin plus fluorouracil/Leucovorin vs sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 39. | Liang RB, Zhao Y, He MK, Wen DS, Bu XY, Huang YX, Lai ZC, Xu YJ, Kan A, Wei W, Zhang YJ, Chen MS, Guo RP, Li QJ, Shi M. Hepatic Arterial Infusion Chemotherapy of Oxaliplatin, Fluorouracil, and Leucovorin With or Without Sorafenib as Initial Treatment for Advanced Hepatocellular Carcinoma. Front Oncol. 2021;11:619461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Zheng K, Zhu X, Fu S, Cao G, Li WQ, Xu L, Chen H, Wu D, Yang R, Wang K, Liu W, Wang H, Bao Q, Liu M, Hao C, Shen L, Xing B, Wang X. Sorafenib Plus Hepatic Arterial Infusion Chemotherapy vs Sorafenib for Hepatocellular Carcinoma with Major Portal Vein Tumor Thrombosis: A Randomized Trial. Radiology. 2022;303:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |