Published online Apr 7, 2024. doi: 10.3748/wjg.v30.i13.1911
Peer-review started: October 10, 2023
First decision: December 27, 2023
Revised: January 7, 2024
Accepted: February 25, 2024
Article in press: February 25, 2024
Published online: April 7, 2024
Processing time: 176 Days and 4.8 Hours
Liuweiwuling Tablet (LWWL) is a Chinese patent medicine approved for the treatment of chronic inflammation caused by hepatitis B virus (HBV) infection. Previous studies have indicated an anti-HBV effect of LWWL, specifically in terms of antigen inhibition, but the underlying mechanism remains unclear.
To investigate the potential mechanism of action of LWWL against HBV.
In vitro experiments utilized three HBV-replicating and three non-HBV-repli
In HepG2.1403F cells, LWWL (0.8 mg/mL) exhibited inhibitory effects on HBV DNA, hepatitis B surface antigen and pregenomic RNA (pgRNA) at rates of 51.36%, 24.74% and 50.74%, respectively. The inhibition rates of LWWL (0.8 mg/mL) on pgRNA/covalently closed circular DNA in HepG2.1403F, HepG2.2.15 and HepG2.A64 cells were 47.78%, 39.51% and 46.74%, respectively. Integration of transcriptomics and metabolomics showed that the anti-HBV effect of LWWL was primarily linked to pathways related to apoptosis (PI3K-AKT, CASP8-CASP3 and P53 pathways). Apoptosis flow analysis revealed that the apoptosis rate in the LWWL-treated group was significantly higher than in the control group (CG) among HBV-replicating cell lines, including HepG2.2.15 (2.92% ± 1.01% vs 6.68% ± 2.04%, P < 0.05), HepG2.A64 (4.89% ± 1.28% vs 8.52% ± 0.50%, P < 0.05) and HepG2.1403F (3.76% ± 1.40% vs 7.57% ± 1.35%, P < 0.05) (CG vs LWWL-treated group). However, there were no significant differences in apoptosis rates between the non-HBV-replicating HepG2 cells (5.04% ± 0.74% vs 5.51% ± 1.57%, P > 0.05), L02 cells (5.49% ± 0.80% vs 5.48% ± 1.01%, P > 0.05) and LX2 cells (6.29% ± 1.54% vs 6.29% ± 0.88%, P > 0.05). TUNEL staining revealed a significantly higher apoptosis rate in the LWWL-treated group than in the CG in the HBV-replicating mouse model, while no noticeable difference in apoptosis rates between the two groups was observed in the non-HBV-replicating mouse model.
Preliminary results suggest that LWWL exerts a potent inhibitory effect on wild-type and drug-resistant HBV, potentially involving selective regulation of apoptosis. These findings offer novel insights into the anti-HBV activities of LWWL and present a novel mechanism for the development of anti-HBV medications.
Core Tip: Liuweiwuling Tablet (LWWL) exerts a potent inhibitory effect on both wild-type and drug-resistant hepatitis B virus (HBV) models. This effect appears to hinge on selective apoptosis regulation-an unprecedented discovery in the realm of anti-HBV drug mechanisms. These revelatory findings not only deepen our understanding of LWWL’s anti-HBV properties but also offer an innovative mechanism to inspire the development of new anti-HBV drugs.
- Citation: Ge FL, Yang Y, Si LL, Li YH, Cao MZ, Wang J, Bai ZF, Ren ZG, Xiao XH, Liu Y. Inhibition of hepatitis B virus via selective apoptosis modulation by Chinese patent medicine Liuweiwuling Tablet. World J Gastroenterol 2024; 30(13): 1911-1925
- URL: https://www.wjgnet.com/1007-9327/full/v30/i13/1911.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i13.1911
Hepatitis B virus (HBV) infection can lead to chronic hepatitis B (CHB) and increase the risk of liver cirrhosis and hepatocellular carcinoma. The estimated 257 million people annually who are positive for hepatitis B surface antigen (HBsAg) suffer > 887000 deaths[1]. China has the highest number of CHB patients globally (70 million), resulting in an annual direct medical burden of up to ¥600 billion[2,3]. Consequently, CHB remains one of the most critical global public health concerns. Interferon and nucleoside/nucleotide analogs (NAs), two types of anti-HBV medications, have obtained licenses for treating HBV-related diseases. Currently, six NAs have been authorized in China for CHB treatment. Despite the considerable benefits these antiviral drugs have offered patients, the challenge of eradicating HBV from individuals with chronic infection using current medication remains substantial. Additional factors affecting treatment success include adverse drug responses and HBV drug resistance. Substantial endeavors have been made to develop more efficacious drugs and therapies[4,5], aiming to facilitate functional cure for CHB.
Liuweiwuling Tablet (LWWL) (approval number: Z20060238) has been granted approval by the China National Medical Products Administration. This is included in the national health insurance program and exhibits notable anti-inflammatory properties[6,7]. In our earlier research using the wild-type HBV model, we observed an anti-HBV effect of LWWL. Particularly notable is the suppression of HBV antigen, which compensates for the absence of NAs[8]. Our meta-analysis of its clinical effects indicates that combination of LWWL with NAs increases the undetectability of HBV DNA and hepatitis B e antigen (HBeAg) when compared with NAs alone[9]. However, the lack of clarity of its antiviral mechanism restricts its potential therapeutic application. The objective of this study was to elucidate the antiviral activity of LWWL. This was achieved by investigating its potential mechanism through the integration of transcriptomics and metabolomics. Additionally, a more comprehensive evaluation of the anti-HBV effect of LWWL was conducted within a drug-resistant HBV model.
We utilized three HBV-replicating cell lines: HepG2.2.15 (wild-type), HepG2.A64 (entecavir-resistant) and HepG2.1403F (multidrug-resistant). Cytotoxicity of LWWL (Shibo Jindu, Zibo, China) was assessed using the Cell Counting Kit-8 (Dojindo Laboratories, Kyushu, Japan). The molecular and cellular experiments were conducted within the biosafety level-2 Laboratory at the Fifth Medical Center of the Chinese PLA General Hospital. All procedures were executed in full adherence to the established protocols. The 50% cytotoxicity concentration (CC50) for cultured cells was calculated.
HepG2.1403F cells were seeded in 48-well plates at 2 × 104 per well with various drug concentrations [0, 0.1, 0.2, 0.4 or 0.8 mg/mL LWWL, or 0, 0.2, 2, 20 or 200 μmol/L tenofovir disoproxil fumarate (TDF)]. During 120-h cultivation, cell supernatants were harvested to measure HBsAg and HBeAg levels using Enzyme-linked immunosorbent assay kits (Wantai Biological Pharmacy Enterprise Co. Ltd., Beijing, China). Concurrently, cells were harvested to determine HBV DNA levels via quantitative PCR (qPCR) assays as described previously[10,11]. The 50% inhibitory concentration (IC50) and selectivity index (ratio between CC50 and IC50) were calculated.
Three HBV-replicating cell lines were individually seeded in 48-well plates at 2 × 104 per well in triplicate with or without 0.8 mg/mL LWWL, or 200 μmol/L. During 120-h cultivation, cell supernatants were collected for HBV pregenomic RNA (pgRNA) analysis using quantitative reverse transcription PCR. Additionally, adherent cells were harvested to quantify HBV covalently closed circular DNA (cccDNA) via qPCR, as previously outlined[12,13].
Male SPF C57BL/6 mice, aged 6-8 wk, weight 18-20 g, were purchased from Sibeifu, Beijing (animal license No. SCXK (Jing) 2019-0016). The animals were kept in the SPF laboratory of the Fifth Medical Center of the Chinese PLA General Hospital and were allowed to eat and drink freely except for fasting tests. The room was kept at 22 ± 2 °C in a 12-h light/dark cycle, with 50% humidity. After 1 wk of adaptive feeding, mice were subjected to follow-up experiments. pAAV-HBV1.2 HBV plasmid (20 mg) was injected into mice through the tail vein within 5-8 s using a high-pressure hydrodynamic method to establish the HBV-replicating mouse model. The mice were divided into four groups (n = 6) and received intraperitoneal treatments once daily for 4 wk. Group 1 served as the control and received normal saline (NS). Group 2 was treated with a low dose of LWWL (1 g/kg/d); group 3 received a high dose of LWWL (2 g/kg/d); and group 4 was treated with TDF (63 mg/kg/d). The levels of HBV antigens and DNA were measured. The animal study was conducted at the Animal Laboratory Center, Fifth Medical Center of the Chinese PLA General Hospital, following strict adherence to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol received approval from the Committee on the Ethics of Animal Experiments at the Fifth Medical Center of the Chinese PLA General Hospital (Permit number: IACUC-2021-0009).
The serum samples were obtained from section “Evaluating anti-HBV efficacy of LWWL in mouse models” of the materials and methods. Then, 200 μL serum was extracted, followed by addition of 600 μL methanol. After 30 s of vortex oscillation, the mixture was centrifuged at 13000 rpm for 10 min, resulting in collection of 450 μL superserum. The superserum was subjected to extraction and concentrated to dryness through a vacuum centrifugal concentrator. The residue was dissolved using 200 μL methanol, followed by 30 s of vortexing. Supernatant samples were obtained by centrifugation at 15000 rpm for 10 min. We used the ACQUITY UPLC HSS T3 chromatographic column (100 mm × 2.1 mm, 1.8 μm). The column temperature was set at 45 °C. The mobile phase comprised A-water (with 0.1% formic acid) and B-acetonitrile (with 0.1% formic acid). The flow rate was 0.35 mL/min, and the injection volume was 2 μL. The elution gradient is detailed in Supplementary Table 1. Regarding the mass spectrum conditions, the ion source used was electrospray ionization, and both positive and negative ion scanning modes were utilized to gather signals for the sample quality spectrum (Supplementary Table 2).
Chromatographic peaks were imported into MetaboAnalyst 3.0, followed by standardized processing based on peak area. The standardized data were analyzed using SIMCA-P-13.0, with principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA). Potential biomarkers were identified by analyzing the two OPLS-DA groups, setting a threshold of P < 0.05 and variable importance in the projection (VIP) > 1. Potential biomarkers underwent biological function annotation and metabolic pathway enrichment analysis through Mass Hunter PCDL Manager software, MetaboAnalyst website, Kyoto Encyclopedia of Genes and Genomes (KEGG), and various other metabolomics and biological databases. The key differential metabolites were selected for receiver operating characteristic curve analysis, aligning with the research objective and biological functions. The area under the curve (AUC) was calculated to determine the sensitivity and specificity of these differential metabolites. A differential metabolite with an AUC closer to 1 indicated its superiority to differentiate between the two groups. Statistical analysis was conducted using MetaboAnalyst 3.0. Experimental data were presented as mean ± SD. One-way ANOVA was used for comparisons between groups, with statistical significance set at P < 0.05.
The mice were categorized into four groups, comprising HBV-replicating mice and non-HBV mice. The HBV-replicating mice were allocated into two groups (n = 6) for intraperitoneal treatment, administered once daily for 4 wk. Group 1 received NS, while group 2 was treated with high-dose LWWL (2 g/kg/d). Simultaneously, the non-HBV mice were also distributed into two groups (n = 6) for intraperitoneal treatment over a 4-wk period. Group 1 received NS) while group 2 was treated with high-dose LWWL (2 g/kg/d). The liver tissue was used to assess apoptosis, with TUNEL staining executed through the utilization of the TUNEL FITC Apoptosis Detection Kit[14].
Apoptosis assessment was conducted using six distinct cell lines, consisting of three HBV-replicating cell lines (HepG2.2.15, HepG2.A64 and HepG2.1403F) as well as three non-HBV-replicating cell lines (LX2, HepG2 and L02). The six cell lines were individually seeded into six-well plates at 2 × 105 per well for Flow cytometry. Following 24 h incubation, each plate underwent treatment with 0.8 mg/mL LWWL for 72 h at 37 °C in a 5% CO2 incubator. Subsequent to treatment, the cells were harvested, rinsed with ice-cold phosphate-buffered saline, and subjected to 5 min incubation with Annexin V-PE and 7-AAD (Multi Sciences, Hangzhou, China). Analysis was conducted utilizing a flow cytometer equipped with the FAC Station data management system and Cell Quest software (Becton Dickinson, San Jose, CA, United States). Data were analyzed using FlowJo v10.
All statistical analyses were conducted using SPSS 16.0. The data are presented as mean ± SD and underwent analysis using a t-test for comparisons between groups. In instances where suitable, one-way ANOVA was used. Statistical significance was defined as P < 0.05.
