Published online May 21, 2024. doi: 10.3748/wjg.v30.i19.2553
Revised: March 5, 2024
Accepted: April 25, 2024
Published online: May 21, 2024
Processing time: 127 Days and 23.5 Hours
The role of exosomes derived from HepG2.2.15 cells, which express hepatitis B virus (HBV)-related proteins, in triggering the activation of LX2 liver stellate cells and promoting liver fibrosis and cell proliferation remains elusive. The focus was on comprehending the relationship and influence of differentially expressed microRNAs (DE-miRNAs) within these exosomes.
To elucidate the effect of exosomes derived from HepG2.2.15 cells on the activation of hepatic stellate cell (HSC) LX2 and the progression of liver fibrosis.
Exosomes from HepG2.2.15 cells, which express HBV-related proteins, were isolated from parental HepG2 and WRL68 cells. Western blotting was used to confirm the presence of the exosomal marker protein CD9. The activation of HSCs was assessed using oil red staining, whereas DiI staining facilitated the observation of exosomal uptake by LX2 cells. Additionally, we evaluated LX2 cell proliferation and fibrosis marker expression using 5-ethynyl-2′-deoxyuracil staining and western blotting, respectively. DE-miRNAs were analyzed using DESeq2. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were used to annotate the target genes of DE-miRNAs.
Exosomes from HepG2.2.15 cells were found to induced activation and enhanced proliferation and fibrosis in LX2 cells. A total of 27 miRNAs were differentially expressed in exosomes from HepG2.2.15 cells. GO analysis indicated that these DE-miRNA target genes were associated with cell differentiation, intracellular signal transduction, negative regulation of apoptosis, extracellular exosomes, and RNA binding. KEGG pathway analysis highlighted ubiquitin-mediated proteolysis, the MAPK signaling pathway, viral carcinogenesis, and the toll-like receptor signaling pathway, among others, as enriched in these targets.
These findings suggest that exosomes from HepG2.2.15 cells play a substantial role in the activation, proliferation, and fibrosis of LX2 cells and that DE-miRNAs within these exosomes contribute to the underlying mechanisms.
Core Tip: This study investigated the effects of exosomes, particularly those derived from HepG2.2.15 cells, on the activation of LX2 stellate cells and the progression of liver fibrosis. Exosomes from HepG2.2.15 cells been found to enhance LX2 cell activation, proliferation, and fibrosis. These exosomes contained 27 differentially expressed microRNAs that target various cellular functions and pathways, including differentiation, signal transduction, and apoptosis regulation. This suggests a significant role for HepG2.2.15-derived exosomes in liver fibrosis, with potential therapeutic implications.
- Citation: Gao Y, Li L, Zhang SN, Mang YY, Zhang XB, Feng SM. HepG2.2.15-derived exosomes facilitate the activation and fibrosis of hepatic stellate cells. World J Gastroenterol 2024; 30(19): 2553-2563
- URL: https://www.wjgnet.com/1007-9327/full/v30/i19/2553.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i19.2553
Introduction of chronic hepatitis B infection (CHB) induced by the hepatitis B virus (HBV) is a considerable public health concern, as it causes substantial morbidity, contributing to nearly one-third of liver cirrhosis cases worldwide[1-3]. Hepatocyte damage and subsequent necrosis are the primary clinical manifestation of CHB that occur due to the immune response generated by the HBV-infected liver cells[4]. Moreover, there is a strong association between liver injury and the onset of liver fibrosis. Current therapeutic measures prioritize the use of antioxidants and hepatoprotective agents. However, these treatments have limited efficacy in attenuating liver damage and inhibiting the advancement of fibrosis[5]. Therefore, thorough research on HBV-induced fibrogenesis and the pursuit of superior therapeutic approaches are indispensable.
Hepatic stellate cells (HSCs), which are widely dispersed throughout the liver as perisinusoidal cells, perform various functions ranging from vascular regulation to drug metabolism in the healthy liver[5]. Typically, quiescent cells are activated upon liver injury, proliferate, and exhibit heightened contractility, inflammation, chemotaxis, and an increase in extracellular matrix (ECM) production, which critically contribute to fibrosis[6]. Consequently, elucidating the role of HSCs in fibrogenesis may herald novel treatments for liver fibrosis.
Exosomes are small extracellular vesicles, approximately 100 nm in size, secreted by various cells and present ubiquitously in biological fluids[7]. Their composition includes diverse proteins and nucleic acids such as double-stranded DNA, mRNA, microRNAs (miRNAs), and long non-coding RNA (lncRNAs), enabling them to merge with target cells and modulate their function[8]. Exosomes actively engage in the pathophysiological mechanisms of numerous diseases[9,10]. Recent evidence suggests that exosomes from hepatitis C virus-infected hepatocytes containing specific miRNAs induce HSC activation, thereby facilitating fibrogenesis[11]. They are integral to the immune defense against HBV, carrying virus-derived nucleic acids that provoke macrophages[12], whereas cholangiocyte-originating exosomes containing H19 increase HSC differentiation and activation, promoting fibrosis[13]. However, the specific roles of exosomes in HBV-related liver fibrosis require further investigation.
In this study, we found that exosomes emanating from HBV-infected hepatocytes induced the activation and proliferation of LX2 cells, an immortalized line of human HSCs. Using small RNA sequencing, differentially expressed microRNAs (DE-miRNAs) in HepG2.2.15 cell-derived exosomes (an HBV-infected hepatocellular carcinoma cell line) and their non-infected counterparts were identified and profiled. Subsequent annotations based on Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) illuminated the miRNA target gene functions, thus presenting the pathways implicated in HBV-mediated liver pathology.
On February 21, 2022, the Ethics Committee of The Affiliated Calmette Hospital of Kunming Medical University and the First Hospital of Kunming approved the ethical review of this research. All procedures were conducted in accordance with the relevant laws and institutional guidelines.
HepG2.2.15, HepG2, and WRL68 cell lines were obtained from the American Type Culture Collection (United States). WRL-68 cells, which resemble hepatocytes, are suitable for in vitro liver studies because of their similar morphology, protein secretion, cytokeratin patterns, and key liver enzyme activities[14]. HepG2.2.15 cells were cultured in DMEM (Thermo Fisher Scientific, Waltham, MA, United States) supplemented with 10% fetal bovine serum (FBS; GIBCO BRL, Grand Island, NY, United States) and G418 (Thermo Fisher Scientific, United States). HepG2 cells were grown in DMEM supplemented with 10% FBS. WRL68 cells were maintained in DMEM supplemented with 10% FBS serum and 1 mmol/L sodium pyruvate (Thermo Fisher Scientific, United States). All cell lines were cultivated at 37 °C in a humidified atmosphere containing 5% CO2.
Exosomes were isolated from the culture media of HepG2.2.15, HepG2, and WRL68 cells following a previously described protocol[15]. Initially, media samples were centrifuged at 2000 g for 30 min at 4 °C, followed by a secondary centrifugation at 10000 g for 45 min at 4 °C. Afterward, the supernatant was filtered through a 0.45 μM filter and ultracentrifuged at 100000 g for 70 min at 4 °C. The pellet was suspended in 100 μL of chilled PBS. Portions of the resuspended exosomes (20 μL for electron microscopy, 10 μL for particle size analysis) were set aside, with the remainder stored at -80 °C. LX2 cells were co-cultured with exosomes using a Transwell system, following the methods outlined in a previous study[16].
For transmission electron microscopy (TEM) analysis, 10 μL of exosome suspension and acetate dioxide were applied to a copper grid and left to settle for 1 min. After drying with a filter paper for 2 min at room temperature, the samples were examined using a Hitachi H-7650 transmission electron microscope (Hitachi, Tokyo, Japan) at an acceleration voltage of 100 kV. A NanoSight NS300 system (Malvern Panalytical, Shanghai, China) was employed to measure exosome size distribution and concentration by leveraging Brownian motion[17].
RIPA buffer (Abcam) was used to extract total protein from LX2 cells exposed to exosomes. Protein concentrations were quantified using the BCA Protein Assay Kit (Abcam, China). Proteins were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto PVDF membranes, which were blocked with 5% fat-free milk at room temperature overnight. Primary antibodies used were CD9, α-smooth muscle actin (α-SMA), COL1A1, MMP-2, TIMP-1, CTGF, and β-actin, all at 1:1000 dilutions (Abcam), followed by incubation with Goat Anti-Rabbit immunoglobulin G H&L (HRP, 1:10000, ab7090, Abcam) for 2 h at room temperature. Beta-actin served as a loading control. Relative expression levels were calculated using grayscale values of target proteins normalized to β-actin, with analysis conducted in Image J software (National Institutes of Health, United States).
Cell proliferation was measured using 5-ethynyl-2′-deoxyuracil (EDU) kit (RiboBio, Guangzhou, China), and the ratio of EDU staining cells (with red fluorescence) to Hoechst33342C stained cells (with blue fluorescence) was used to evaluate cell proliferation rate. LX2 cells were seeded into 96-well plates at a concentration of 2 × 103 per well and incubated with EDU reagent. After incubation for 2 h, the experiments were performed according to the kit instruction[18]. Finally, Hoechst staining was performed for 30 min, the stained cells were observed under a microscope, and ImageJ software (National Institutes of Health, Bethesda, MD, United States) was used for data analysis.
For Dil staining, exosomes were incubated with 10 μL of Dil solution (Invitrogen, Carlsbad, CA, United States) at 37° for 30 min and then washed with PBS. After 10 μg/mL of Dil-labeled exosomes were co-cultured with LX2 cells for 24 h, the cells were fixed with 4% paraformaldehyde. Cytoplasm was stained with α-SMA, nucleus was stained with DAPI, and uptake of exosomes were observed by an inverted fluorescence microscope.
For Oil red staining, cells were fixed in 10% formalin, washed with 60% propylene glycerol, and then stained with 0.5% oil red (Sigma-Aldrich, St. Louis, MO, United States) with propylene glycerol for 10 min at 60 °C. The red lipid droplets were observed under a microscope and photographed.
Small-RNA sequencing and subsequent bioinformatics analyses were performed by Shanghai Oebiotech Co., LTD (China). Differential expression analysis was based on the DESeq2 package of R software[19]. DE-miRNAs (P < 0.05, log2FC > 1) were selected for subsequent analyses. The prediction of target genes of DE-miRNAs was performed in the miRNADA database[20] with threshold parameters of S ≥ 150 and ΔG ≤ −30 kcal/moL. GO function enrichment analysis and KEGG pathway enrichment analysis of targets were performed using ClusterProfiler package[21]. P value ≤ 0.05 were considered statistically significant.
GraphPad Prism version 8.0 was used for the statistical analysis. To ascertain the significance between two distinct groups, an unpaired t-test was employed, whereas one-way analysis of variance was used to analyze the significance among multiple groups. Statistical significance was set at P < 0.05.
Exosomes were isolated from HepG2.2.15, HepG2, and WRL68 cells and their presence was confirmed. Initially, the exosome marker protein CD9 was identified by western blot analysis, which demonstrated its expression in exosomes from all three cell types (Figure 1A). TEM and particle size analyses were performed for further verification. The exosomes displayed characteristic double-layered membranes and predominantly cup-shaped morphologies, with diameters ranging between 50 and 140 nm and an average size of approximately 82.47 nm (Figure 1B and C), thereby confirming that the particles were exosomes.
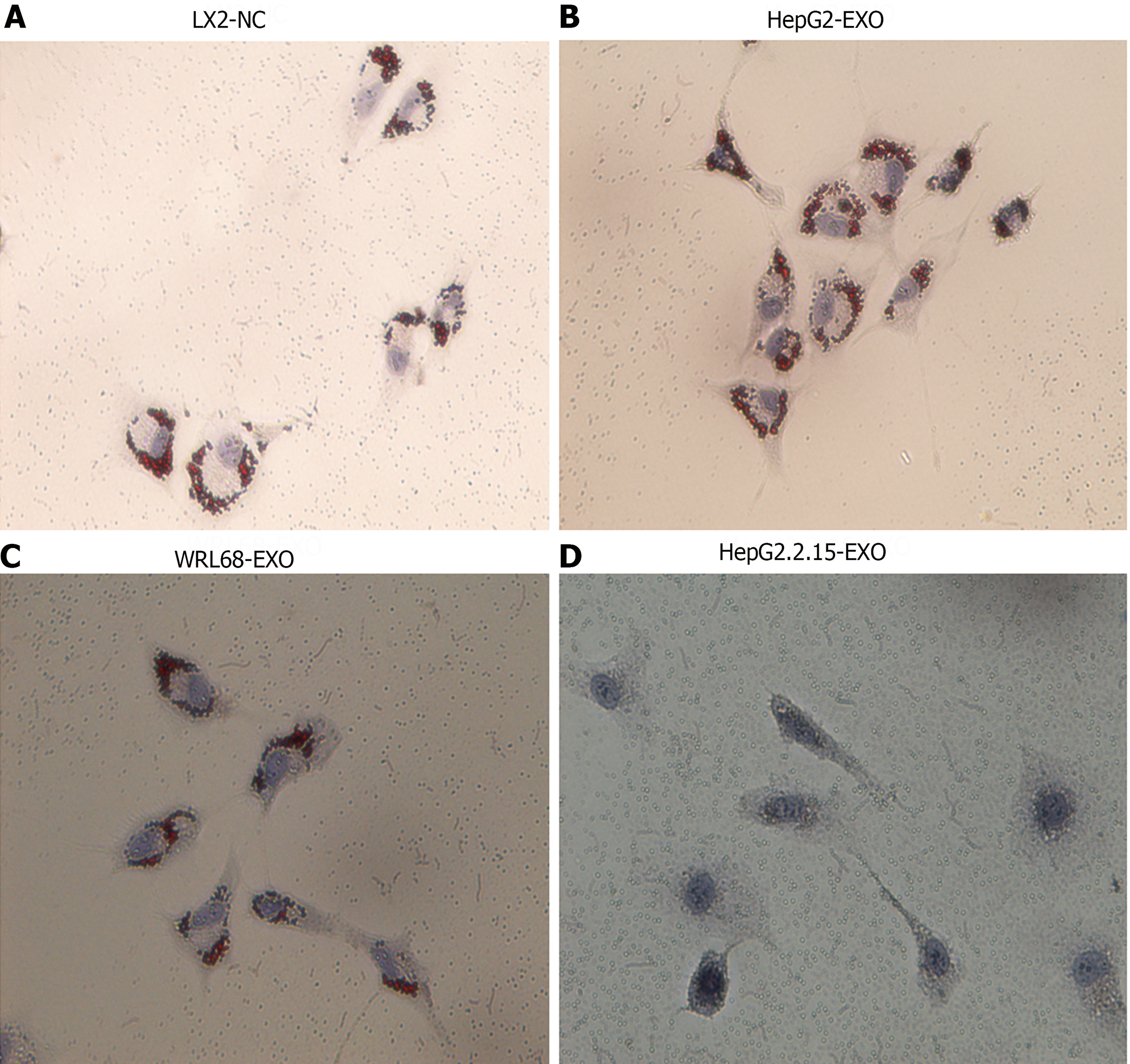
Resting HSCs are rich in lipid droplets. However, during HSC activation, these droplets either decrease or disappear. Exosomes derived from HepG2.2.15, HepG2, and WRL68 cells (termed HepG2.2.15-exo, HepG2-exo, and WRL68-exo, respectively) were co-cultured with LX2 cells. After 24 h of co-culture, compared to the control and other exosome-treated LX2 cells (Figure 2A-C), oil red staining indicated the lack of lipid droplets in LX2 cells treated with HepG2.2.15 exosomes (Figure 2D). This suggests that exosomes from HBV-infected hepatocytes potentiate HSC activation.
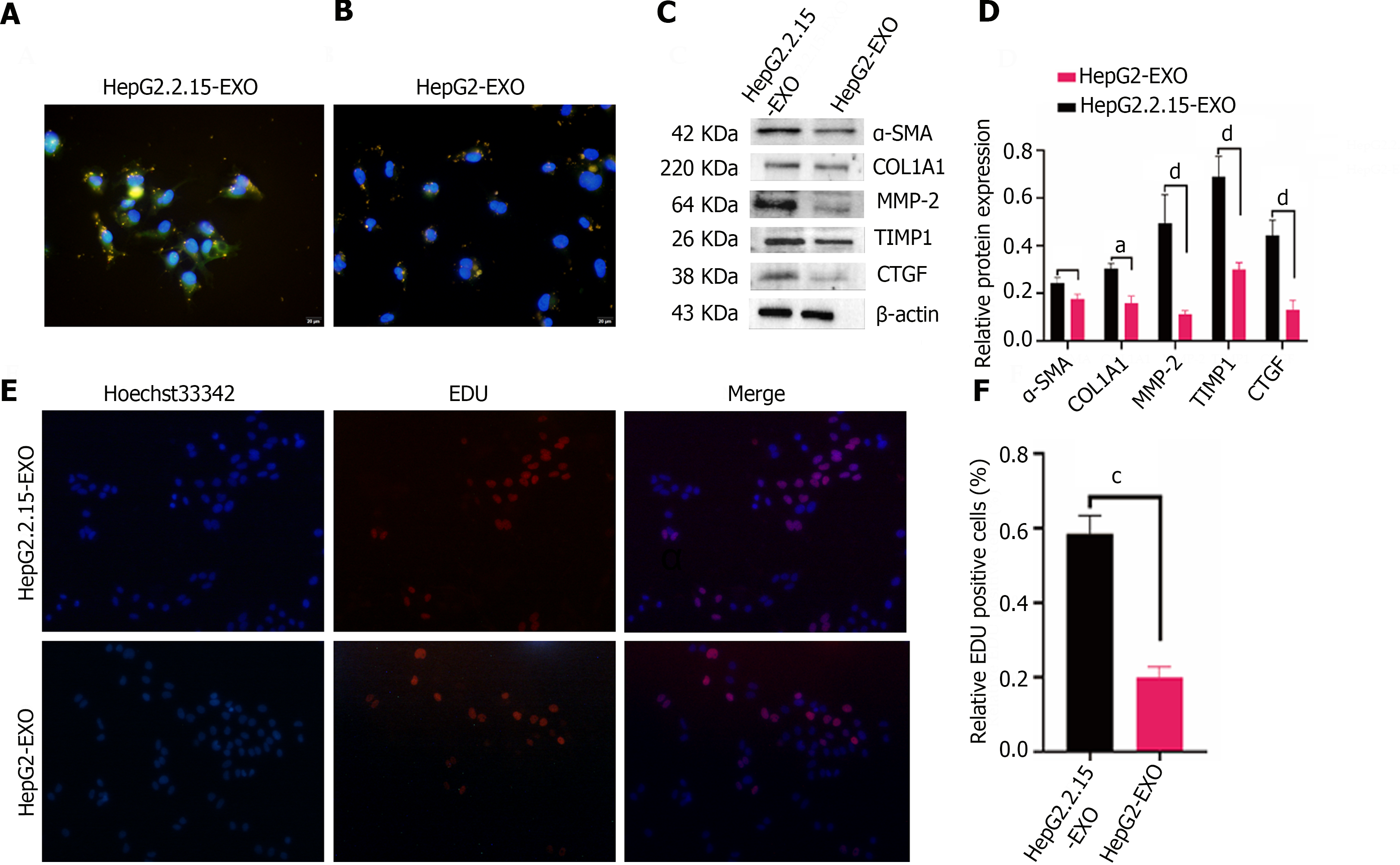
Exosomes from HepG2.2.15 and HepG2 cells were tagged with DiI and co-cultured with LX2 cells for 24 h. The fibrogenic marker α-SMA was detected in the LX2 cytoplasm, as indicated by green fluorescence, whereas the nuclei were stained blue with DAPI. Morphologically, LX2 cells exhibited a spindle shape and prominent pseudopods upon up-taking HepG2.2.15-derived exosomes (yellow), as evidenced by a higher expression of α-SMA. This was in contrast to the lower expression of α-SMA in LX2 cells treated with HepG2-derived exosomes, which retained the majority of exosomes within the cytoplasm (Figure 3A and B). These findings illustrate that LX2 cells internalize exosomes from both cell lines in vitro, but it is primarily the HepG2.2.15-derived exosomes that augment α-SMA expression in HSCs.
In assessing the effect of HepG2.2.15-derived exosomes on the fibrotic processes of LX2 cells, the expression of fibrosis-associated proteins such as α-SMA, COL1A1, MMP2, TIMP-1, and CTGF was found to be elevated in the HepG2.2.15-exo group compared to the HepG2-exo group (Figure 3C and D), suggesting that exosomes from HepG2.2.15 hasten LX2 fibrosis[22].
The proliferation of LX2 cells was examined after co-incubation with HepG2.2.15-exo and HepG2-exo for 24 h. EDU staining was conducted to discriminate between proliferating and non-proliferating cells; the nuclei of proliferating cells were stained red, while all nuclei were counterstained blue. The proliferation rate of LX2 cells in the HepG2.2.15-exo group was substantially higher than that in the HepG2-exo group (Figure 3E and F), indicating that HepG2.2.15-derived exosomes facilitated both fibrosis and proliferation in LX2 cells.
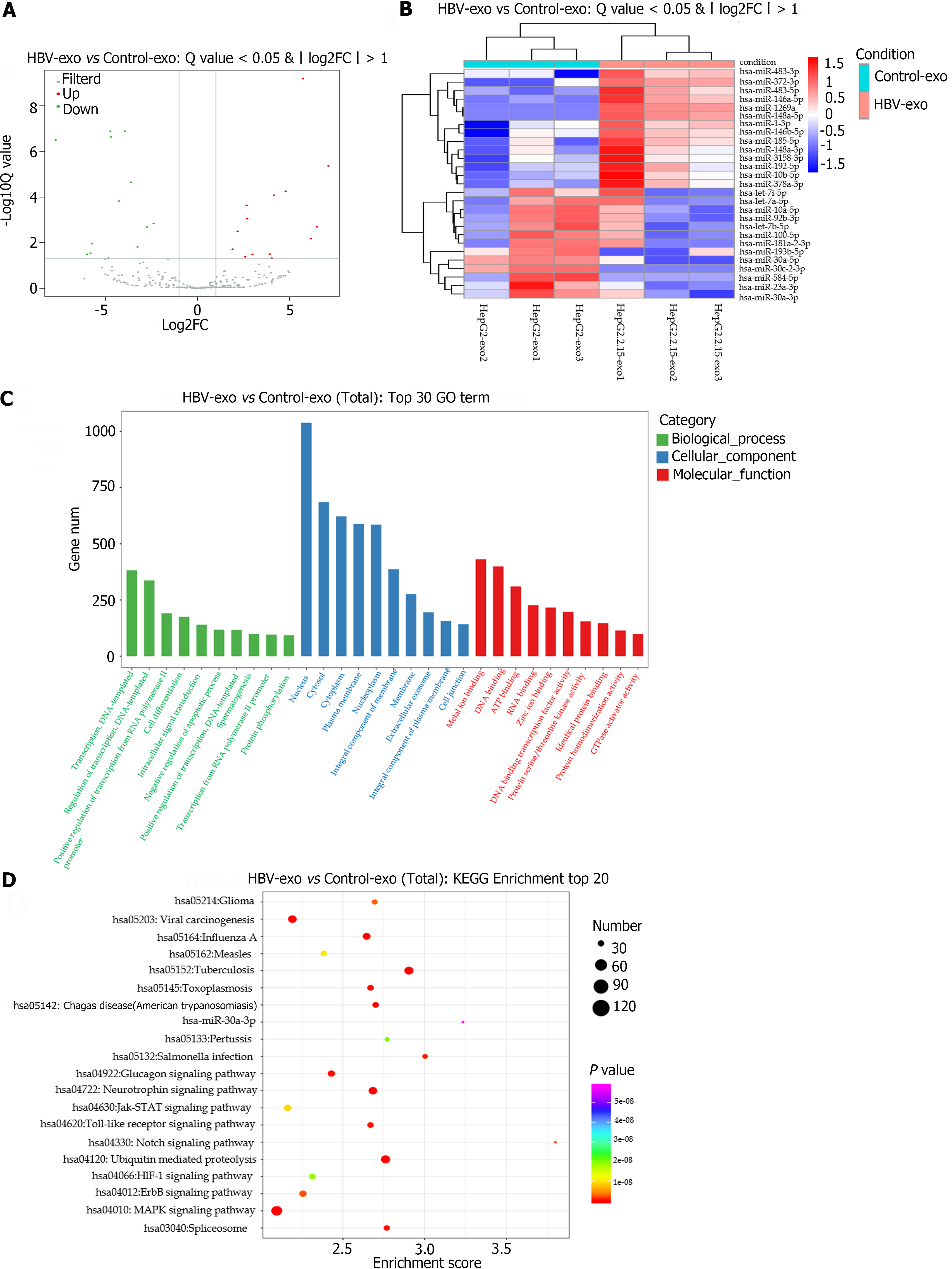
The miRNA expression profiles of exosomes derived from HepG2.2.15 and HepG2 cells were studied using small RNA sequencing. The subsequent volcano plot revealed 27 DE-miRNAs between the two exosome populations, with 14 miRNAs upregulated and 13 downregulated in exosomes derived from HepG2.2.15 (Figure 4A). Detailed information regarding the DE-miRNAs is provided in Table 1. The heatmap in Figure 4B illustrates the variation in DE-miRNAs between the HepG2.2.15-and HepG2 exosome-treated groups.
| miRNA_ID | log2FC | Q value | Sequence |
| hsa-let-7a-5p | -2.73154 | 0.00204 | TGAGGTAGTAGGTTGTATAGTT |
| hsa-let-7b-5p | -4.69951 | 2.33E-07 | TGAGGTAGTAGGTTGTGTGGTT |
| hsa-let-7i-5p | -2.38322 | 0.00143 | TGAGGTAGTAGTTTGTGCTGTT |
| hsa-miR-1-3p | 4.033866 | 0.047597 | TGGAATGTAAAGAAGTATGTAT |
| hsa-miR-100-5p | -3.94205 | 1.30E-07 | AACCCGTAGATCCGAACTTGTG |
| hsa-miR-10a-5p | -3.59889 | 2.26E-05 | TACCCTGTAGATCCGAATTTGTG |
| hsa-miR-10b-5p | 2.666194 | 0.000232 | TACCCTGTAGAACCGAATTTGTG |
| hsa-miR-1269a | 6.487066 | 0.002002 | CTGGACTGAGCCGTGCTACTGG |
| hsa-miR-146a-5p | 5.734318 | 6.49E-10 | TGAGAACTGAATTCCATGGGTT |
| hsa-miR-146b-5p | 4.145809 | 8.31E-05 | TGAGAACTGAATTCCATAGGCTG |
| hsa-miR-148a-3p | 1.906654 | 0.019359 | TCAGTGCACTACAGAACTTTGT |
| hsa-miR-148a-5p | 6.152472 | 0.006559 | AAAGTTCTGAGACACTCCGACT |
| hsa-miR-181a-2-3p | -4.83032 | 0.047597 | ACCACTGACCGTTGACTGTACC |
| hsa-miR-185-5p | 2.986783 | 0.03238 | TGGAGAGAAAGGCAGTTCCTGA |
| hsa-miR-192-5p | 2.596432 | 0.041346 | CTGACCTATGAATTGACAGCC |
| hsa-miR-193b-5p | -5.73663 | 0.011076 | CGGGGTTTTGAGGGCGAGATGA |
| hsa-miR-23a-3p | -3.25416 | 0.01536 | ATCACATTGCCAGGGATTTCC |
| hsa-miR-30a-3p | -4.72925 | 1.30E-07 | CTTTCAGTCGGATGTTTGCAGC |
| hsa-miR-30a-5p | -7.69272 | 3.18E-07 | TGTAAACATCCTCGACTGGAAG |
| hsa-miR-30c-2-3p | -5.80039 | 0.028767 | CTGGGAGAAGGCTGTTTACTCT |
| hsa-miR-3158-3p | 2.179298 | 0.003177 | AAGGGCTTCCTCTCTGCAGGAC |
| hsa-miR-372-3p | 7.10648 | 4.32E-06 | AAAGTGCTGCGACATTTGAGCGT |
| hsa-miR-378a-3p | 2.695553 | 0.000885 | ACTGGACTTGGAGTCAGAAGGC |
| hsa-miR-483-3p | 3.922906 | 0.031633 | TCACTCCTCTCCTCCCGTCTT |
| hsa-miR-483-5p | 4.78546 | 5.57E-05 | AAGACGGGAGGAAAGAAGGGAG |
| hsa-miR-584-5p | -5.99766 | 0.03238 | TTATGGTTTGCCTGGGACTGAG |
| hsa-miR-92b-3p | -4.26701 | 0.00015 | TATTGCACTCGTCCCGGCCTCC |
Predictive analysis using the miRanda database allowed the identification of potential target genes modulated by the DE-miRNAs (Table 2). Further enrichment analyses using GO and KEGG elucidated the biological functions of these targets (Figure 4C).
| miRNA_ID | Targets | n |
| hsa-miR-1269a | ADORA1, CPLX2, MECR, ADAR, EXTL2, NCKAP5L, ZXDC, HSF4, ENGASE, CEMP1, TMEM231, CREBBP, PLIN4, WIPF3, TRMT9B, FOXRED2, PTGER3, LTBP3, KCNK16, INAVA, RBM24, OTOA, CELF5, ATP8B3, IL6ST, CDIP1, TGIF2, POC1B-GALNT4, RIMS4, RHOBTB1, TRRAP, PASK, CDC20, STON2, TP53I11, CHRDL2, BTD, H6PD, ANLN, C12orf43, MYCN, BRD1, CRYGN KCNH3, ZNF618, NFAM1, MAST2, KY, DLAT, TPR, GALNT4, WASL, CDC25B, VPS9D1, ZFHX3, TMEM115, PATZ1, CCT8L2, PCLO, SOX8, ATP11A, CLUH, ELAC2, MRGBP, SMG9, ROBO3, VWA1, WDR82, ZNF696, C1QTNF6, ALPK2, GIPC3, SDK1, N4BP1, SLC39A3, KLF15, LYL1, LHX3, METTL25, LOC101927262, MLPH, DNAI1, GSTO2, ACSM1, GPBAR1, DGKD, LOC107986805, IQANK1, DAPK2, NLRC3, CHST8, EMID1, LOC112268219, ZNF331 | 93 |
| hsa-miR-584-5p | DHDDS, IL4I1 | 2 |
| hsa-miR-30c-2-3p | AK1, IGFBP3, PARP3, CT45A5, CTDSPL, FAM227A, CT45A1, CT45A3, CT45A6, GAB4, ELMSAN1, FAM160A2, ZNF30, EPHA10, ECE1, ZNF84, RAI14, CCDC166, CHAMP1, C6orf132, FREM3, THRA, TFR2, SYT7, CABLES1, NOL4L, GPR161, KIAA2012, ATG4D, KCNK9, SIPA1L1, ADAP1, CT45A8, CT45A9, CT45A7 , LOC79999, LOC388436, MAPK4, CCR1, PCDHB6, ARMC7, XXYLT1, SLC22A14, ZSCAN22, KCNS1, LOC643802, SETD1B, ELAVL3, CSPG4, CHST3, CRK, KCNA10, MPHOSPH10, SPEG, SF3B3, ATG4B, KCNV1, KLHL20, SUSD6, ZDHHC17, DNAH1, PCDHB5, YTHDF1, AJAP1, PCDHB10, PCDHB11, PCDHB12, PCDHB13, PCDHB14, PCDHB2, PCDHB3, PCDHB7, PCDHB9, PCDHB8, WRNIP1, PCDHB16, NSD1, NOL12, TREML2, LRRC3, HUWE1, PRPF38A, TP53RK, SAMD10, CT45A2, MT1E, MT1M, KCNK3, P4HA2, ZNF467, SLC28A3, LOC102724624, IL17REL, DOCK2, ZDHHC11, C8orf88, LOC107987099, SUOX | 98 |
| hsa-miR-193b-5p | OR8J3, SH3D19, RPS19, ABCF1, SLC35E2B, CITED2, DNAI2, SLC35E2A, KIF21B, ZNF541, CACHD1, BRD1, ZNF677, GPR83, DLC1, DLG2, GOT1, KCNQ4, CCPG1, PIK3R2, TIMM44, SEPHS2, NLGN4Y, TNXB, ARHGAP27, GALNT12, FHOD3, CIC, UBE2S, EAF1, RNASEH2B, RPH3AL, MIB2, COQ8A, CIB4, C11orf91, GAREM1 | 37 |
| hsa-miR-146a-5p | EARS2, GPATCH11, GPAT3 | 3 |
| hsa-miR-181a-2-3p | GCGR, EPN1, GTF2A1L, STON1-GTF2A1L, ARHGEF26, REXO4, TJP1, ATXN2, MIER2, PRSS8, COPB2, SF3A1, CLEC4E, KIF26A, INO80D, USP53, MIIP, WDR46 | 18 |
| hsa-miR-483-5p | UBE3A, ZBTB16, DTX2, EPN1, MAMSTR, ESRRG, PABPC4, TTC39C, NFKBIL1, NONO, HOPX, ZNF701, DNAJC27, RBM14, MAP4K4, ARFGAP2, UBAP2, LOC403312, SHROOM2, PTK2, SEC16B, CRYZL2P-SEC16B, GCNT1, HDAC2, NOVA2, TIMP2, CNTNAP1, NEURL1, HOXA11, NHLH2, MUC3A, CLDN16, NR2E3, GPATCH1, TMEM33, CTC1, TIMM29, MFSD6L, PAN3, THNSL1, VPS9D1, NKAIN4, FAM172A, DTHD1, RNF112, HS3ST1, GRIN2D, GNG8, ADRM1, PRDM15, LOC285500, UNC5A, PRKAG3, ROBO1, RNF180, RNF39, C1QTNF4, TBX5, CD27, SRL, PRCD, LOC390937, ZNF273 | 62 |
| hsa-let-7b-5p | HMGCR, TRPM3, FOXB2, WDR62, BUB1B-PAK6, CMKLR1, GPR85, DICER1, SPHK2, TRRAP, RAB18, SOX5, PFKFB1, PAK6, HDAC6, PDP2, DNASE1, TAF5, IFT172, ZNF589, SLC35B4, RIMKLA, OTOP1, KMT5C, ZNF546, LRTOMT | 26 |
| hsa-miR-92b-3p | RPL28, ST20, SOAT1, UBXN1, MFGE8, IREB2, AURKA, PIAS2, NTN4, HTD2, KDSR, TLK1, HINT3, DNHD1, ARID3A, MYO3A, IGDCC4, CEP170B, RGS6 | 19 |
| hsa-miR-146b-5p | BBS12, MSH5, INTS4, LOC101929322 | 4 |
GO analysis identified the biological processes most enriched in targets, which were notably linked to cellular differentiation, intracellular signal transduction, and negative regulation of apoptosis. The cellular components that were enriched included exosomes, plasma membrane components, and cell junctions. The predominant molecular functions were RNA binding, DNA-binding transcription factor activity, and homologous protein binding. The detailed GO enrichment findings are presented in Supplementary Tables 1-3. KEGG analysis highlighted the top 20 enriched pathways, with the most significant being the tuberculosis, ubiquitin-mediated proteolysis, MAPK signaling, and neurotrophin signaling pathways (Figure 4D).
Liver fibrosis, a common outcome of chronic liver disease, is associated with significant morbidity and mortality; however, there remains a dearth of effective treatments for this condition[23]. While traditionally considered irreversible, recent research has suggested that liver fibrosis can be reversed, as evidenced by certain experimental models and human cirrhosis studies[24]. Currently the principal approaches to managing liver disease include the interruption of harmful stimuli and, ultimately, liver transplantation[25]. HBV core proteins has been shown to induce the production of cytokines and trigger an immune response. In our investigation, we discovered that exosomes derived from the HepG2.2.15 cell line activated LX2 cells, thereby exacerbating their fibrogenic potential[26].
HSCs exist in two states, quiescent HSC (qHSCs) and activated HSC (aHSCs). qHSCs are responsible for vitamin A storage in the liver. aHSCs are the primary source of myofibroblasts, the primary source of ECM in the damaged liver. Notably, HSC is activated after liver injury, and recent single-cell RNA sequencing studies have confirmed that activation of stellate cells involves different phenotypic changes[27]. Our results demonstrated that the expression of the fibrosis marker proteins α-SMA, COL1A1, MMP2, TIMP-1, and CTGF in the HepG2.2.15 group was higher than that in the HepG2 group, HepG2.2.15-derived exosomes accelerated LX2 HSC fibrosis and promoted LX2 proliferation in vitro.
Exosomes mediate intercellular communication and can package and transport lncRNAs, mRNA, proteins, and other bioactive substances. Exosome also plays a role in the occurrence and development of many diseases such as tumor metastasis and neurological diseases[28]. Exosomes containing miRNAs in the liver regulate HSC activation to control the pathogenesis of liver fibrosis. For example, the research has shown that miR-214 is transferred from HSC donor cells to HSC recipient cells via exosomes, which regulates the development of CCN2-dependent fibrosis[29]. Seo et al[30] found that TLR3 may be a new target for fibrosis, and the possible mechanism is that γδ T cells promote interleukin-17A production in liver injury, further activating exosome-mediated TLR3. Our study showed that HepG2.2.15 cells could secrete exosomes and LX2 in HepG2.2.15-derived exosomes in vitro and further promote the fibrosis of LX2.
miRNAs are the most abundant small RNA that can be detected in almost all animal models and have potential therapeutic effects and prognosis in many diseases. miRNAs recruit Argonaute protein complexes to complementary target mRNA, resulting in translation inhibition or mRNA degradation[31]. Many miRNAs are considered fibrous and are involved in cardiac fibrosis, such as miR-29, which targets a series of mRNAs that encode fibrosis-related proteins[32]. The treatment of liver fibrosis is mainly based on miRNAs (miR-29b and miR-150, etc.), small drug molecules (Hh inhibitors and TGF-β inhibitors, etc.), antibody therapy (TIMP-1 and Simtuzumab, etc.), antigen therapy (ODNs and TFO, etc.)[33]. Common miRNA analysis methods include microarrays, reverse transcription quantitative polymerase chain reaction, and RNA sequencing (RNA-seq)[34]. Small RNA sequencing (sRNA-seq) can detect more than one billion RNAs in a single run and unearth new miRNA genes because of its untargeted character. sRNA-seq analysis of HepG2-derived exosomes and HepG2.2.15-derived exosomes was performed to further investigate miRNA expression and function in exosomes. We found 27 DE-miRNAs (14 upregulated miRNAs and 13 downregulated miRNAs) and 27 miRNAs were used to predict their targets, followed by target gene GO enrichment analysis and KEGG enrichment analysis. We revealed that these targets are mainly involved in the regulation of functions such as cell differentiation, intracellular signaling, negative regulation of apoptotic processes, cellular exosome and RNA binding, mediation of protein hydrolysis, MAPK signaling pathways, viral oncogenesis, and toll-like receptor signaling pathways.
We investigated the effects of HBV-infected hepatocytes on HSC activation, proliferation, and fibrosis. However, this study has several limitations. For example, there is no elucidation of the pathway through which HepG2.2.15-derived exosome-transported DE-miRNAs mediate phenotypic changes in HSCs. In addition, experiments in vivo are lacking to validate the effects of HepG2.2.15-derived exosomes and the DE-miRNAs carried by them on the phenotype of HSCs and liver fibrosis are lacking.
This study highlights the pronounced effect of HBV-infected hepatocytes on HSC activation, proliferation, and fibrosis. This also highlights the magnifying role of HepG2.2.15-derived exosomes in fibrogenic potential. Despite the significance of these findings, the study falls short of including in vivo experiments and leaves the pathway through which exosomes mediate HSCs’phenotypic changes of HSCs unresolved. Further research is required to elucidate these aspects fully.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade A
Creativity or Innovation: Grade A
Scientific Significance: Grade A
P-Reviewer: Michalopoulos GK, United States S-Editor: Fan JR L-Editor: A P-Editor: Cai YX
| 1. | Nguyen MH, Wong G, Gane E, Kao JH, Dusheiko G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin Microbiol Rev. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 2. | Xiao J, Wang F, Wong NK, He J, Zhang R, Sun R, Xu Y, Liu Y, Li W, Koike K, He W, You H, Miao Y, Liu X, Meng M, Gao B, Wang H, Li C. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J Hepatol. 2019;71:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 387] [Article Influence: 64.5] [Reference Citation Analysis (1)] |
| 3. | Chang X, Wang J, Chen Y, Long Q, Song L, Li Q, Liu H, Shang Q, Yu Z, Jiang L, Xiao G, Li L, Chen L, Wang X, Li Z, Chen D, Dong Z, An L, Tan L, Yang Y. A novel nomogram to predict evident histological liver injury in patients with HBeAg-positive chronic hepatitis B virus infection. EBioMedicine. 2021;67:103389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Tsukuda S, Watashi K. Hepatitis B virus biology and life cycle. Antiviral Res. 2020;182:104925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 5. | Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 781] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 6. | Yang F, Li H, Li Y, Hao Y, Wang C, Jia P, Chen X, Ma S, Xiao Z. Crosstalk between hepatic stellate cells and surrounding cells in hepatic fibrosis. Int Immunopharmacol. 2021;99:108051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Sato K, Meng F, Glaser S, Alpini G. Exosomes in liver pathology. J Hepatol. 2016;65:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 8. | Isaac R, Reis FCG, Ying W, Olefsky JM. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021;33:1744-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 457] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 9. | Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6920] [Cited by in RCA: 6553] [Article Influence: 1310.6] [Reference Citation Analysis (0)] |
| 10. | Tran PHL, Xiang D, Tran TTD, Yin W, Zhang Y, Kong L, Chen K, Sun M, Li Y, Hou Y, Zhu Y, Duan W. Exosomes and Nanoengineering: A Match Made for Precision Therapeutics. Adv Mater. 2020;32:e1904040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 11. | Khatun M, Ray RB. Mechanisms Underlying Hepatitis C Virus-Associated Hepatic Fibrosis. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 12. | Wang J, Cao D, Yang J. Exosomes in Hepatitis B Virus Transmission and Related Immune Response. Tohoku J Exp Med. 2020;252:309-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Liu R, Li X, Zhu W, Wang Y, Zhao D, Wang X, Gurley EC, Liang G, Chen W, Lai G, Pandak WM, Robert Lippman H, Bajaj JS, Hylemon PB, Zhou H. Cholangiocyte-Derived Exosomal Long Noncoding RNA H19 Promotes Hepatic Stellate Cell Activation and Cholestatic Liver Fibrosis. Hepatology. 2019;70:1317-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 14. | Gutiérrez-Ruiz MC, Bucio L, Souza V, Gómez JJ, Campos C, Cárabez A. Expression of some hepatocyte-like functional properties of WRL-68 cells in culture. In Vitro Cell Dev Biol Anim. 1994;30A:366-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int J Nanomedicine. 2020;15:6917-6934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 827] [Article Influence: 165.4] [Reference Citation Analysis (0)] |
| 16. | Li X, Chen R, Kemper S, Brigstock DR. Dynamic Changes in Function and Proteomic Composition of Extracellular Vesicles from Hepatic Stellate Cells during Cellular Activation. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Bachurski D, Schuldner M, Nguyen PH, Malz A, Reiners KS, Grenzi PC, Babatz F, Schauss AC, Hansen HP, Hallek M, Pogge von Strandmann E. Extracellular vesicle measurements with nanoparticle tracking analysis - An accuracy and repeatability comparison between NanoSight NS300 and ZetaView. J Extracell Vesicles. 2019;8:1596016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 379] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 18. | Chu G, Zhou X, Hu Y, Shi S, Yang G. Rev-erbα Inhibits Proliferation and Promotes Apoptosis of Preadipocytes through the Agonist GSK4112. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34752] [Cited by in RCA: 57448] [Article Influence: 5744.8] [Reference Citation Analysis (0)] |
| 20. | Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010;11:R90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1182] [Cited by in RCA: 1284] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 21. | Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, Feng T, Zhou L, Tang W, Zhan L, Fu X, Liu S, Bo X, Yu G. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb). 2021;2:100141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 522] [Cited by in RCA: 4765] [Article Influence: 1191.3] [Reference Citation Analysis (0)] |
| 22. | Lai M, Afdhal NH. Liver Fibrosis Determination. Gastroenterol Clin North Am. 2019;48:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 23. | Roehlen N, Crouchet E, Baumert TF. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 751] [Article Influence: 150.2] [Reference Citation Analysis (0)] |
| 24. | Caligiuri A, Gentilini A, Pastore M, Gitto S, Marra F. Cellular and Molecular Mechanisms Underlying Liver Fibrosis Regression. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 160] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 25. | Friedman SL, Pinzani M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology. 2022;75:473-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 259] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 26. | Dawood RM, El-Meguid MA, Salum GM, El Awady MK. Key Players of Hepatic Fibrosis. J Interferon Cytokine Res. 2020;40:472-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 27. | Kamm DR, McCommis KS. Hepatic stellate cells in physiology and pathology. J Physiol. 2022;600:1825-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 150] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 28. | Zhang XW, Zhou JC, Peng D, Hua F, Li K, Yu JJ, Lv XX, Cui B, Liu SS, Yu JM, Wang F, Jin CC, Yang ZN, Zhao CX, Hou XY, Huang B, Hu ZW. Disrupting the TRIB3-SQSTM1 interaction reduces liver fibrosis by restoring autophagy and suppressing exosome-mediated HSC activation. Autophagy. 2020;16:782-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 29. | Chen L, Charrier A, Zhou Y, Chen R, Yu B, Agarwal K, Tsukamoto H, Lee LJ, Paulaitis ME, Brigstock DR. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology. 2014;59:1118-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 30. | Seo W, Eun HS, Kim SY, Yi HS, Lee YS, Park SH, Jang MJ, Jo E, Kim SC, Han YM, Park KG, Jeong WI. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by γδ T cells in liver fibrosis. Hepatology. 2016;64:616-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 31. | Ferragut Cardoso AP, Banerjee M, Nail AN, Lykoudi A, States JC. miRNA dysregulation is an emerging modulator of genomic instability. Semin Cancer Biol. 2021;76:120-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 32. | Tadokoro T, Morishita A, Masaki T. Diagnosis and Therapeutic Management of Liver Fibrosis by MicroRNA. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 33. | Kumar V, Mahato RI. Delivery and targeting of miRNAs for treating liver fibrosis. Pharm Res. 2015;32:341-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Benesova S, Kubista M, Valihrach L. Small RNA-Sequencing: Approaches and Considerations for miRNA Analysis. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |