Copyright
©The Author(s) 2024.
World J Gastroenterol. Apr 7, 2024; 30(13): 1911-1925
Published online Apr 7, 2024. doi: 10.3748/wjg.v30.i13.1911
Published online Apr 7, 2024. doi: 10.3748/wjg.v30.i13.1911
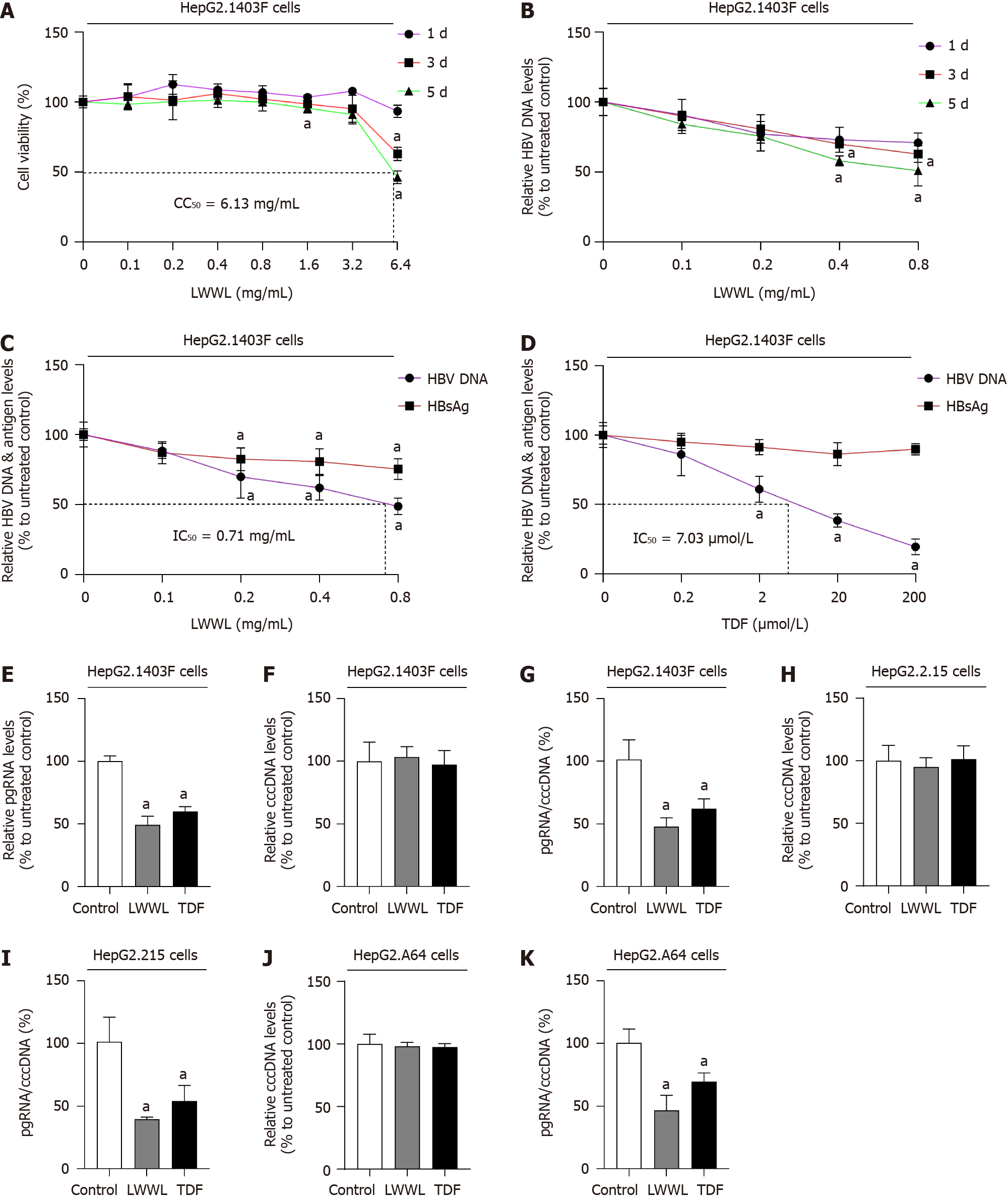
Figure 1 Effects of Liuweiwuling Tablet on hepatitis B virus DNA/covalently closed circular DNA/RNA and antigen in cell models.
A: The safe concentration of Liuweiwuling Tablet (LWWL), defined as maintaining ≥ 95% cell viability compared with the untreated controls, was evaluated in HepG2.1403F cells; B: The optimal effective time for anti-hepatitis B virus (HBV) activity of LWWL, determined as the time-point demonstrating the strongest suppression of HBV DNA during a 5-d observation period, was evaluated in HepG2.1403F cells; C: The inhibitory effects of LWWL on HBV DNA and supernatant hepatitis B surface antigen (HBsAg) were evaluated in HepG2.1403F cells; D: The inhibitory effects of tenofovir disoproxil fumarate (TDF) on HBV DNA and supernatant HBsAg were assessed in HepG2.1403F cells; E: The inhibitory effects of both LWWL and TDF on pregenomic RNA (pgRNA) were measured in HepG2.1403F cells; F: The inhibitory effects of both LWWL and TDF on covalently closed circular DNA (cccDNA) were measured in HepG2.1403F cells; G: The inhibitory effects of both LWWL and TDF on pgRNA/cccDNA were measured in HepG2.1403F cells; H: The inhibitory effects of LWWL and TDF on cccDNA were evaluated in HepG2.2.15 cells; I: The inhibitory effects of LWWL and TDF on pgRNA/cccDNA were evaluated in HepG2.2.15 cells; J: The inhibitory effects of LWWL and TDF on cccDNA were investigated in HepG2.A64 cells; K: The inhibitory effects of LWWL and TDF on pgRNA/cccDNA were investigated in HepG2.A64 cells. aP < 0.05 vs control.
- Citation: Ge FL, Yang Y, Si LL, Li YH, Cao MZ, Wang J, Bai ZF, Ren ZG, Xiao XH, Liu Y. Inhibition of hepatitis B virus via selective apoptosis modulation by Chinese patent medicine Liuweiwuling Tablet. World J Gastroenterol 2024; 30(13): 1911-1925
- URL: https://www.wjgnet.com/1007-9327/full/v30/i13/1911.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i13.1911