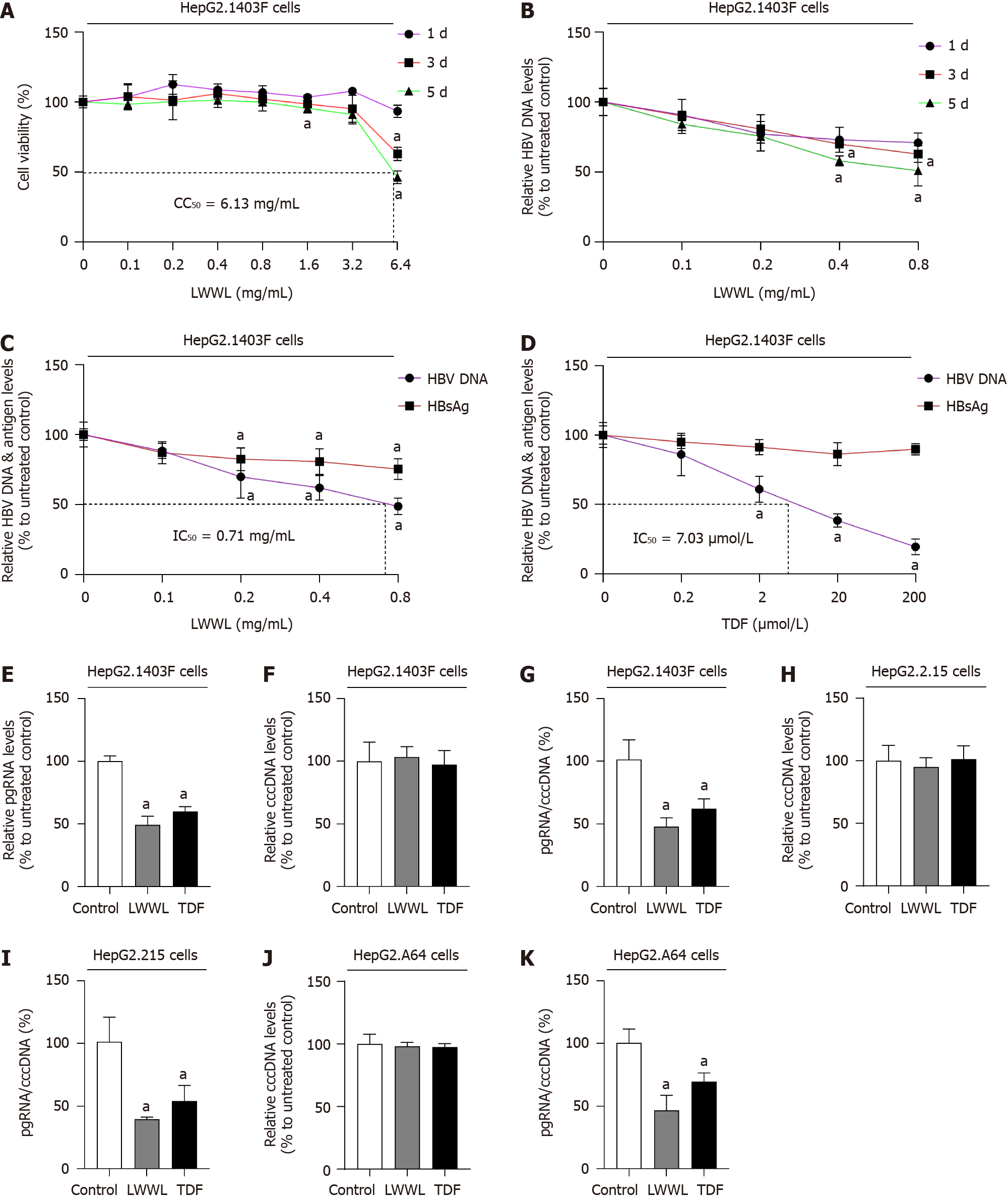
The cytotoxicity assay demonstrated that 0.8 mg/mL LWWL did not have any noticeable impact on cell viability. For HepG2.1403F cells, CC50 was 6.13 mg/mL (Figure 1A). Evaluation of the antiviral effect of LWWL was conducted on days 1, 3 and 5, under safe concentrations. The optimal antiviral effect of LWWL was observed on day 5 in HepG2.1403F cells (Figure 1B).
In HepG2.1403F cells, 0.8 mg/mL LWWL exhibited inhibitory rates of 51.36%, 24.74% on HBV DNA and HBsAg respectively (Figure 1C). In HepG2.1403F cells, 200 μmol/L TDF showed inhibitory rates of 80.42%, 10.22% on HBV DNA and HBsAg respectively (Figure 1D). In HepG2.1403F cells, 0.8 mg/mL LWWL and 200 μmol/L TDF exhibited inhibitory rates of 50.74%, 40.02% on pgRNA respectively (Figure 1E). In HepG2.1403F cells, neither LWWL nor TDF demonstrated any inhibitory effect on cccDNA (Figure 1F). In HepG2.1403F cells, 0.8 mg/mL LWWL and 200 μmol/L TDF exhibited rates of 47.78%, 62.09% for pgRNA/cccDNA respectively (Figure 1G). In HepG2.215 cells, neither LWWL nor TDF demonstrated any inhibitory effect on cccDNA (Figure 1H). In HepG2.215 cells, 0.8 mg/mL LWWL and 200 μmol/L TDF exhibited rates of 39.51%, 54.15% for pgRNA/cccDNA respectively (Figure 1I). In HepG2.A64 cells, neither LWWL nor TDF demonstrated any inhibitory effect on cccDNA (Figure 1J). In HepG2.A64 cells, 0.8 mg/mL LWWL and 200 μmol/L TDF exhibited rates of 46.74%, 69.73% for pgRNA/cccDNA respectively (Figure 1K).
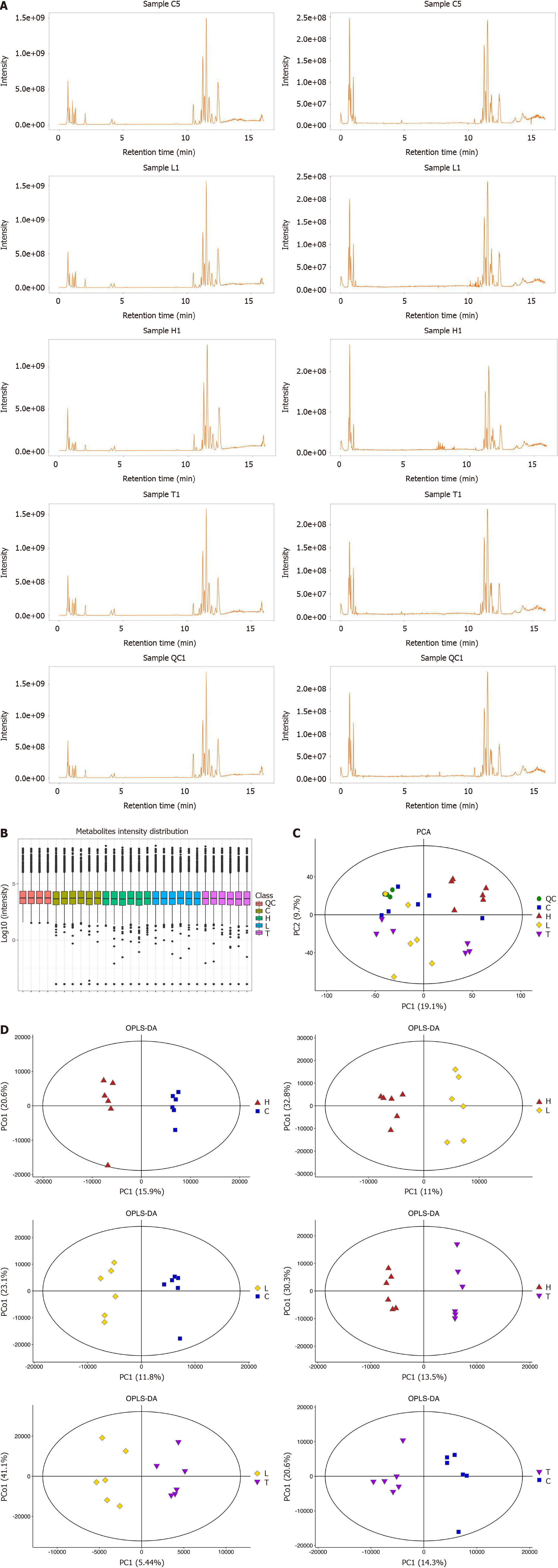
The results from the Base Peak Chromatogram (Figure 2A) and Boxplot (Figure 2B) indicated that the expression of total metabolites was consistent across all groups, meeting the requirements for intergroup metabolite comparison. As per the PCA results, the metabolic profiles of the control group (CG), low-dose LWWL group (LG), high-dose LWWL group (HG), and TDF group (TG) displayed distinct differences, highlighting effective differentiation among the groups. Of particular note was the distinct metabolic profile of HG/CG (Figure 2C). For a more refined identification of differentially expressed metabolites (DEMs), the metabolic profile between the two groups was refined through OPLS-DA, building upon the foundation provided by PCA to present a comprehensive metabolic overview of the four groups. OPLS-DA clearly demonstrated the distinct separation of the two groups (Figure 2D). The analyses of metabolic pathway enrichment and screening of DEMs were robustly corroborated by OPLS-DA and PCA.
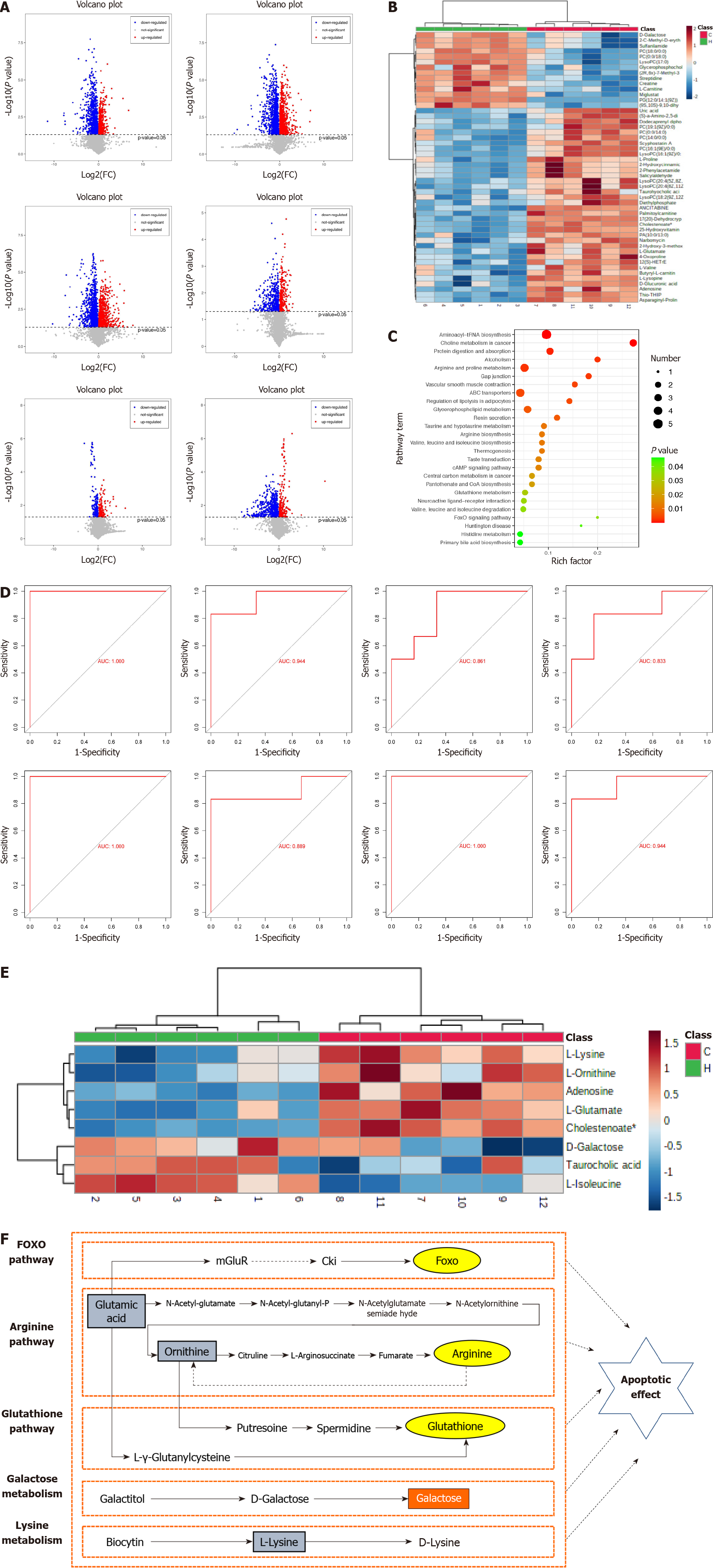
The criteria used for identifying DEMs in this study included VIP > 1 and P < 0.05. In HG/CG, HG/LG, HG/TG, LG/CG, LG/TG and TG/CG groups, 107, 118, 162, 55, 26 and 94 distinct metabolites were identified, respectively (Figure 3A). Hierarchical clustering illustrated a significant distinction in DEMs between HG and CG, effectively discerning the two groups (Figure 3B). As indicated by KEGG analysis, the DEMs within HG/CG exhibited prominent enrichment in pathways such as taurine and hypotaurine metabolism, arginine biosynthesis, and the FoxO signaling pathway, alongside other pathways (Figure 3C).
This study focused on the DEMs within the HG/CG comparison. Through consultation of pertinent literature and functional annotations of metabolites, eight key DEMs-adenosine, D-galactose, L-lysine, L-isoleucine, L-glutamate, L-ornithine, taurocholic acid, and 7α-dihydroxy-5-cholestenoate were identified. Their respective AUC values were 1, 0.944, 0.889, 0.944, 1, 0.861, 0.833 and 1, respectively (Figure 3D). The eight key DEMs could potentially serve as pivotal metabolites contributing to the anti-HBV effects of LWWL. This supposition is supported by heat map analysis, which highlighted their notable specificity and sensitivity in distinguishing between HG and CG (Figure 3E). Among the eight major DEMs, taurocholic acid, L-ornithine and L-lysine exhibited a substantial correlation with apoptosis (Figure 3F).
Transcriptomic analysis has been extensively described in our prior work, with a particular emphasis on elucidating apoptosis-related gene expression[8]. The transcriptomic results revealed 24 differentially expressed genes (DEGs) associated with anti-HBV effects. Through experimental validation, we identified 13 DEGs that were specifically related to anti-HBV activity. Thirteen DEGs between the LWWL group and the CG were confirmed using reverse transcriptase-PCR. In comparison with the CG, the LWWL group exhibited significantly lower expression levels of EGR2 and FOS, while the expression of IKBKE, CCNE2, AKT3, CREB3L2, PIK3R3, CREBBP, BCL2, PCNA, E2F1, CASP8 and P53 was significantly higher. Among these 13 genes, AKT3, PIK3R3, BCL2, PCNA, FOS, CASP8 and P53 were associated with the PI3K-AKT, CASP8-CASP3 and P53 pathways; all of which are apoptosis related pathways (Table 1).
| Gene | Fold change | P value | Is it related with apoptosis |
| E2F1 | 4.44770094 | 0.008685393 | No |
| PCNA | 4.047933076 | 0.002898892 | No |
| CASP8 | 3.570355797 | 0.003000082 | Yes |
| TP53 | 3.452487306 | 0.016679865 | Yes |
| CREBBP | 2.885252648 | 0.003455666 | No |
| IKBKE | 2.849255028 | 0.016173753 | No |
| PIK3R3 | 2.815615387 | 0.011672063 | Yes |
| CCNE2 | 2.506258474 | 0.014722413 | No |
| BCL2 | 2.239207884 | 0.007566661 | Yes |
| AKT3 | 2.023770673 | 0.003487678 | Yes |
| CREB3L2 | 0.621889559 | 0.006920633 | No |
| EGR2 | 0.44857779 | 0.001957335 | No |
| FOS | 0.183742709 | 0.02708347 | Yes |
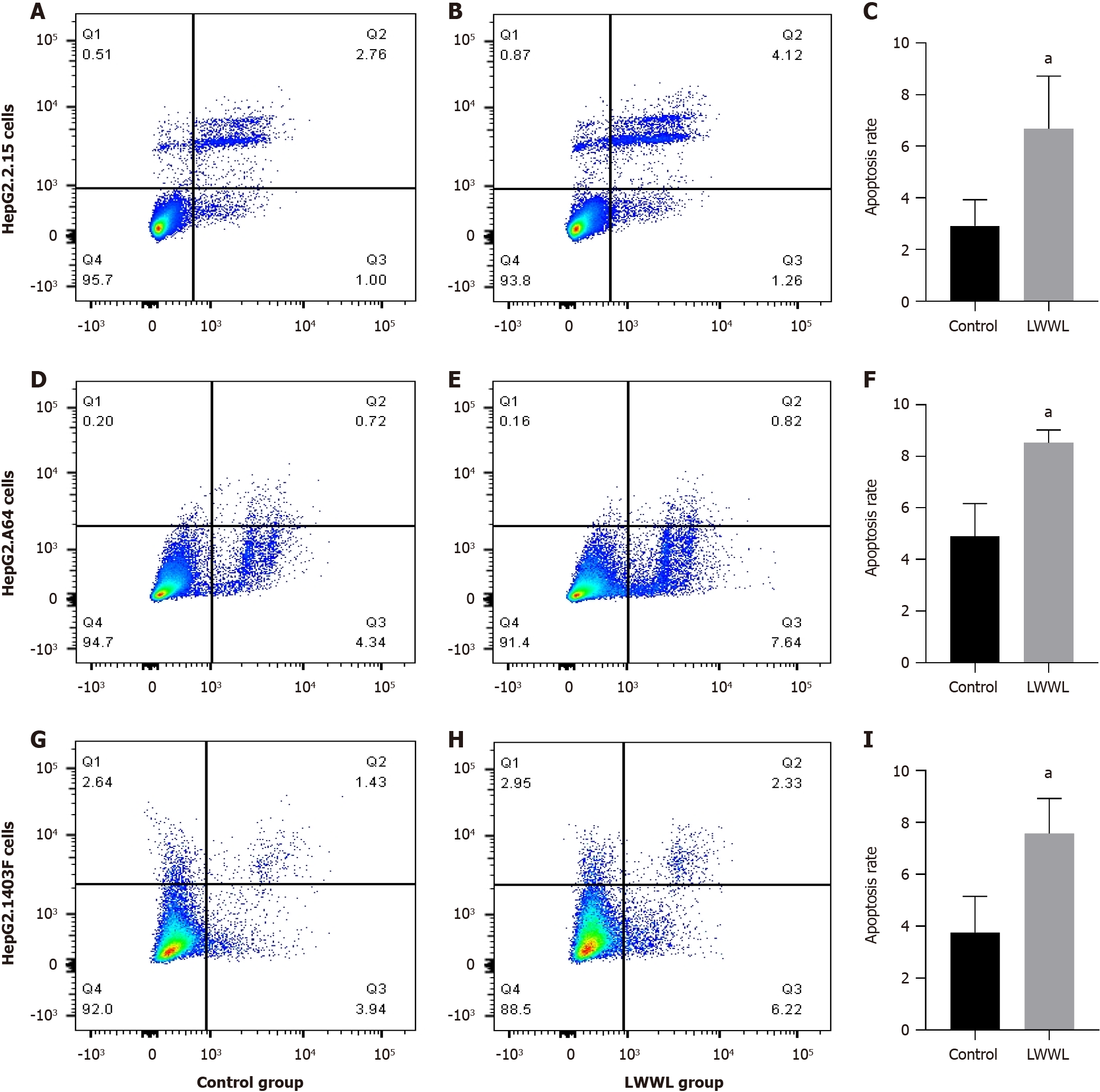
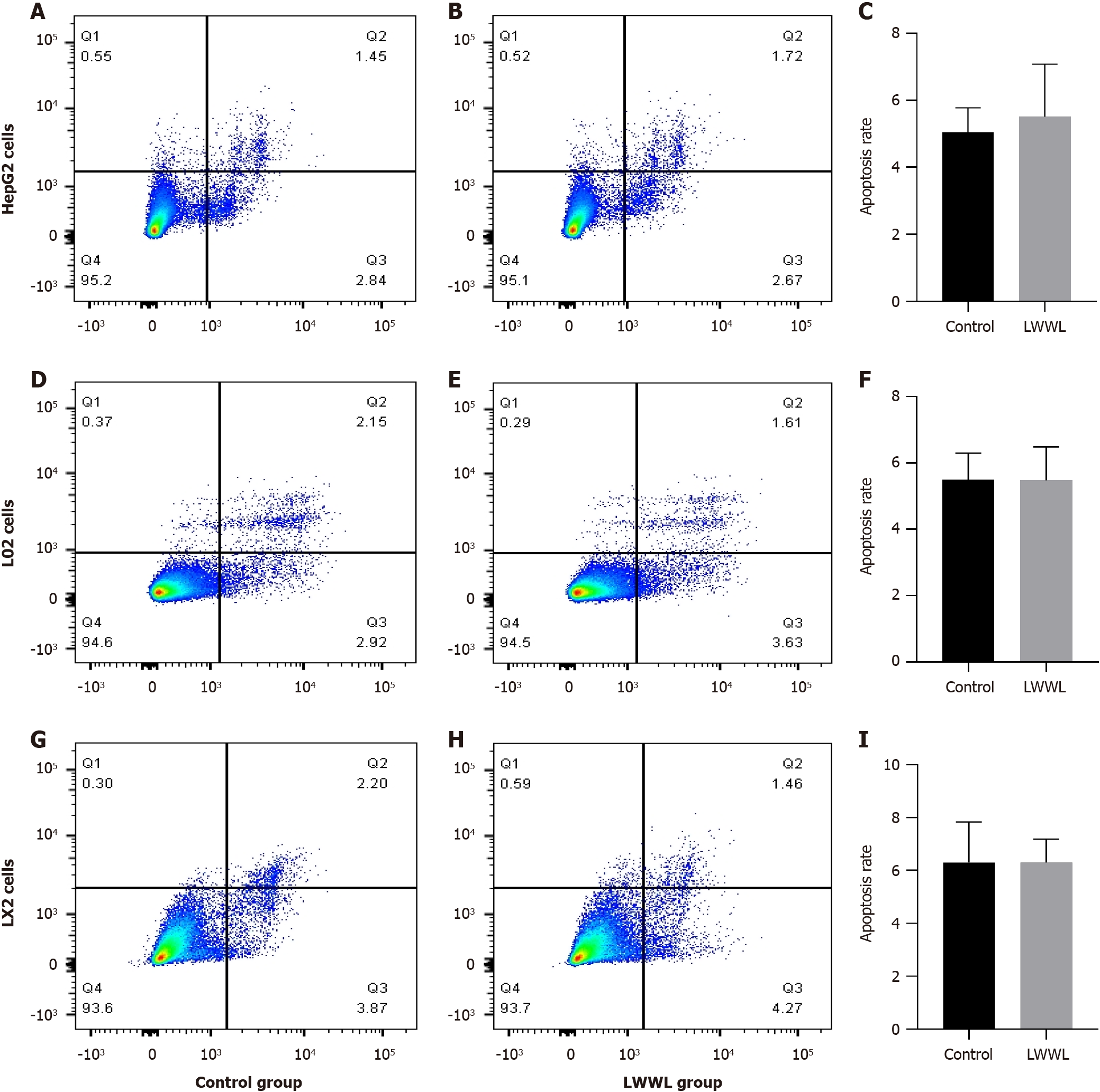
Based on analysis of apoptosis flow, the apoptosis rate in the LWWL-treated group was significantly increased compared with the CG in HepG2.2.15 cells (2.92% ± 1.01% vs 6.68 ± 2.04%, P < 0.05), HepG2.A64 cells (4.89% ± 1.28% vs 8.52% ± 0.50%, P < 0.05) and HepG2.1403F cells (3.76% ± 1.40% vs 7.57% ± 1.35%, P < 0.05) (CG vs LWWL-treated group) (Figure 4). However, no notable difference in apoptosis rate was observed between the LWWL-treated and CG in HepG2 cells (5.04% ± 0.74% vs 5.51% ± 1.57%, P > 0.05), L02 cells (5.49% ± 0.80% vs 5.48% ± 1.01%, P > 0.05) and LX2 cells (6.29% ± 1.54% vs 6.29% ± 0.88%, P > 0.05) (CG vs LWWL-treated group) (Figure 5).
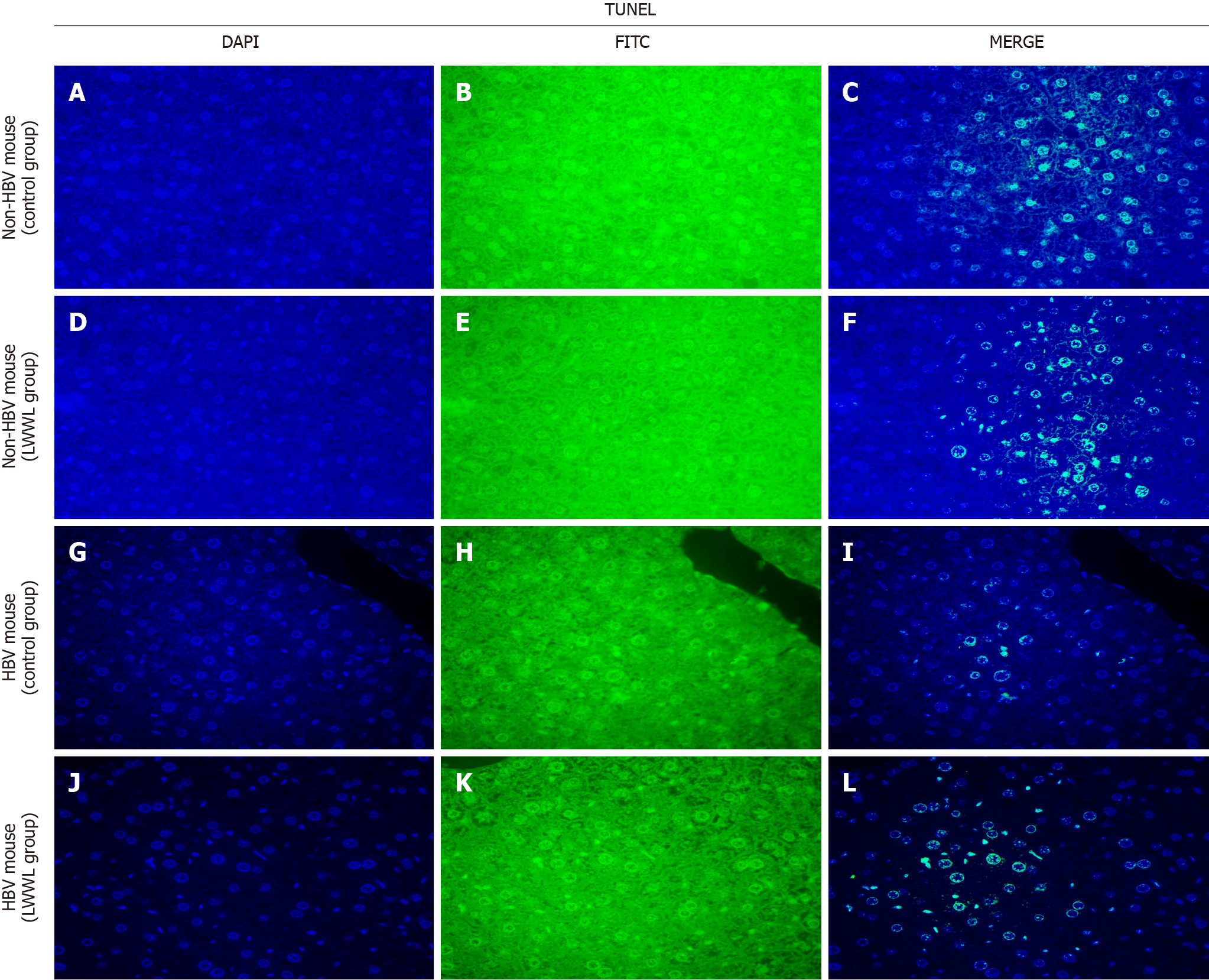
TUNEL staining was used to assess the impact of LWWL on liver apoptosis in the HBV and non-HBV-replicating mouse models. In the HBV-replicating mouse model, LWWL enhanced the apoptotic response of the liver as compared with the CG. In the non-HBV-replicating mouse model, LWWL had no significant apoptotic effect on the liver when compared with the CG (Figure 6).
Previously, we assessed the antiviral efficacy of LWWL in animal and cell models of wild-type HBV infection, using established virological indicators such as HBV DNA, HBsAg, HBeAg, and hepatitis B core antigen[8]. To provide a more comprehensive assessment of the anti-HBV potential of LWWL, the present study expanded to encompass a multidrug-resistant HBV cell model in addition to the wild-type HBV cell model. The evaluation of novel HBV indicators including pgRNA, cccDNA and pgRNA/cccDNA were integrated alongside conventional virological markers. LWWL demon
We extended our comprehensive assessment of the activity of LWWL against HBV and used a combined approach of transcriptomics and metabolomics to delve into its underlying mechanism, revealing a predominant involvement of apoptosis-related pathways. Transcriptomics and metabolomics revealed that the anti-HBV effect of LWWL primarily involved apoptosis-associated pathways, encompassing the PI3K-AKT, CASP8-CASP3 and P53 pathways, in conjunction with apoptosis-related metabolites including D-galactose, lysine, L-glutamic acid and ornithine[8]. Subsequent examination of the terminal outcomes of the apoptosis-related pathways of LWWL revealed that LWWL selectively triggered apoptosis in HBV-infected cells but had no effect on non-HBV-infected hepatic cells in vitro and in vivo.
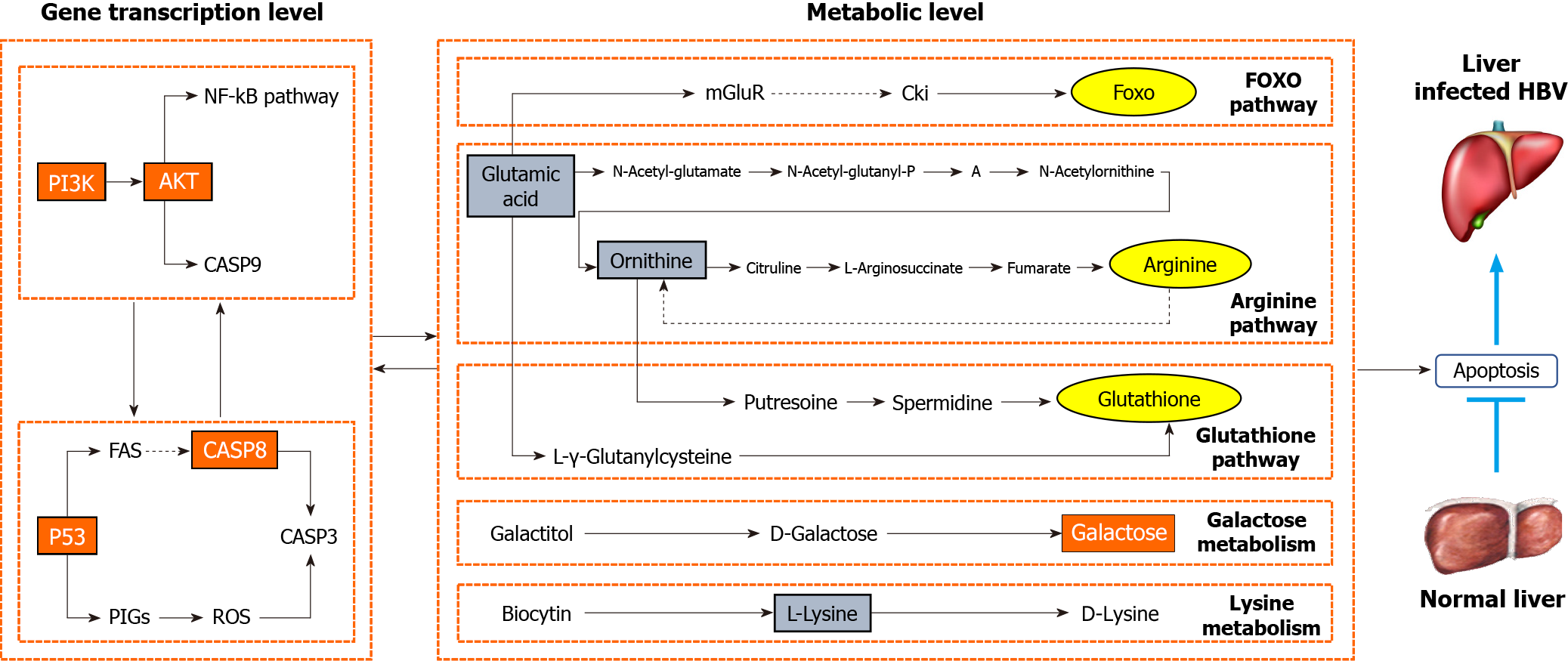
The pathogenesis of CHB is complex. Alongside the extensively studied immunological mechanism, the apoptotic pathway of HBV has progressively gained prominence as a focal area of interest. Following HBV infection of liver cells, the equilibrium of the body is disrupted, culminating in diverse disorders, among which, disruption of apoptosis homeostasis plays a pivotal role. HBV inhibits liver cell apoptosis to sustain its conducive survival microenvironment[4,15,16]. By building upon this foundation, some investigations have delved into the anti-HBV mechanism of drugs through the modulation of apoptosis-associated pathways. Notably, polysaccharides derived from Sipunculus nudus and humic acid were found to modulate the CASP3 pathway[17,18], while Folium Artemisiae Argyi exhibited the ability to regulate the Wnt/β-catenin pathway[19], inducing apoptosis in a dose-dependent manner in HepG2.2.15 cells and hindering HBV DNA replication. Ascentage Pharma APG-1387, currently advancing to phase II clinical trials, stands as a cutting-edge anti-HBV therapeutic that centers on stimulating the apoptosis of HBV-infected cells[20]. Consequently, the exploration and development of anti-HBV drugs through the lens of apoptosis is scientifically important and clinically relevant. In contrast to prior medications that induce anti-HBV effects via apoptosis induction, we have unearthed an innovative selective apoptosis mechanism against HBV. This signifies that LWWL can target and eliminate HBV-infected cells while sparing normal cells. This may account for the favorable safety profile of LWWL in clinical settings. The activation of apoptosis-associated pathways (PI3K-AKT, CASP8-CASP3 and P53) by LWWL likely intertwines with the modulation of apoptosis-related metabolites (D-galactose, lysine, L-glutamate and ornithine), resulting in apoptosis of HBV-infected hepatic cells (Figure 7).
In conclusion, our study establishes, for the first time, the potent HBV-suppressive capability of the Chinese patent medicine LWWL. These suppressive effects surpassed those of TDF in terms of antigen expression in vitro and in vivo. A foundational insight into the mechanism of the anti-HBV action of LWWL suggests that it selectively induces apoptosis in HBV-replicating hepatic cells while not affecting non-HBV-replicating hepatic cells. These findings have revealed novel insights into the anti-HBV potential of LWWL, offering a prospect of enhancing combination therapy with LWWL and established NAs. Additionally, these insights could serve as a novel mechanistic reference for the advancement of anti-HBV medications.
Nucleoside/nucleotide analogs (NAs) are the most commonly-used anti-hepatitis B virus (HBV) agents. They effectively inhibit viral replication, whereas the suppressive effects are much weaker on HBV antigen and drug-resistant HBV. Therefore, it is very important and meaningful to find new drugs to make up for the deficiency of NAs. Liuweiwuling Tablet (LWWL) is a licensed Chinese patent medicine for anti-inflammation of chronic HBV infection. We previous found that LWWL has an anti-HBV effect in wild-type HBV model for the first time, but the mechanism is still unclear.
The objective of this study was to elucidate the scientific significance of LWWL’s antiviral advantages, in order to provide a reference for the clinical application of LWWL against HBV.
The study aimed to explore the potential mechanism of anti-HBV, and further comprehensively evaluate its anti-HBV effect in drug-resistant HBV model.
In vitro experiments utilized three HBV-replicating cell lines and three non-HBV-replicating cell lines, while an in vivo experiment involved a hydrodynamic injection-mediated mouse model with HBV replication. Transcriptomics and metabolomics were employed to investigate the underlying mechanisms.
Our study establishes the potent HBV-suppressive capability of the Chinese patent medicine LWWL. Furthermore, these suppressive effects surpassed those of tenofovir disoproxil fumarate in terms of antigen expression in both in vitro and in vivo. A foundational insight into the mechanism of LWWL’s anti-HBV action suggests its involvement in the regulation of selective apoptosis, selectively inducing apoptosis in HBV-replicating hepatic cells while not affecting non-HBV-replicating hepatic cells.
The preliminary revelation in the anti-HBV pharmacological mechanism is that LWWL exerts a potent inhibitory impact on both wild-type and drug-resistant HBV, potentially involving a selective regulation of apoptosis. These findings offer novel insights into the anti-HBV activities of LWWL and present a novel mechanism for the development of anti-HBV medications.
Novel anti-HBV drugs were developed by using a selective regulation of apoptosis mechanism.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciftci S, Turkey; Tovo CV, Brazil S-Editor: Qu XL L-Editor: A P-Editor: Yuan YY
| 1. | Revill PA, Chisari FV, Block JM, Dandri M, Gehring AJ, Guo H, Hu J, Kramvis A, Lampertico P, Janssen HLA, Levrero M, Li W, Liang TJ, Lim SG, Lu F, Penicaud MC, Tavis JE, Thimme R; Members of the ICE-HBV Working Groups; ICE-HBV Stakeholders Group Chairs; ICE-HBV Senior Advisors, Zoulim F. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol Hepatol. 2019;4:545-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 379] [Article Influence: 63.2] [Reference Citation Analysis (1)] |
| 2. | Ye J, Chen J. Interferon and Hepatitis B: Current and Future Perspectives. Front Immunol. 2021;12:733364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 3. | Nguyen MH, Wong G, Gane E, Kao JH, Dusheiko G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin Microbiol Rev. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 4. | Ge F, Yang Y, Bai Z, Si L, Wang X, Yu J, Xiao X, Liu Y, Ren Z. The role of Traditional Chinese medicine in anti-HBV: background, progress, and challenges. Chin Med. 2023;18:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Reference Citation Analysis (0)] |
| 5. | Seo JW, Kim K, Jun KI, Kang CK, Moon SM, Song KH, Bang JH, Kim ES, Kim HB, Park SW, Kim NJ, Choe PG, Park WB, Oh MD. Recovery of Tenofovir-induced Nephrotoxicity following Switch from Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide in Human Immunodeficiency Virus-Positive Patients. Infect Chemother. 2020;52:381-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Hepatobiliary Specialized Committee of China Association of Chinese Medicine; Liver Diseases Specialized Committee of China Medical Association of Minorities. The clinical guidelines of diagnosis and treatment of chronic hepatitis B with traditional Chinese medicine. Zhongxiyi Jiehe Ganbing Zazhi. 2019;29:97-102. |
| 7. | Hepatobiliary Specialized Committee of China Association of Chinese Medicine; Chinese Patent Medicine Committee of China Association of Chinese Medicine; Clinical Pharmacy Professional Committee of Chinese Pharmaceutical Association. Clinical application expert consensus of treatment of chronic hepatitis B with Liuwei wuling tablet. Zhongxiyi Jiehe Ganbing Zazhi. 2020;30:482-485. |
| 8. | Ge FL, Si LL, Yang Y, Li YH, Lv ZL, Liu WH, Liao H, Wang J, Zou J, Li L, Li H, Zhang ZL, Wang JB, Lu XC, Xu DP, Bai ZF, Liu Y, Xiao XH. Chinese Patent Medicine Liuweiwuling Tablet had Potent Inhibitory Effects on Both Wild-Type and Entecavir-Resistant Hepatitis B Virus (HBV) in vitro and Effectively Suppressed HBV Replication in Mouse Model. Front Pharmacol. 2021;12:756975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Ge FL, Yang Y, Si LL, Li YH, Zhao WX, Bai ZF, Liu Y, Xiao XH. The efficacy and safety of Chinese patent medicine LWWL combined with nucleoside/nucleotide analogues in treatment of chronic hepatitis B: A systematic review and meta-analysis. Gastro End. 2023;165-173. [DOI] [Full Text] |
| 10. | Liu Y, Li X, Xin S, Xu Z, Chen R, Yang J, Liu L, Wong VW, Yang D, Chan HL, Xu D. The rtA181S mutation of hepatitis B virus primarily confers resistance to adefovir dipivoxil. J Viral Hepat. 2015;22:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Wang J, Du M, Huang H, Chen R, Niu J, Jiang J, Zhuang H, Lu F. Reply to: "Serum HBV pgRNA as a clinical marker for cccDNA activity": Consistent loss of serum HBV RNA might predict the "para-functional cure" of chronic hepatitis B. J Hepatol. 2017;66:462-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Si LL, Li XD, Li L, Niu M, Wang JB, Lan JC, Xu DP, Liu Y. Inhibitory effect of Suduxing extracts on covalently closed circular DNA of hepatitis B virus. Zhonghua Shiyan And Linchuang Ganranbing Zazhi (Dianziban). 2020;14:265-271. |
| 13. | Ji D, Liu Y, Si LL, Li L, Chen GF, Xin SJ, Zhao JM, Xu D. Variable influence of mutational patterns in reverse-transcriptase domain on replication capacity of hepatitis B virus isolates from antiviral-experienced patients. Clin Chim Acta. 2011;412:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Yang Y, Ge FL, Zhan XY, Mu WQ, Li ZY, Lin L, Wei ZY, Bai ZF, Sun Q, Xiao XH. Schisandra chinensis Oil Attenuates Aristolochic Acid I-Induced Nephrotoxicity in vivo and in vitro. Chin J Integr Med. 2022;28:603-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Ebert G, Allison C, Preston S, Cooney J, Toe JG, Stutz MD, Ojaimi S, Baschuk N, Nachbur U, Torresi J, Silke J, Begley CG, Pellegrini M. Eliminating hepatitis B by antagonizing cellular inhibitors of apoptosis. Proc Natl Acad Sci U S A. 2015;112:5803-5808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 16. | He P, Zhang B, Liu D, Bian X, Li D, Wang Y, Sun G, Zhou G. Hepatitis B Virus X Protein Modulates Apoptosis in NRK-52E Cells and Activates Fas/FasL Through the MLK3-MKK7-JNK3 Signaling Pathway. Cell Physiol Biochem. 2016;39:1433-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Su J, Jiang L, Wu J, Liu Z, Wu Y. Anti-tumor and anti-virus activity of polysaccharides extracted from Sipunculus nudus(SNP) on Hepg2.2.15. Int J Biol Macromol. 2016;87:597-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Pant K, Yadav AK, Gupta P, Rathore AS, Nayak B, Venugopal SK. Humic acid inhibits HBV-induced autophagosome formation and induces apoptosis in HBV-transfected Hep G2 cells. Sci Rep. 2016;6:34496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Wang K, Yan WM. [Effect of extract of folium artemisiae argyi on HepG2.2.15 cell apoptosis and hepatitis B virus replication]. Zhongguo Linchuang Yaolixue Zazhi. 2021;37:1992-1995. |
| 20. | Pan W, Luo Q, Yan X, Yuan L, Yi H, Zhang L, Li B, Zhang Y, Sun J, Qiu MZ, Yang DJ. A novel SMAC mimetic APG-1387 exhibits dual antitumor effect on HBV-positive hepatocellular carcinoma with high expression of cIAP2 by inducing apoptosis and enhancing innate anti-tumor immunity. Biochem Pharmacol. 2018;154:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |