Published online Apr 7, 2024. doi: 10.3748/wjg.v30.i13.1887
Peer-review started: January 12, 2024
First decision: January 30, 2024
Revised: February 7, 2024
Accepted: March 14, 2024
Article in press: March 14, 2024
Published online: April 7, 2024
Processing time: 82 Days and 3.8 Hours
Differences in the preoperative characteristics and weight loss outcomes after sleeve gastrectomy (SG) between patients with familial aggregation of obesity (FAO) and patients with sporadic obesity (SO) have not been elucidated.
To explore the impact of SG on weight loss and the alleviation of obesity-related comorbidities in individuals with FAO.
A total of 193 patients with obesity who underwent SG were selected. Patients with FAO/SO were matched 1:1 by propensity score matching and were cate
We defined FAO as the presence of two or more first-degree relatives with obesity. Patients with FAO did not initially show significant differences in baseline data, short-term postoperative weight loss, or obesity-related comorbidities when compared to patients with SO preoperatively. However, distinctions between the two groups became evident at the two-year mark, with statistically significant differences in both percentage of total weight loss (P = 0.006) and percentage of excess weight loss (P < 0.001). The FAO group exhibited weaker remission of type 2 diabetes mellitus (T2DM) (P = 0.031), hyperlipidemia (P = 0.012), and non-alcoholic fatty liver disease (NAFLD) (P = 0.003) as well as a lower incidence of acid reflux (P = 0.038).
FAO patients is associated with decreased mid-to-long-term weight loss outcomes; the alleviation of T2DM, hyperlipidemia and NAFLD; and decreased incidence of acid reflux postoperatively.
Core Tip: This was a retrospective study. We aimed to compare preoperative characteristics and postoperative outcomes between patients with familial aggregation of obesity (FAO) and those with sporadic obesity. The following data were examined: Baseline characteristics, weight changes at postoperative intervals (1, 3, 6, 12, 24, and 36 months), alleviation of obesity-related complications, and the occurrence of surgery-related complications. Such a comparative analysis provides valuable insights for guiding postoperative treatment and health education tailored to individuals with FAO.
- Citation: Wang ZY, Qu YF, Yu TM, Liu ZL, Cheng YG, Zhong MW, Hu SY. Novel subtype of obesity influencing the outcomes of sleeve gastrectomy: Familial aggregation of obesity. World J Gastroenterol 2024; 30(13): 1887-1898
- URL: https://www.wjgnet.com/1007-9327/full/v30/i13/1887.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i13.1887
The prevalence of overweight and obesity is steadily increasing in China[1], making it the country with the largest population of overweight and obese individuals globally. It is expected that the prevalence of overweight [body mass index (BMI) 24.0-28.0 kg/m²] and obesity (BMI ≥ 28.0 kg/m²) in adults may reach 65.3% by 2030[2], thus leading to a significant public health concern[3]. Obesity can lead to a myriad of multisystem abnormalities, encompassing cardiovascular disease, type 2 diabetes mellitus (T2DM), hyperlipidemia, hyperuricemia, nonalcoholic fatty liver disease, polycystic ovary syndrome, mental disorders, locomotor and joint disorders, and respiratory diseases, among other comorbidities.
Obesity results from the intricate interplay of genetic, environmental, lifestyle, and sociocultural factors[4]. These factors affect fat accumulation or consumption by influencing several physiologic mediators of food intake and energy expenditure[5]. An individual’s family–often representing a microcosm of closely aligned genetic profiles, lifestyle behaviors, environmental exposures, and sociocultural outlooks–exerts a substantial influence on the emergence of obesity and the outcomes of weight management. Sleeve gastrectomy (SG), which accounted for 84.9% of bariatric surgical procedures[6], effectively facilitates weight loss in patients with obesity while markedly enhancing metabolic processes and ameliorating obesity-related comorbidities[7].
The familial aggregation of diseases is a focal point of research across several disciplines, including psychiatry[8], neurology[9] and oncology[10,11]. By studying the characteristics of first-degree relatives of individuals with obesity, we can gain deeper insights into the pathogenesis of obesity, thereby contributing to the search for novel treatments or prevention methods[12]. This study provides a theoretical foundation for precision prevention and treatment of obesity.
We selected a cohort of 193 patients with obesity who met the criteria for SG and who underwent surgery at our medical center between December 2019 and April 2023 for this observational study. Following surgery, all patients received uniform postoperative guidance and health education.
Inclusion criteria: (1) Patients aged between 16 and 65 years; (2) patients met the surgical indications outlined in the Chinese Guidelines for the Surgical Management of Obesity and T2DM (2019 edition)[13]; and (3) patients were capable of undergoing normal follow-up after SG.
Exclusion criteria: (1) Patients lacking information about first-degree relatives; (2) patients requiring obesity-inducing medications for their medical conditions after the operation; (3) patients who became pregnant shortly after the procedure; and (4) patients on appetite suppressants (such as metformin) following surgery.
The familial aggregation of obesity (FAO) group and the sporadic obesity (SO) group: We divided the participants into groups according to the number of first-degree relatives (parents, children and siblings of the proband) with obesity (BMI > 28 kg/m2). The grouping criteria for FAO were as follows.
1FAO (FAO group 1): Except for the proband, the number of first-degree relatives with obesity ≥ 1. 1SO (SO group 1): Except for the proband, the number of first-degree relatives with obesity = 0.
2FAO (FAO group 2): Except for the proband, the number of first-degree relatives with obesity ≥ 2. 2SO (SO group 2): Except for the proband, the number of first-degree relatives with obesity < 2.
The data were collected independently by two individuals. Perioperative data for all patients, including sex, age, BMI, family history, waist circumference, hip circumference, and obesity-related comorbidities (such as hypertension, T2DM, and hyperlipidemia), were systematically recorded using the electronic case management system. Following SG, we conducted thorough postoperative follow-ups at 1, 3, 6, 12, 24, and 36 months through a combination of hospital visits and telephone interviews. These follow-ups involved evaluating postoperative weight, conducting blood tests, assessing surgery-related complications, and monitoring the improvement of preoperative obesity-related comorbidities. To assess the effectiveness of weight loss surgery, we employed the percentage of total weight loss (%TWL) and percentage of excess weight loss (%EWL) as evaluation criteria.
%TWL = (initial body weight - final body weight)/initial weight × 100%.
%EWL = [(initial body weight - final body weight)/(initial weight - ideal body weight)] × 100%.
Ideal BMI (IBMI): 23 kg/m2 (Asian standard), ideal body weight: IBMI × (height)2.
All clinical data were analyzed using SPSS statistical software (version 26.0; SPSS, Inc., Chicago, IL, United States). Propensity score matching (PSM)[14] was employed for 1:1 matching of FAO/SO groups. Independent samples t tests were also conducted to compare preoperative baseline data and postoperative weight loss outcomes at each follow-up interval between patients in the FAO group and the SO group. Linear regression was employed to identify factors influencing %TWL and %EWL. To compare the prevalence of obesity-related comorbidities and surgery-related complications in patients in the FAO and SO groups, χ2 or Fisher’s exact test was employed both preoperatively and at the 6-month postoperative assessment. P values < 0.05 indicated statistical significance.
This observational study included a total of 193 patients who underwent SG (male: 64, 33.2%; female: 129, 66.8%), with a mean BMI of 41.3 ± 7.0 kg/m². Among the obese patients, various obesity-related comorbidities were prevalent, including metabolic syndrome (88, 45.6%), hypertension (67, 34.7%), T2DM (94, 48.7%), hyperlipidemia (81, 42%), sleep apnea hypopnea syndrome (128, 66.3%), polycystic ovary syndrome (31, 24.0%, n = 129), nonalcoholic fatty liver disease (163, 84.5%), gout (10, 5.2%), and hyperuricemia (114, 59.1%). Additionally, 113 patients (58.5%) were in the 1FAO group, while 58 (30.0%) were in the 2FAO group. Specific indicators of obesity-related comorbidities are detailed in Table 1.
| Baseline | Total, n = 193 |
| Sex (Females) | 129 (66.8) |
| Age (yr, mean ± SD) | 31.5 ± 8.2 |
| Height (cm, mean ± SD) | 169.3 ± 8.0 |
| Weight (kg, mean ± SD) | 119.6 ± 27.7 |
| Body mass index (kg/m2, mean ± SD) | 41.3 ± 7.0 |
| Waistline (cm, mean ± SD) | 123.2 ± 17.2 |
| Hipline (cm, mean ± SD) | 129.4 ± 15.7 |
| 1FAO | 113 (58.5) |
| 2FAO | 58 (30.0) |
| Obesity-related comorbidities | |
| Metabolic syndrome | 88 (45.6) |
| Hypertension | 67 (34.7) |
| Cardiovascular disease | 10 (5.2) |
| Type 2 diabetes mellitus | 94 (48.7) |
| Impaired glucose tolerance | 78 (40.4) |
| Hyperlipoidemia | 81 (42.0) |
| Obstructive sleep apnea | 128 (66.3) |
| Polycystic ovarian syndrome (n = 129) | 31 (24.0) |
| Non-alcoholic fatty liver disease | 163 (84.5) |
| Gout | 10 (5.2) |
| Hyperuricemia | 114 (59.1) |
Preoperative baseline information: We applied PSM analysis to pair patients in the 1SO/1FAO and 2SO/2FAO groups utilizing predictors of major obesity-related comorbidities (metabolic syndrome, hypertension, T2DM, hyperlipidemia, and nonalcoholic fatty liver disease). The matched groups exhibited no significant differences in patient age, height, weight, or BMI, as shown in Table 2.
| 1SO, n = 75 | 1FAO, n = 75 | P value | 2SO, n = 54 | 2FAO, n = 54 | P value | |
| Sex [female, n (%)] | 50 (66.7) | 48 (64.0) | 0.731 | 36 (66.7) | 32 (59.3) | 0.425 |
| Age (yr) | 29.5 ± 6.8 | 31.8 ± 9.1 | 0.234 | 31.7 ± 8.6 | 30.7 ± 8.6 | 0.548 |
| Height (cm) | 170.3 ± 7.9 | 170.1 ± 7.7 | 0.884 | 169.9 ± 7.2 | 170.4 ± 8.3 | 0.711 |
| Body Weight (kg) | 123.5 ± 30.4 | 122.2 ± 29.9 | 0.840 | 120.9 ± 28.6 | 124.2 ± 31.3 | 0.562 |
| BMI (kg/m2) | 42.2 ± 8.1 | 41.8 ± 7.5 | 0.795 | 41.5 ± 7.4 | 42.3 ± 7.9 | 0.591 |
| Waistline (cm) | 126.0 ± 18.3 | 124.1 ± 19.9 | 0.731 | 124.8 ± 19.8 | 124.3 ± 19.4 | 0.916 |
| Hipline (cm) | 128.3 ± 12.3 | 129.8 ± 16.5 | 0.744 | 128.0 ± 13.3 | 130.7 ± 17.4 | 0.459 |
Preoperative obesity-related comorbidities: We conducted PSM analysis again to compare patients within the matched 1SO/1FAO and 2SO/2FAO groups utilizing sex and BMI as predictors. The analysis revealed no significant differences in preoperative obesity-related comorbidities between patients in the matched groups, as indicated in Table 3.
| n | 1SO, n = 80 | 1FAO, n = 80 | χ2 | P value | n | 2SO, n = 58 | 2FAO, n = 58 | χ2 | P value | ||
| MS | Without | 89 | 43 (53.8) | 46 (57.5) | 0.228 | 0.633 | 67 | 34 (58.6) | 33 (56.9) | 0.035 | 0.851 |
| With | 71 | 37 (46.3) | 34 (42.5) | 49 | 24 (41.4) | 25 (43.1) | |||||
| HTN | Without | 105 | 54 (67.5) | 51 (63.8) | 0.249 | 0.618 | 75 | 33 (56.9) | 42 (72.4) | 3.056 | 0.080 |
| With | 55 | 26 (32.5) | 29 (36.3) | 41 | 25 (43.1) | 16 (27.6) | |||||
| T2DM | Without | 86 | 43 (53.8) | 43 (53.8) | > 0.999 | 59 | 31 (53.4) | 28 (48.3) | 0.310 | 0.577 | |
| With | 74 | 37 (46.3) | 37 (46.3) | 57 | 27 (46.6) | 30 (51.7) | |||||
| IGT | Without | 89 | 45 (56.3) | 44 (55.0) | 0.025 | 0.874 | 71 | 33 (56.9) | 38 (65.5) | 0.908 | 0.341 |
| With | 71 | 35 (43.8) | 36 (45.0) | 45 | 25 (43.1) | 20 (34.5) | |||||
| HLP | Without | 96 | 51 (63.8) | 45 (56.3) | 0.938 | 0.333 | 68 | 39 (67.2) | 29 (50.0) | 3.554 | 0.059 |
| With | 64 | 29 (36.3) | 35 (43.8) | 48 | 19 (32.8) | 29 (50.0) | |||||
| PCOS | Without | 87 | 41 (74.5) | 46 (80.7) | 0.612 | 0.434 | 52 | 27 (77.1) | 25 (73.5) | 0.157 | 0.924 |
| With | 25 | 14 (25.5) | 11 (19.3) | 17 | 8 (22.9) | 9 (26.5) | |||||
| NAFLD | Without | 26 | 16 (20.0) | 10 (12.5) | 1.653 | 0.199 | 19 | 8 (13.8) | 11 (19.0) | 0.566 | 0.452 |
| With | 134 | 64 (80.0) | 70 (87.5) | 97 | 50 (86.2) | 47 (81.0) | |||||
| OSA | Without | 53 | 27 (33.8) | 26 (32.5) | 0.028 | 0.867 | 37 | 14 (24.1) | 23 (39.7) | 3.215 | 0.073 |
| With | 107 | 53 (66.3) | 54 (67.5) | 79 | 44 (75.9) | 35 (60.3) | |||||
| HUA | Without | 68 | 37 (46.3) | 31 (38.8) | 0.921 | 0.337 | 42 | 18 (31.0) | 24 (41.4) | 1.344 | 0.246 |
| With | 92 | 43 (53.8) | 49 (61.3) | 74 | 40 (69.0) | 34 (58.6) | |||||
Comparison of postoperative information: All 193 patients completed 1/3/6 months of postoperative follow-up, 107 patients completed 12 months of postoperative follow-up, 60 patients completed 24 months of postoperative follow-up, and 21 patients completed 36 months of postoperative follow-up (analysis at 36 months was primarily focused on trend interpretation).
We conducted PSM analysis to align patients in the 1SO/1FAO and 2SO/2FAO groups. We employed sex, preoperative BMI, and major obesity-related comorbidities (metabolic syndrome, hypertension, T2DM, hyperlipidemia, and nonalcoholic fatty liver disease) as predictors to minimize differences between the groups and mitigate the impact of variations in these factors on surgical outcomes.
After PSM analysis, the patient counts were as follows:
1SO vs1FAO = 73 vs 73; 2SO vs2FAO = 53 vs 53 (1/3/6 months after surgery).
1SO vs1FAO = 37 vs 43; 2SO vs2FAO = 52 vs 31 (12 months after surgery).
1SO vs1FAO = 22 vs 22; 2SO vs2FAO = 37 vs 17 (24 months after surgery).
1SO vs1FAO = 6 vs 7; 2SO vs2FAO = 11 vs 8 (36 months after surgery).
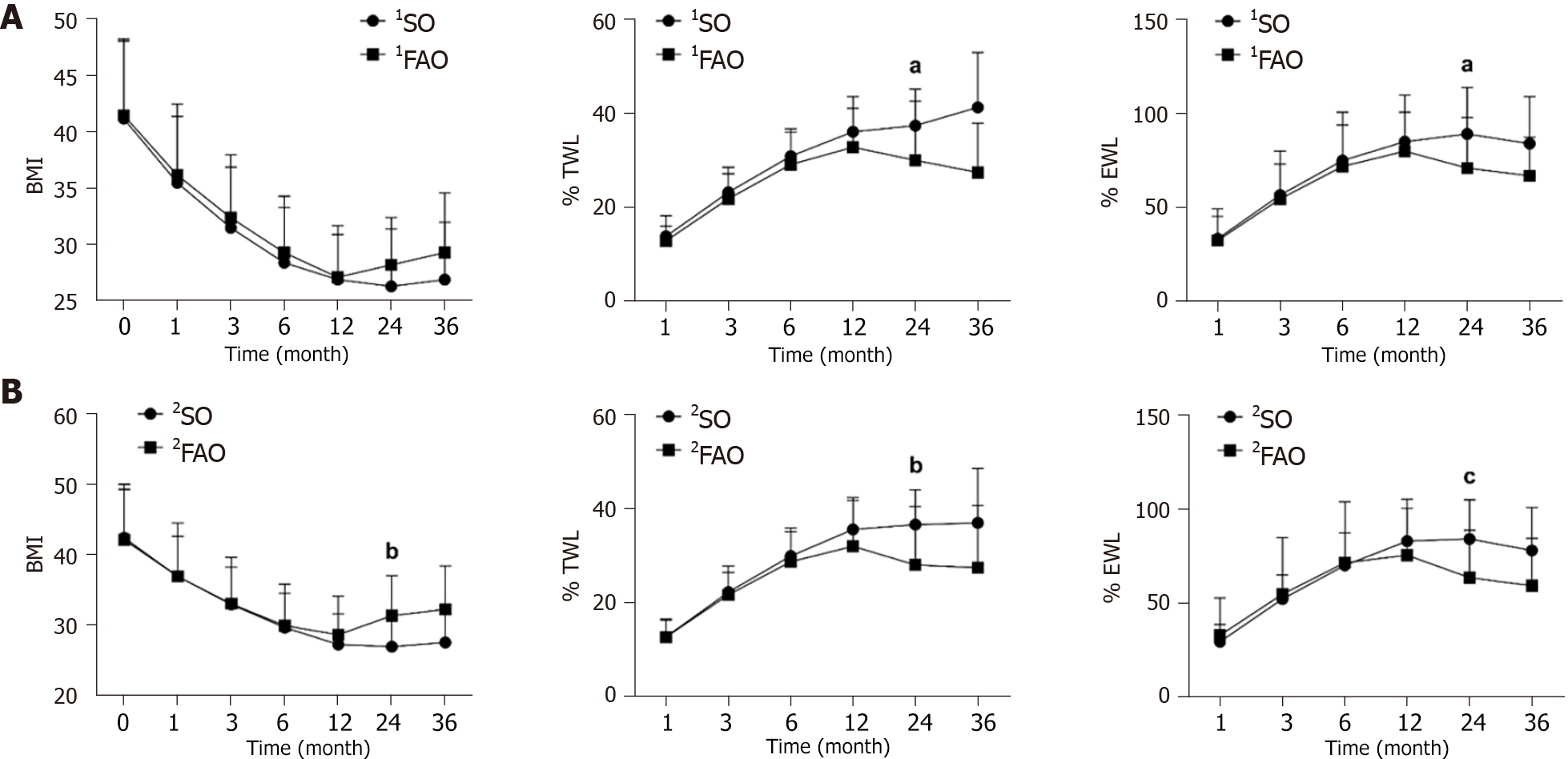
Weight loss: (1) SG results in a substantial weight reduction in the majority of patients after the procedure, as shown in Table 4. There was no significant difference in short-term postoperative weight loss between patients in the 1FAO group and those in the 1SO group. Nevertheless, over time, notable differences became evident at 24 months postsurgery, with patients in the 1FAO group experiencing less weight loss after SG than their counterparts in the 1SO group (%TWL: P = 0.025; %EWL: P = 0.025). Comparatively, patients in the 2FAO group exhibited similar but more pronounced differences than did those in the 2SO group (BMI: P = 0.003, %TWL: P = 0.006, %EWL: P < 0.001). Several line graphs are shown in Figure 1. These lines of view visually illustrate the difference above. Patients with FAO regain weight to some extent at the two-year postoperative mark, while patients with SO are able to maintain a more favorable weight loss outcome.
| 1SO, n = 73 | 1FAO, n = 73 | P value | 2SO, n = 53 | 2FAO, n = 53 | P value | ||
| Baseline | Sex [female, n (%)] | 49 (67.1) | 49 (67.1) | > 0.999 | 36 (67.9) | 33 (62.3) | 0.541 |
| Age (yr) | 30.5 ± 7.7 | 31.0 ± 8.5 | 0.706 | 31.6 ± 8.7 | 30.6 ± 8.3 | 0.537 | |
| Height (cm) | 169.2 ± 8.4 | 169.9 ± 8.1 | 0.617 | 169.8 ± 7.5 | 170.2 ± 8.5 | 0.817 | |
| Body weight (kg) | 118.8 ± 26.7 | 120.7 ± 27.4 | 0.660 | 123.2 ± 27.3 | 123.2 ± 31.3 | 0.995 | |
| BMI (kg/m2) | 41.2 ± 6.9 | 41.5 ± 6.8 | 0.802 | 42.4 ± 6.9 | 42.1 ± 7.9 | 0.842 | |
| Waistline (cm) | 123.0 ± 16.0 | 123.4 ± 17.3 | 0.892 | 124.9 ± 18.4 | 123.4 ± 19.3 | 0.725 | |
| Hipline (cm) | 128.8 ± 16.3 | 131.0 ± 14.4 | 0.439 | 131.0 ± 18.1 | 129.8 ± 17.1 | 0.771 | |
| BMI | Pre-op (kg/m2) | 41.2 ± 6.9 | 41.5 ± 6.8 | 0.802 | 42.4 ± 6.9 | 42.1 ± 7.9 | 0.842 |
| 1 month (kg/m2) | 35.5 ± 5.9 | 36.2 ± 6.3 | 0.465 | 36.9 ± 5.7 | 36.9 ± 7.6 | 0.974 | |
| 3 months (kg/m2) | 31.5 ± 5.4 | 32.4 ± 5.6 | 0.364 | 32.9 ± 5.3 | 33.0 ± 6.6 | 0.950 | |
| 6 months (kg/m2) | 28.4 ± 4.9 | 29.3 ± 5.0 | 0.246 | 29.6 ± 4.9 | 29.9 ± 5.9 | 0.784 | |
| 12 months (kg/m2) | 26.9 ± 4.8 | 27.1 ± 3.9 | 0.830 | 27.2 ± 4.4 | 28.6 ± 5.5 | 0.213 | |
| 24 months (kg/m2) | 26.3 ± 5.1 | 28.2 ± 4.2 | 0.201 | 26.9 ± 4.2 | 31.3 ± 5.7 | 0.003 | |
| 36 months (kg/m2) | 26.9 ± 5.1 | 29.3 ± 5.3 | 0.428 | 27.5 ± 4.0 | 32.2 ± 6.2 | 0.061 | |
| %TWL | 1 month | 13.8 ± 4.4 | 12.8 ± 3.2 | 0.116 | 12.7 ± 3.5 | 12.6 ± 3.9 | 0.896 |
| 3 months | 23.2 ± 5.3 | 21.8 ± 5.3 | 0.112 | 22.2 ± 4.2 | 21.6 ± 6.2 | 0.571 | |
| 6 months | 30.9 ± 5.8 | 29.0 ± 6.8 | 0.072 | 29.9 ± 5.2 | 28.7 ± 7.2 | 0.323 | |
| 12 months | 36.1 ± 7.5 | 32.7 ± 8.3 | 0.063 | 35.6 ± 6.8 | 32.0 ± 9.8 | 0.081 | |
| 24 months | 37.4 ± 7.8 | 30.0 ± 12.6 | 0.025 | 36.6 ± 7.4 | 26.7 ± 12.5 | 0.006 | |
| 36 months | 41.3 ± 11.7 | 27.4 ± 10.5 | 0.044 | 37.0 ± 11.6 | 26.4 ± 13.3 | 0.079 | |
| %EWL | 1 month | 33.2 ± 12.1 | 32.2 ± 16.9 | 0.675 | 29.1 ± 9.2 | 32.5 ± 19.9 | 0.262 |
| 3 months | 56.5 ± 16.5 | 54.4 ± 25.5 | 0.561 | 51.8 ± 12.8 | 54.5 ± 30.0 | 0.541 | |
| 6 months | 74.9 ± 18.9 | 71.5 ± 28.9 | 0.399 | 69.8 ± 17.4 | 71.2 ± 32.5 | 0.771 | |
| 12 months | 84.9 ± 24.8 | 79.8 ± 20.8 | 0.322 | 82.7 ± 22.4 | 75.2 ± 25.0 | 0.162 | |
| 24 months | 89.1 ± 24.8 | 70.9 ± 27.0 | 0.025 | 83.9 ± 20.8 | 59.2 ± 25.2 | < 0.001 | |
| 36 months | 83.9 ± 25.1 | 66.7 ± 20.6 | 0.202 | 77.8 ± 22.8 | 56.9 ± 25.3 | 0.084 | |
And (2) Multiple linear regression analysis. To further explore the factors affecting weight loss outcomes and assess the impact of FAO, we conducted linear regression analyses on %TWL and %EWL at various postoperative time points (Tables 5 and 6). The %TWL, %EWL and BMI exhibited normal distributions. Factors affecting %TWL and %EWL showed no significant multicollinearity. After controlling for the effects of age and obesity-related comorbidities on surgery, we observed that the impact of 1FAO on weight loss outcomes was not significantly different at 24 months postsurgery, whereas 2FAO and preoperative BMI exhibited statistically significant differences in their influence on weight loss outcomes, as indicated in Tables 5 and 6 (%TWL: 2FAO: P < 0.001, BMI: P = 0.001; %EWL: 2FAO: P < 0.001).
| 1 month | 3 months | 6 months | 12 months | 24 months | ||
| %TWL | 1FAO | -1.036 | -1.352 | -1.838 | -1.542 | -5.123 |
| Sex | -0.361 | -1.054 | -1.798 | -1.229 | -1.227 | |
| BMI | 0.0280 | 0.096 | 0.250b | 0.444a | 0.672a | |
| MS | 0.091 | -0.962 | -1.006 | -3.808 | 0.829 | |
| HTN | 0.876 | 0.864 | 0.082 | -0.844 | -5.728 | |
| T2DM | -0.370 | -0.283 | -0.087 | -0.376 | -3.427 | |
| HLP | 0.980 | 1.030 | -0.817 | -1.203 | -4.127 | |
| NAFLD | -0.517 | -0.410 | -1.568 | 0.065 | -0.958 | |
| OSA | -0.371 | 0.939 | 1.033 | 2.752 | 3.023 | |
| HUA | 0.506 | 0.028 | -0.039 | 2.115 | 2.590 | |
| %TWL | 2FAO | -0.272 | -0.671 | -1.385 | -3.164 | -9.486b |
| Sex | 1.046 | 0.461 | 1.013 | 2.693 | 2.206 | |
| BMI | -0.039 | 0.054 | 0.156 | 0.285 | 0.618a | |
| MS | -0.891 | -0.860 | -1.852 | -1.634 | -4.611 | |
| HTN | 0.306 | 0.810 | -0.088 | -2.749 | -3.853 | |
| T2DM | -0.338 | -1.478 | -1.548 | 0.198 | -1.050 | |
| HLP | 1.318 | 1.867 | 0.690 | -2.605 | -0.980 | |
| NAFLD | -0.113 | -0.303 | 0.482 | 0.338 | 2.968 | |
| OSA | -0.483 | 0.747 | 0.288 | 2.558 | 0.332 | |
| HUA | -0.367 | -1.384 | -1.298 | 0.120 | -0.298 | |
| 1 month | 3 months | 6 months | 12 months | 24 months | ||
| %EWL | 1FAO | -0.698 | -1.238 | -2.329 | -4.439 | -14.637 |
| Sex | 3.531 | 4.486 | 2.864 | -4.092 | -1.340 | |
| BMI | -1.100 c | -1.751 c | -1.903 c | -1.397 b | -0.757 | |
| MS | -1.272 | -4.902 | -6.480 | -10.983 | 2.548 | |
| HTN | 4.388 | 5.435 | 4.388 | 1.123 | -13.696 | |
| T2DM | 2.464 | 3.318 | 4.445 | 0.240 | -11.171 | |
| HLP | 0.807 | 1.271 | -2.716 | -4.837 | -11.703 | |
| NAFLD | -5.720 | -8.292 | -11.392 a | 0.987 | 1.587 | |
| OSA | -2.937 | -0.133 | -0.533 | 5.991 | 6.384 | |
| HUA | 0.940 | -0.329 | -1.269 | 2.565 | 2.079 | |
| %EWL | 2FAO | 2.067 | 1.089 | -0.691 | -7.224 | -23.513 c |
| Sex | 6.912 a | 8.730 | 10.880 | 5.487 | 7.279 | |
| BMI | -1.145 c | -1.806 c | -2.054 c | -1.539 c | -0.666 | |
| MS | -7.068 | -8.342 | -12.334 | -6.298 | -5.596 | |
| HTN | 5.569 | 9.484 | 8.283 | -2.062 | -8.179 | |
| T2DM | 3.291 | 0.925 | 0.717 | -0.222 | -10.684 | |
| HLP | 2.784 | 4.219 | 1.786 | -7.448 | -5.069 | |
| NAFLD | -3.588 | -6.218 | -5.107 | 1.598 | 7.874 | |
| OSA | -5.186 | -3.097 | -5.148 | 2.368 | -2.031 | |
| HUA | -2.054 | -5.344 | -5.365 | -2.837 | -5.233 | |
Alleviation of obesity-related comorbidities: SG significantly alleviates a wide range of obesity-related comorbidities, including metabolic syndrome, hypertension, T2DM, hyperlipoidemia (HLP), non-alcoholic fatty liver disease (NAFLD), and hyperuricemia, in the majority of patients 6 months postsurgery. Nevertheless, the extent of remission varies between patients with SO or FAO. As shown in Table 7, the incidence of NAFLD was greater in the 1FAO group than in the 1SO group (P = 0.015). The 2FAO group exhibited a higher prevalence of T2DM (P = 0.031), HLP (P = 0.012), and NAFLD (P = 0.003) than the 2SO group.
| n | 1SO, n = 73 | 1FAO, n = 73 | χ2 | P value | n | 2SO, n = 53 | 2FAO, n = 53 | χ2 | P value | ||
| MS | Without | 133 | 65 (89) | 68 (93.2) | 0.760 | 0.383 | 100 | 52 (98.1) | 48 (90.6) | 0.205 | |
| With | 13 | 8 (11.0) | 5 (6.8) | 6 | 1 (1.9) | 5 (9.4) | |||||
| HTN | Without | 132 | 66 (90.4) | 66 (90.4) | > 0.999 | 99 | 50 (94.3) | 49 (92.5) | > 0.999 | ||
| With | 14 | 7 (9.6) | 7 (9.6) | 7 | 3 (5.7) | 4 (7.5) | |||||
| T2DM | Without | 131 | 66 (90.4) | 65 (89.0) | 0.074 | 0.785 | 97 | 52 (98.1) | 45 (84.9) | 0.031 | |
| With | 15 | 7 (9.6) | 8 (11.0) | 9 | 1 (1.9) | 8 (15.1) | |||||
| HLP | Without | 118 | 60 (82.2) | 58 (79.5) | 0.177 | 0.674 | 91 | 50 (94.3) | 41 (77.4) | 6.290 | 0.012 |
| With | 28 | 13 (17.8) | 15 (20.5) | 15 | 3 (5.7) | 12 (22.6) | |||||
| NAFLD | Without | 96 | 55 (75.3) | 41 (56.2) | 5.962 | 0.015 | 65 | 40 (75.5) | 25 (47.2) | 8.949 | 0.003 |
| With | 50 | 18 (24.7) | 32 (43.8) | 41 | 13 (24.5) | 28 (52.8) | |||||
| HUA | Without | 98 | 46 (63.0) | 52 (71.2) | > 0.999 | 78 | 41 (77.4) | 37 (69.8) | 1.603 | 0.205 | |
| With | 48 | 27 (37.0) | 21 (28.8) | 28 | 12 (22.6) | 16 (30.2) | |||||
Surgery-related complications: We compared major surgery-related comorbidities (acid reflux, nausea/vomiting, alopecia, and constipation) postsurgery among the different groups of patients. There was no significant difference in surgery-related complications between patients in the 1FAO group and the 1SO group (P > 0.05). However, the prevalence of acid reflux symptoms was lower in the 2FAO group than in the 2SO group (2SO:2FAO = 24.5%:9.4%, P = 0.038). There was no significant difference in nausea/vomiting, alopecia, or constipation between the two groups.
Obesity and its severity are influenced primarily by genetic, environmental, lifestyle, and sociocultural factors[5]. Families, as fundamental units in the context of obesity, often share common genetic traits, lifestyle behaviors, and sociocultural perceptions. While many studies have focused on the family history of obesity in adolescents and children[4], there is a lack of research investigating the impact of FAO on SG.
Our study examined the impact of family history on patients with obesity and introduced the novel concept of FAO. After using PSM analysis to eliminate the possible influence of sex, preoperative BMI, and major obesity-related comorbidities on surgical outcomes, we found a significant difference in the weight loss outcomes of SG between patients with FAO, defined as two or more first-degree relatives with obesity, and those with SO. Specifically, patients with FAO experienced worse weight loss outcomes as well as lower remission rates of T2DM and NAFLD after SG. These findings suggest a potential association between FAO and weight regain after SG.
In terms of genetics, families of patients with FAO may share common obesity susceptibility genes. These genes included single-gene obesity genes, such as those encoding leptin (Lep) and its receptor (Lepr), the melanocortin-4 receptor, and proopiomelanocortin, and polygenic obesity genes (FTO loci), among others[5]. These genes influence weight by regulating the energy balance in the central nervous system, ultimately affecting body weight[15,16]. However, it is crucial to note that genetics alone cannot fully explain the differences in surgical outcomes between the two groups[17]. The disparities in surgical outcomes result from the combined influence of genetic and environmental factors.
In terms of environmental exposures, diet and lifestyle, patients who undergo SG and their family members share common obesity-inducing factors, such as similar dietary and exercise habits. All of these conditions exhibit many similarities, as both patients and their family members suffer from obesity and related comorbidities, which are often accompanied by a sedentary lifestyle[12]. In terms of cognition, similar cognitive levels within the family[18] determine the development of obesity and weight loss outcome of bariatric surgery. The combination of these factors results in weaker dietary and exercise maintenance abilities among patients with FAO[19] than in those with SO, possibly contributing to their mid-to-long-term postoperative weight regain.
SG significantly improves various metabolic processes[20], including glucose metabolism, lipid metabolism, and amino acid metabolism, in patients with obesity. Patients with FAO exhibit lower remission rates for T2DM, hyperlipidemia and NAFLD. This difference may be related to the extent of improvement in glucose and lipid metabolism. By aggregating information about patients with FAO, we aimed to investigate and identify factors influencing the postoperative remission of glucose and lipid metabolism. This research may lead to the use of novel therapeutic approaches for individuals with primary or secondary metabolic disorders.
The incidence of de novo gastroesophageal reflux disease (GORD) after SG is approximately 24.8%[21]. We observed a significantly lower incidence of postoperative acid reflux in patients with FAO than in those with SO. This difference may be associated with reduced intra-abdominal pressure[22]. The International Federation for the Surgery of Obesity and Metabolic Disorders recommends performing an endoscopy at 1 year after surgery, followed by subsequent screenings every 2 to 3 years based on the results of the initial examination[23]. Our findings may further contribute to the precise prevention and treatment of postoperative de novo GORD.
Impaired family functioning may be one of the factors influencing surgical outcomes[24]. A bidirectional relationship exists between family members and patients. Family members can play a supportive role in assisting patients in achieving and sustaining weight loss[12]. The 'halo effect'[25] of patients extends to their family members, resulting in positive changes. This includes improvements in family members' dietary and lifestyle habits[26,27] and an enhancement in their quality of life[28]. Interventions targeting obesity, by incorporating a family systems framework, can also extend the benefits of surgery to the family members of individuals with obesity[29].
The concept of familial aggregation of diseases helps in identifying groups of individuals with shared disease characteristics. For instance, individuals with a family history of type 2 diabetes are more likely to experience overweight/obesity and are susceptible to adverse metabolic consequences of fat accumulation[30]. Patients with a family history of Alzheimer's disease may experience limitations in cognitive function improvement after SG[31]. Moreover, these findings could aid in identifying susceptibility genes for related diseases and gaining deeper insights into potential pathophysiological mechanisms[32], ultimately leading to the discovery of new preventive or therapeutic strategies for obesity[5]. Currently, large-scale genome-wide association studies have identified more than 1100 obesity-associated genetic loci[33]. This study offers a novel perspective. By studying families as units of investigation rather than isolated individuals, it is possible to further discover susceptibility genes for obesity, predict the development of obesity, and enhance strategies for diagnosing and treating obesity[34].
Limitations: (1) Based on our observational study, differences in patients with FAO gradually emerge only in the mid-to-long-term postsurgery. We are actively investigating longer-term surgical outcomes as part of our ongoing research; (2) We excluded a few patients for whom it was difficult to trace first-degree relative information (e.g., adopted, stepparents, or deceased first-degree relatives). These patients exhibited weight loss results equal to or below the average, possibly due to impaired family functioning[24], posing challenges for detailed analysis. We intend to increase the sample size to further explore potential underlying factors; and (3) This study was conducted at a single center, acknowledging variations in familial lifestyles across countries and regions. Therefore, initiating a multicenter study involving multiple regions could provide more patients with precise treatment options.
SG can significantly reduce body weight and alleviate obesity-related comorbidities in the majority of patients. Familial aggregation in individuals with obesity impacts the mid-to-long-term weight loss outcomes of SG; affects the alleviation of T2DM, hyperlipidemia and NAFLD; and leads to a decreased incidence of acid reflux postoperatively. By studying the familial association of obesity, we can gain further insights into the pathogenesis of obesity. Moreover, offering stratified diagnostic and treatment plans for patients with obesity, along with more personalized and targeted health education, can enhance the precision of postoperative prevention and treatment.
Sleeve gastrectomy (SG) significantly reduces weight and improves obesity-related comorbidities in patients with obesity. However, differences in surgical outcomes between patients with familial aggregation of obesity (FAO) and those with sporadic obesity (SO) have not been elucidated.
To investigate whether FAO influences the surgical outcomes of SG.
To compare preoperative characteristics, postoperative weight loss, resolution of obesity-related comorbidities, and surgical complications between the FAO and SO groups.
In this retrospective study, we recruited 193 patients who underwent SG and categorized them into FAO and SO groups based on the presence of obesity in their first-degree relatives. Propensity score matching analysis was used to match the patients at a 1:1 ratio to eliminate confounding factors.
The baseline data and incidence of obesity-related comorbidities did not significantly differ between FAO patients and SO patients. Two years postsurgery, the FAO group exhibited a lower total weight loss percentage (P < 0.001) and excess weight loss percentage (P < 0.001) than did the SO group. Significant differences were observed between the two groups in terms of remission rates of type 2 diabetes mellitus (T2DM) (P = 0.031), hyperlipidemia (P = 0.012), nonalcoholic fatty liver disease (P = 0.003), and postoperative reflux occurrence rate (P = 0.038).
Compared to those in the SO group, the FAO patients in the SO group demonstrated slightly weaker medium-term weight loss outcomes; reduced symptoms of T2DM, hyperlipidemia, and nonalcoholic fatty liver disease; and a decreased postoperative reflux rate.
This study provides a theoretical basis for the treatment, surgical method selection, and postoperative health management of patients with FAO.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tolmanis I, Latvia S-Editor: Li L L-Editor: A P-Editor: Yu HG
| 1. | Wu Y, Xue H, Wang H, Su C, Du S, Wang Y. The impact of urbanization on the community food environment in China. Asia Pac J Clin Nutr. 2017;26:504-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 2. | Wang Y, Zhao L, Gao L, Pan A, Xue H. Health policy and public health implications of obesity in China. Lancet Diabetes Endocrinol. 2021;9:446-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 289] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 3. | Wang Y, Wang L, Qu W. New national data show alarming increase in obesity and noncommunicable chronic diseases in China. Eur J Clin Nutr. 2017;71:149-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Park SH, Park H. Relationships of family history of disease and child weight status to child routines: Multi-mediating effect of parental feeding practices and perception of child's weight. Nurs Health Sci. 2019;21:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | González-Muniesa P, Mártinez-González MA, Hu FB, Després JP, Matsuzawa Y, Loos RJF, Moreno LA, Bray GA, Martinez JA. Obesity. Nat Rev Dis Primers. 2017;3:17034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 805] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 6. | Tian P, Fu J, Li M, Liu Y, Bian S, Zhang M, Liu J, Jin L, Zhang Z, Zhang P. Metabolic and bariatric surgery in China: A summary of the Greater China Metabolic and Bariatric Surgery Database and comparison with other international registry databases. Diabetes Obes Metab. 2023;25:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 7. | Feng W, Zhu Z, Li X, Zhou Z, Qu S, Sun X, Zhu D. Weight loss and metabolic benefits of bariatric surgery in China: A multicenter study. J Diabetes. 2023;15:787-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Chou IJ, Kuo CF, Huang YS, Grainge MJ, Valdes AM, See LC, Yu KH, Luo SF, Huang LS, Tseng WY, Zhang W, Doherty M. Familial Aggregation and Heritability of Schizophrenia and Co-aggregation of Psychiatric Illnesses in Affected Families. Schizophr Bull. 2017;43:1070-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Gaare JJ, Skeie GO, Tzoulis C, Larsen JP, Tysnes OB. Familial aggregation of Parkinson's disease may affect progression of motor symptoms and dementia. Mov Disord. 2017;32:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Rossides M, Grunewald J, Eklund A, Kullberg S, Di Giuseppe D, Askling J, Arkema EV. Familial aggregation and heritability of sarcoidosis: a Swedish nested case-control study. Eur Respir J. 2018;52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Heikkinen SMM, Madanat-Harjuoja LM, Seppä KJM, Rantanen ME, Hirvonen EM, Malila NK, Pitkäniemi JM. Familial aggregation of early-onset cancers. Int J Cancer. 2020;146:1791-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Lent MR, Bailey-Davis L, Irving BA, Wood GC, Cook AM, Hirsch AG, Still CD, Benotti PN, Franceschelli-Hosterman J. Bariatric Surgery Patients and Their Families: Health, Physical Activity, and Social Support. Obes Surg. 2016;26:2981-2988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Chinese Society for Metabolic & Bariatric Surgery. Chinese Guidelines for the Surgical Management of Obesity and Type 2 Diabetes Mellitus (2019 edition). Zhongguo Shiyong Waike Zazhi. 2019;39:301-306. [DOI] [Full Text] |
| 14. | Chen JW, Maldonado DR, Kowalski BL, Miecznikowski KB, Kyin C, Gornbein JA, Domb BG. Best Practice Guidelines for Propensity Score Methods in Medical Research: Consideration on Theory, Implementation, and Reporting. A Review. Arthroscopy. 2022;38:632-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 15. | Perakakis N, Farr OM, Mantzoros CS. Leptin in Leanness and Obesity: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;77:745-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 16. | van der Klaauw AA, Farooqi IS. The hunger genes: pathways to obesity. Cell. 2015;161:119-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 269] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 17. | Loos RJ. The genetics of adiposity. Curr Opin Genet Dev. 2018;50:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 18. | Matheson BE, Colborn D, Bohon C. Bariatric Surgery in Children and Adolescents with Cognitive Impairment and/or Developmental Delay: Current Knowledge and Clinical Recommendations. Obes Surg. 2019;29:4114-4126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Fipps DC, Holder SM, Schmalz DL, Scott J. Family history of obesity and the influence on physical activity and dietary adherence after bariatric surgery. J Perioper Pract. 2022;32:230-233. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Kinlen D, Cody D, O'Shea D. Complications of obesity. QJM. 2018;111:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 21. | Hajibandeh S, Hajibandeh S, Ghassemi N, Evans D, Cheruvu CVN. Meta-analysis of Long-term De Novo Acid Reflux-Related Outcomes Following Sleeve Gastrectomy: Evidence Against the Need for Routine Postoperative Endoscopic Surveillance. Curr Obes Rep. 2023;12:395-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Sheppard CE, Sadowski DC, de Gara CJ, Karmali S, Birch DW. Rates of reflux before and after laparoscopic sleeve gastrectomy for severe obesity. Obes Surg. 2015;25:763-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Fisher OM, Chan DL, Talbot ML, Ramos A, Bashir A, Herrera MF, Himpens J, Shikora S, Higa KD, Kow L, Brown WA. Barrett's Oesophagus and Bariatric/Metabolic Surgery-IFSO 2020 Position Statement. Obes Surg. 2021;31:915-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Pratt KJ, Kiser H, Ferber MF, Whiting R, Needleman B, Noria S. Impaired Family Functioning Affects 6-Month and 12-Month Postoperative Weight Loss. Obes Surg. 2021;31:3598-3605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 25. | Rebibo L, Verhaeghe P, Cosse C, Dhahri A, Maréchal V, Regimbeau JM. Does longitudinal sleeve gastrectomy have a family "halo effect"? A case-matched study. Surg Endosc. 2013;27:1748-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Rex SM, Russel K, Reiter-Purtill J, Zeller MH, Courcoulas A, West-Smith L, Robson SM. A cross-sectional examination of the home food environments of mothers who have undergone metabolic and bariatric surgery: a pilot study. Surg Obes Relat Dis. 2020;16:2016-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Willmer M, Berglind D, Thorell A, Sundbom M, Uddén J, Raoof M, Hedberg J, Tynelius P, Ghaderi A, Näslund E, Rasmussen F. Changes in BMI and psychosocial functioning in partners of women who undergo gastric bypass surgery for obesity. Obes Surg. 2015;25:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Ibrahim C, Matta J, Lurbe I Puerto K, Sacre Y. Evaluation of Eating Habits and Quality of Life in Postbariatric Surgery Patients and Their Family Members: A Case-Control Study. J Nutr Metab. 2021;2021:6657567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 29. | Kitzman-Ulrich H, Wilson DK, St George SM, Lawman H, Segal M, Fairchild A. The integration of a family systems approach for understanding youth obesity, physical activity, and dietary programs. Clin Child Fam Psychol Rev. 2010;13:231-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 203] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 30. | Cederberg H, Stančáková A, Kuusisto J, Laakso M, Smith U. Family history of type 2 diabetes increases the risk of both obesity and its complications: is type 2 diabetes a disease of inappropriate lipid storage? J Intern Med. 2015;277:540-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Alosco ML, Spitznagel MB, Strain G, Devlin M, Crosby RD, Mitchell JE, Gunstad J. Family history of Alzheimer's disease limits improvement in cognitive function after bariatric surgery. SAGE Open Med. 2014;2:2050312114539477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Grinbaum R, Beglaibter N, Mitrani-Rosenbaum S, Kaplan LM, Ben-Zvi D. The Obesogenic and Glycemic Effect of Bariatric Surgery in a Family with a Melanocortin 4 Receptor Loss-of-Function Mutation. Metabolites. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 33. | Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, McMahon A, Morales J, Mountjoy E, Sollis E, Suveges D, Vrousgou O, Whetzel PL, Amode R, Guillen JA, Riat HS, Trevanion SJ, Hall P, Junkins H, Flicek P, Burdett T, Hindorff LA, Cunningham F, Parkinson H. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47:D1005-D1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3312] [Cited by in RCA: 2701] [Article Influence: 450.2] [Reference Citation Analysis (0)] |
| 34. | Loos RJF, Yeo GSH. The genetics of obesity: from discovery to biology. Nat Rev Genet. 2022;23:120-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 621] [Article Influence: 207.0] [Reference Citation Analysis (1)] |