Published online Mar 28, 2024. doi: 10.3748/wjg.v30.i12.1739
Peer-review started: January 19, 2024
First decision: February 5, 2024
Revised: February 19, 2024
Accepted: March 6, 2024
Article in press: March 6, 2024
Published online: March 28, 2024
Processing time: 69 Days and 4.9 Hours
The incidence of patients with early-onset pancreatic cancer (EOPC; age ≤ 50 years at diagnosis) is on the rise, placing a heavy burden on individuals, families, and society. The role of combination therapy including surgery, radiotherapy, and chemotherapy in non-metastatic EOPC is not well-defined.
To investigate the treatment patterns and survival outcomes in patients with non-metastatic EOPC.
A total of 277 patients with non-metastatic EOPC who were treated at our institution between 2017 and 2021 were investigated retrospectively. Overall survival (OS), disease-free survival, and progression-free survival were estimated using the Kaplan–Meier method. Univariate and multivariate analyses with the Cox proportional hazards model were used to identify prognostic factors.
With a median follow-up time of 34.6 months, the 1-year, 2-year, and 3-year OS rates for the entire cohort were 84.3%, 51.5%, and 27.6%, respectively. The median OS of patients with localized disease who received surgery alone and adjuvant therapy (AT) were 21.2 months and 28.8 months, respectively (P = 0.007). The median OS of patients with locally advanced disease who received radiotherapy-based combination therapy (RCT), surgery after neoadjuvant therapy (NAT), and chemotherapy were 28.5 months, 25.6 months, and 14.0 months, respectively (P = 0.002). The median OS after regional recurrence were 16.0 months, 13.4 months, and 8.9 months in the RCT, chemotherapy, and supportive therapy groups, respectively (P = 0.035). Multivariate analysis demonstrated that carbohydrate antigen 19-9 level, pathological grade, T-stage, N-stage, and resection were independent prognostic factors for non-metastatic EOPC.
AT improves postoperative survival in localized patients. Surgery after NAT and RCT are the preferred therapeutic options for patients with locally advanced EOPC.
Core Tip: Young adults are an important subgroup of the pancreatic cancer (PC) patient population. This article describes the comprehensive treatment patterns and survival outcomes for patients with non-metastatic early-onset PC (EOPC) from a high-volume center. We demonstrated that adjuvant therapy significantly improves postoperative survival in patients with limited EOPC. We also found that radiotherapy-based combination therapy achieved favorable outcomes in patients with locally advanced and postoperative recurrence. Our findings support an aggressive multimodal treatment strategy for these unique patients.
- Citation: Zhang LT, Zhang Y, Cao BY, Wu CC, Wang J. Treatment patterns and survival outcomes in patients with non-metastatic early-onset pancreatic cancer. World J Gastroenterol 2024; 30(12): 1739-1750
- URL: https://www.wjgnet.com/1007-9327/full/v30/i12/1739.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i12.1739
Pancreatic cancer (PC) is a clinically challenging disease with a 5-year survival rate of only 12.5%[1] because of its insensitivity to therapy and rapid progress. It is estimated that PC will become the second-leading cause of cancer-related deaths by 2030[2]. The incidence and mortality rate of PC tend to increase in young people in many countries[3-5]. Early-onset PC (EOPC) is generally defined as PC diagnosed before the age of 50 years and accounts for approximately 4%-18%. Although EOPC is less common than late-onset PC, it greatly increases the burden on individuals, families, and society of PC patients.
A study reported that EOPC is responsible for 20%-30% of the total number of years of life lost due to the disease[6]. Several studies have demonstrated that smoking, obesity, diabetes, and alcohol consumption are key modifiable risk factors for EOPC[7]. According to older studies, the clinicopathological features of young patients with PC are generally similar to those of older patients[8]. Genomic studies have shown that EOPC has a unique molecular genetic profile with a lower incidence of KRAS mutations and a higher incidence of pathogenic germline variants[9-11].
Population-based studies have shown that patients with EOPC often experience multimodal and more intense regimens[12,13]. Patients with non-metastatic EOPC are likely to benefit from local plus systemic therapy. However, very little data exist regarding the treatment outcomes of non-metastatic EOPC. Clinical guidelines do not provide treatment recommendations for young PC patients, and the optimal therapy remains unclear. This study investigated the clinical features, treatment patterns, and survival outcomes of patients with non-metastatic EOPC treated with multimodal therapy at a high-volume center in Beijing, China.
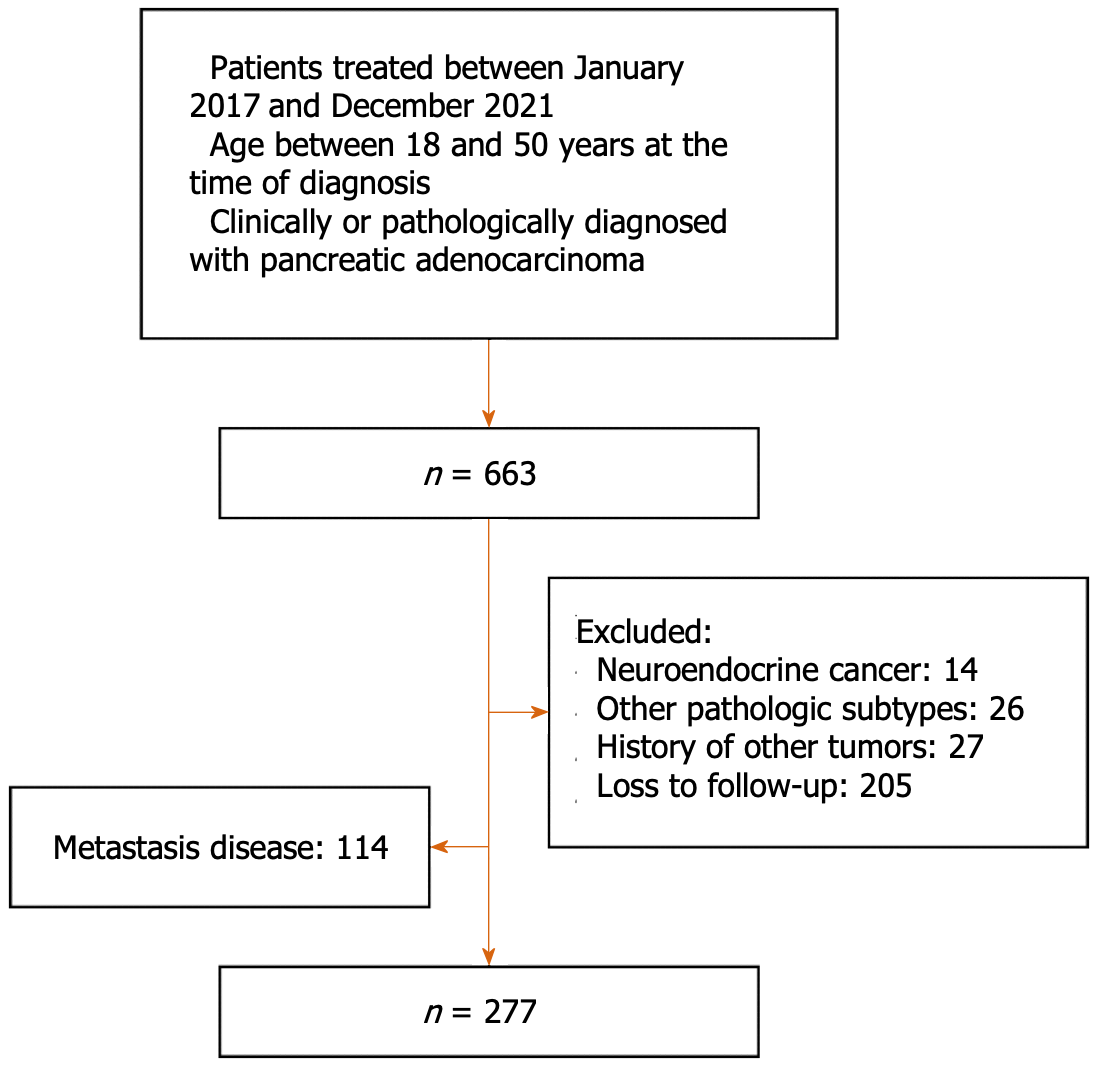
Between January 2017 and December 2021, 277 patients with non-metastatic EOPC who had been treated at the Chinese PLA General Hospital were retrospectively enrolled in our study. PC was diagnosed based on clinical, radiological, and pathological findings and was confirmed by multidisciplinary consultation. The inclusion criteria were as follows: (1) Initial consultation between January 2017 and December 2021; (2) ≤ 50 years and ≥ 18 years of age; (3) Clinical or pathological diagnosis of pancreatic adenocarcinoma; and (4) An Eastern Cooperative Oncology Group performance status score ≤ 2. The exclusion criteria were as follows: (1) Metastatic disease; (2) Pathological subtype of non-adenocarcinoma; (3) History of malignancies at other sites; and (4) Loss to follow-up. The detailed patient selection process is shown in Figure 1. The study protocol was approved by the Medical Ethics Committee of Chinese PLA General Hospital. Patient consent was waived, given the retrospective nature of the study.
Radical resection was the primary treatment for localized (resectable/borderline resectable) EOPC. Preoperative neoadjuvant therapy (NAT) generally involved 4-6 cycles of gemcitabine plus nab-paclitaxel (commonly referred to as GnP) or S-1 (an oral drug of fluorouracil) plus nab-paclitaxel (commonly referred to as SnP). Adjuvant therapy (AT) generally involved six cycles of a single or multiagent regimen based on S-1. For patients with locally advanced disease, treatment included surgery after NAT, radiotherapy-based combination therapy (RCT), and chemotherapy. Individualized radiotherapy target volumes were designed according to the tumor size, lymph node involvement, and adjacent organs at risk. Treatment doses of 50 Gy to the planning target volume and 60-70 Gy to the gross tumor target volume were prescribed with 30 fractions in intensity-modulated radiation therapy and 5 fractions in stereotactic body radiation therapy (SBRT). The first-line chemotherapy regimens mainly included GnP, SnP, and 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX). Immunotherapy mainly included immune checkpoint inhibitors. Targeted therapies included poly ADP-ribose polymerase inhibitors, epidermal growth factor receptor inhibitors, and vascular endothelial growth factor receptor inhibitors.
Patient demographic, clinical, pathological, and serological data were collected from the database and confirmed by chart review. The patients were restaged according to the National Comprehensive Cancer Network (commonly known as NCCN) Guidelines[14] and the American Joint Committee on Cancer 8th edition staging system. The primary endpoint was overall survival (OS). The secondary endpoints included tumor disease-free survival (DFS) and progression-free survival (PFS). OS was defined as the time from diagnosis to death or last follow-up. DFS or PFS was measured from the start of treatment to tumor recurrence or progression, last follow-up, or death. Recurrence and progression were assessed by experienced oncologists according to the Response Evaluation Criteria in Solid Tumors guidelines (version 1.1)[15]. The last follow-up was confirmed up to July 1, 2023.
Statistical analyses were conducted using R software (version 4.2.0). Clinical characteristics and treatment patterns were summarized using medians and ranges for continuous variables and frequencies for categorical descriptors. OS, DFS, and PFS were estimated using the Kaplan-Meier method and compared between subgroups using the log-rank test. Univariate and multivariate analyses were performed using the Cox proportional hazard model. Statistical tests were two-sided, and P < 0.05 was considered statistically significant.
A total of 277 patients with non-metastatic EOPC were enrolled in this study. The patient characteristics are presented in Table 1. The median age of all patients was 46 years (range: 20-50 years), and 68.6% were males. Tumors in the head of the pancreas accounted for 69.4%. The initial symptoms often presented with abdominal pain (49.1%), jaundice (30%), new-onset diabetes (4.3%), back pain (3.2%), and no symptoms (10.1%). History of tobacco, alcohol, obesity, diabetes, and chronic pancreatitis accounted for 36.8%, 27.9%, 8.9%, 5.9%, and 2.9%, respectively. Patients with baseline carbohydrate antigen 19-9 (CA19-9) ≥ 150 U/mL accounted for 26.1%. Among the 222 patients with pathological grading, poor differentiation adenocarcinoma accounted for 50.3%. Localized and locally advanced disease accounted for 77.6% and 22.4%, respectively. Overall, 78.7% of patients were treated with tumor resection, 74.7% with chemotherapy, 27.1% with radiotherapy, 31.0% with immunotherapy, and 19.9% with targeted therapy.
| Characteristics | n (%) |
| Age (yr) | |
| Median (range) | 46 (20-50) |
| < 45 | 115 (41.5) |
| ≥ 45 | 162 (58.5) |
| Sex | |
| Male | 190 (68.6) |
| Female | 87 (31.4) |
| Tumor site | |
| Body and tail | 85 (30.6) |
| Head | 193 (69.4) |
| Clinical manifestation | |
| Abdominal pain | 136 (49.1) |
| Jaundice | 83 (30.0) |
| New-onset diabetes | 12 (4.3) |
| Back pain | 9 (3.2) |
| No symptoms | 28 (10.1) |
| Others | 9 (3.2) |
| History of tobacco | 102 (36.8) |
| History of alcohol | 72 (26.0) |
| Obesity | 19 (6.9) |
| Pre-existing diabetes | 10 (3.6) |
| History of chronic pancreatitis | 6 (2.2) |
| Baseline CA19-9 (U/mL) | |
| ≥ 150 | 126 (51.2) |
| < 150 | 120 (48.8) |
| Unknown | 31 |
| Pathological grade | |
| Well | 12 (5.4) |
| Moderate | 110 (49.5) |
| Poor | 100 (45.0) |
| Unknown | 55 |
| T-stage | |
| 1 | 33 (11.9) |
| 2 | 124 (44.8) |
| 3 | 56 (20.2) |
| 4 | 62 (22.4) |
| X | 2 (0.7) |
| N-stage | |
| 0 | 172 (62.1) |
| 1 | 95 (34.3) |
| 2 | 10 (3.6) |
| Clinical stage | |
| Localized | 215 (77.6) |
| Locally advanced | 62 (22.4) |
| Resection | 218 (78.7) |
| Chemotherapy | 207 (74.7) |
| Radiotherapy | 75 (27.1) |
| Immunotherapy | 86 (31.0) |
| Targeted therapy | 55 (19.9) |
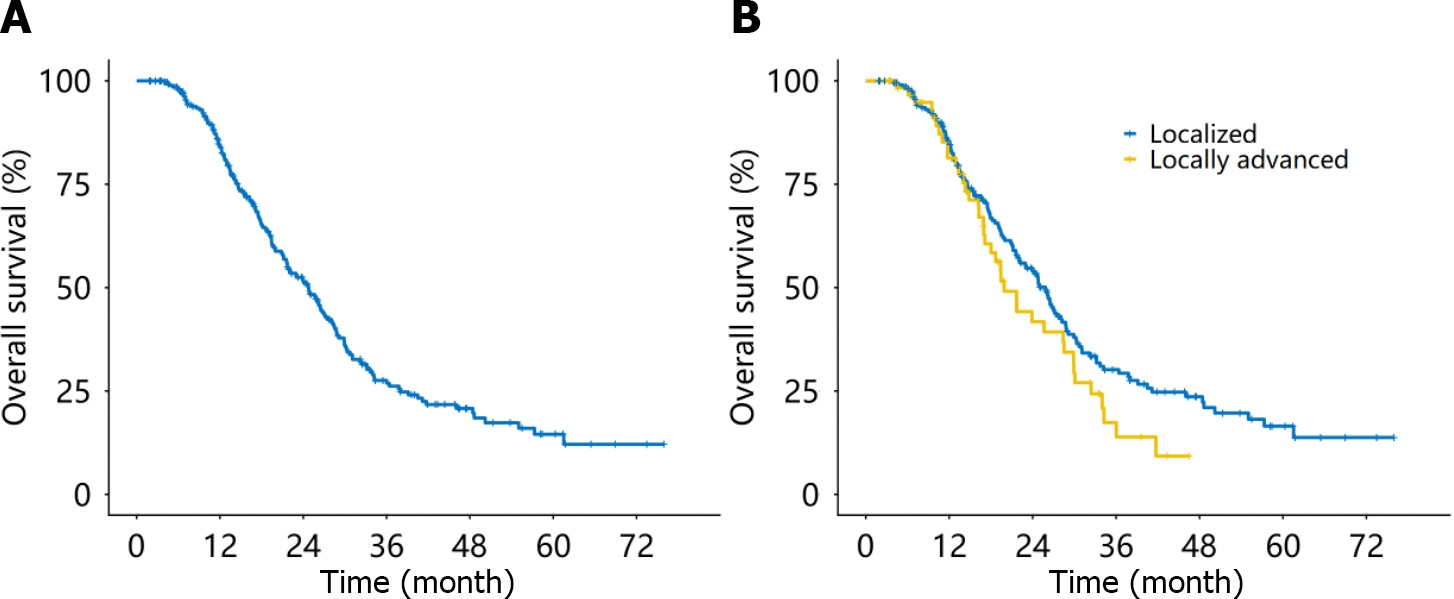
With a median follow-up time of 34.6 months, 167 patients died due to tumor progression. The estimated median OS (mOS) for patients with non-metastatic EOPC was 24.8 months 95%CI: 21.6-27.4 months (Figure 2A). The corresponding 1-year, 2-year, and 3-year OS rates were 84.3% (95%CI: 79.9%-88.9%), 51.5% (95%CI: 45.3%-58.5%), and 27.6% (95%CI: 21.8%-34.8%), respectively. The mOS was 25.8 months (95%CI, 22.1-28.7 months) for patients with localized disease and 19.9 months (95%CI: 17.1-29.9 months) for patients with locally advanced disease (Figure 2B).
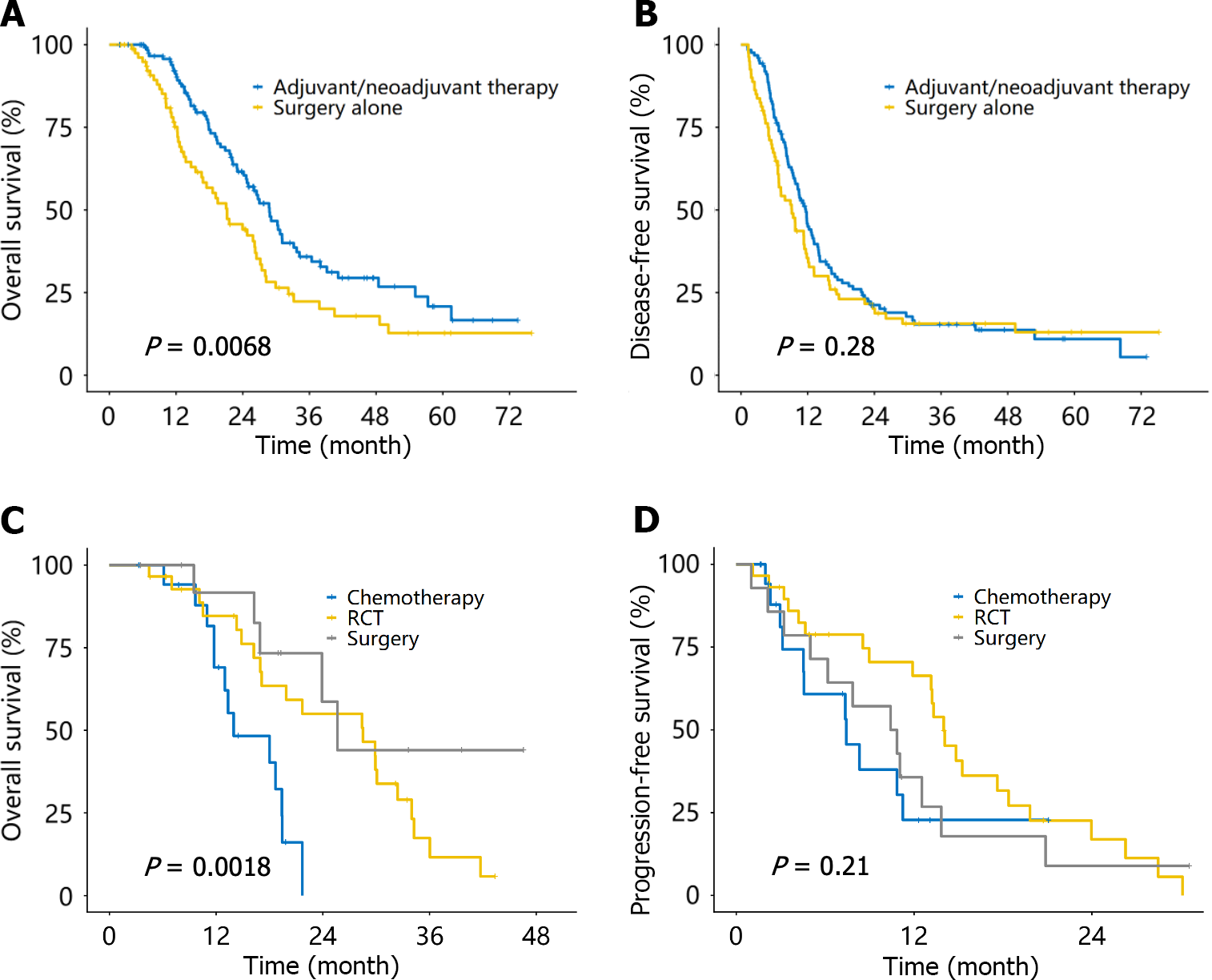
Among the 215 patients with localized disease, all except 11 underwent pancreatic tumor resection. Among them, 80 (39.2%), 10 (4.9%), and 120 (58.8%) patients received surgery alone, NAT, and AT, respectively (Table 2). The mOS for the NAT/AT group was 28.8 months (95%CI: 24.8-33.7 months), which was significantly longer than that for the surgery alone group (21.2 months, 95%CI: 16.6-26.5 months, P = 0.007; Figure 3A). The median DFS for the NAT/AT group was 11.7 months (95%CI: 9.8-13.2 months), which was similar to the surgery alone group (9.2 months, 95%CI: 6.8-11.7 months, P = 0.28; Figure 3B).
| Treatment | n (%) |
| Localized disease | 215 (77.6) |
| Resection | 204 (94.9) |
| Neoadjuvant and/or adjuvant therapy | 124 (60.8)1 |
| Surgery alone | 80 (39.2) |
| Nonsurgical therapy | 11 (5.1) |
| Locally advanced disease | 62 (22.4) |
| Surgery after neoadjuvant therapy | 14 (22.6) |
| Radiotherapy-based combination therapy | 29 (46.8)2 |
| Chemotherapy | 19 (30.6) |
Of the 62 patients with localized disease, 14 (22.6%), 29 (46.8%), and 19 (30.6%) underwent surgery after NAT, RCT, and chemotherapy, respectively (Table 2). The mOS of the surgery group, RCT group, and chemotherapy group was 25.6 months, 28.5 months, and 14.0 months (P = 0.002), respectively (Figure 3C). The median PFS for each of the three groups was 10.6 months, 14.0 months, and 7.4 months (P = 0.21), respectively (Figure 3D).
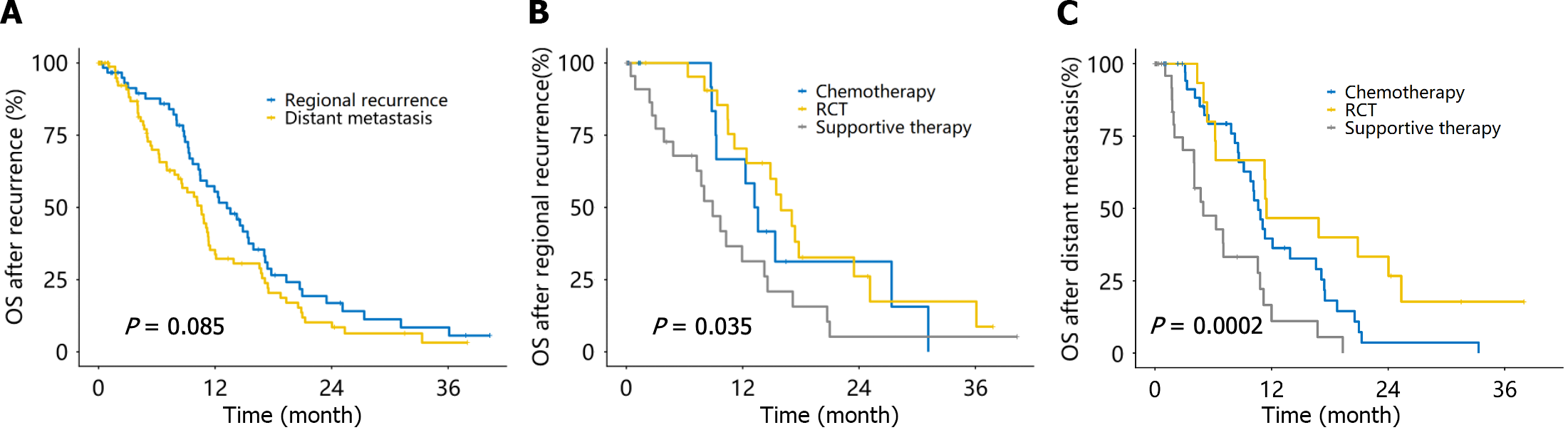
Definite recurrence occurred in 161 of the 218 patients who underwent resection, including isolated regional recurrence (operative area and lymph nodes; 39.7%, 64/161) and distant metastasis with or without regional recurrence (60.3%, 97/161). The mOS after recurrence was 13.2 months (95%CI: 10.4-17.1 months) for regional recurrence patients and 10.6 months (95%CI: 8.2-11.5 months) for distant metastases (Figure 4A). There were 19 patients each with regional recurrence treated with RCT and chemotherapy, 1 patient with repeat surgical resection, and the remaining patients with supportive treatment. The mOS after regional recurrence was 16.0 months, 13.4 months, and 8.9 months in the RCT, chemotherapy, and supportive therapy groups, respectively (P = 0.035; Figure 4B). The numbers of patients with distant metastases who received chemotherapy, RCT, surgical resection, and supportive therapy were 45, 10, 2, and 40, respectively. The mOS after distant metastasis was 11.5 months, 10.9 months, and 5.0 months in the RCT, chemotherapy, and supportive therapy groups, respectively (P < 0.001; Figure 4C).
According to the univariate analysis, baseline CA19-9 level, pathological grade, T-stage, N-stage, and resection were found to be associated with OS. On multivariate analysis, lower CA19-9 level, well and moderate pathological grade, lower T-stage, N0-stage, and resection were independent prognostic factors for OS (Table 3).
| Characteristics | Univariable analysis | Multivariable analysis | ||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| Sex | ||||
| Male | Reference | N/A | N/A | N/A |
| Female | 0.74 (0.53-1.05) | 0.089 | N/A | N/A |
| Age (yr) | ||||
| ≥ 45 | Reference | N/A | N/A | N/A |
| < 45 | 0.82 (0.60-1.12) | 0.217 | N/A | N/A |
| Site | ||||
| Body and tail | Reference | N/A | N/A | N/A |
| Head | 0.83 (0.60-1.15) | 0.271 | N/A | N/A |
| Baseline CA19-9 in U/mL | ||||
| > 150 | Reference | N/A | N/A | N/A |
| ≤ 150 | 0.62 (0.44-0.87) | 0.005a | 0.67 (0.48-0.95) | 0.025a |
| Unknown | 1.05 (0.66-1.66) | 0.841 | 1.17 (0.72-1.91) | 0.532 |
| Pathology grade | ||||
| Well and moderate | Reference | N/A | N/A | N/A |
| Poor | 1.62 (1.15-2.28) | 0.006a | 1.56 (1.08-2.26) | 0.017a |
| Unknown | 1.45 (0.96-2.19) | 0.076 | 0.94 (0.52-1.70) | 0.834 |
| T-stage | ||||
| 1 | Reference | N/A | N/A | N/A |
| 2 | 1.40 (0.82-2.39) | 0.220 | 1.38 (0.80-2.39) | 0.252 |
| 3 | 1.88 (1.06-3.36) | 0.031a | 2.17 (1.18-3.98) | 0.012a |
| 4 | 1.78 (0.99-3.20) | 0.053a | 1.35 (0.67-2.72) | 0.400 |
| X | 2.45 (0.56-10.72) | 0.234 | 2.35 (0.50-11.10) | 0.282 |
| N-stage | ||||
| 0 | Reference | N/A | N/A | N/A |
| 1-2 | 1.85 (1.36-2.51) | < 0.001a | 1.88 (1.36-2.60) | < 0.001a |
| Clinical stage | ||||
| Localized | Reference | N/A | N/A | N/A |
| Locally advanced | 1.34 (0.93-1.92) | 0.117 | N/A | N/A |
| Resection | ||||
| No | Reference | N/A | N/A | N/A |
| Yes | 0.62 (0.44-0.89) | 0.009a | 0.52 (0.29-0.93) | 0.027a |
| Chemotherapy | ||||
| No | Reference | N/A | N/A | N/A |
| Yes | 1.02 (0.74-1.41) | 0.916 | N/A | N/A |
| Radiotherapy | ||||
| No | Reference | N/A | N/A | N/A |
| Yes | 0.81 (0.57-1.14) | 0.223 | N/A | N/A |
| Immunotherapy | ||||
| No | Reference | N/A | N/A | N/A |
| Yes | 1.01 (0.73-1.40) | 0.959 | N/A | N/A |
| Targeted therapy | ||||
| No | Reference | N/A | N/A | N/A |
| Yes | 0.85 (0.59-1.23) | 0.385 | N/A | N/A |
The present study analyzed the treatment patterns, survival outcomes, and prognostic factors of 227 patients with non-metastatic EOPC using real-world data from a high-volume center in China. The mOS of all patients was 24.8 months, and the 1-year, 2-year, and 3-year OS rates were 84.3%, 51.5%, and 27.6%, respectively. The mOS for patients with localized and locally advanced disease was 25.8 months and 19.9 months, respectively. Compared with a retrospective population-based Dutch database study, younger patients had significantly longer survival than patients of all ages (mOS: 8 months)[16]. The 1-year OS in our cohort was better than that of the EOPC cohort from the National Cancer Database (stage I/II: 72.4%, stage III: 47.6%)[12]. These findings suggest that modern multimodal therapy can provide survival benefits.
Surgical resection is the only potential curative treatment for PC. AT can eradicate occult metastatic disease in patients with localized disease. NAT may lead to downstaging before surgery and facilitating a margin-negative resection. We found that 60.8% of patients with localized disease received NAT and/or AT based on fluorouracil or gemcitabine. The mOS was significantly better than that of patients who underwent surgery alone (28.8 months vs 21.2 months, P = 0.007), and the median DFS tended to improve (11.7 months vs 9.2 months, P = 0.28). The benefit of AT in patients with PC was demonstrated in the CONKO-001 trial[17]. Patients who received postoperative gemcitabine single-agent chemotherapy had significantly better OS and DFS than patients who received surgery-alone. The PRODIGE 24 trial further compared adjuvant chemotherapy with modified FOLFIRINOX to gemcitabine[18]. After a median follow-up of 30.5 months, the mOS was 54.4 months in the modified FOLFIRINOX arm and 35.0 months in the gemcitabine arm. The modified FOLFIRINOX had much greater toxicity than other regimens and might be ideal for younger patients with good performance status. In addition, the PREOPANC trial demonstrated that gemcitabine-based neoadjuvant chemoradiotherapy improved OS in resectable and borderline resectable PC compared with upfront surgery[19]. It suggests that early interventional radiotherapy is an effective treatment option in localized patients.
For locally advanced disease, the NCCN guidelines recommend radiotherapy as an optional localized treatment[14]. Our previous studies showed that definitive radiotherapy for inoperable non-metastatic PC patients had favorable and encouraging survival outcomes (mOS: 18 months)[20]. This strategy is also applicable to patients with EOPC. We found that nearly half of the patients with locally advanced disease received RCT. Compared to surgery and chemotherapy, RCT achieved the longest median PFS among the three groups, and the mOS was similar to that of pancreatectomy. A meta-analysis of SBRT for the treatment of locally advanced PC showed a 1-year survival rate of 51.6%, an mOS of 17 months (range: 5.7-47.0 months), and the incidence of serious adverse events of no more than 10%[21]. This finding suggests that SBRT can achieve satisfactory efficacy and safety for the treatment of inoperable PC. However, efficacy of SBRT in EOPC still needs to be further validated in clinical trials.
The increasing use of NAT and advances in surgical techniques have rendered some locally advanced patients eligible for surgical resection. In our study, approximately 20% of patients with locally advanced disease underwent pancreatectomy after NAT, with an mOS of 25.6 months. An international dual-center study showed that EOPC patients who underwent pancreatectomy with American Joint Committee on Cancer III-T4 tumors had an mOS of 29.5 months [22]. Even with locally advanced disease, patients can achieve satisfactory results at high-volume centers by NAT combined with surgery.
Several studies have shown that the use of a multidrug regimen of modified FOLFIRINOX, GnP, and SnP prolongs survival in patients with advanced PC[23-25]. In our study, locally advanced patients in the chemotherapy group were treated primarily with a multiagent regimen based on gemcitabine or fluorouracil, with an mOS of 14 months. Our result is similar to survival outcomes reported in previous studies.
Although AT and NAT significantly improve survival in patients with non-metastatic EOPC, regional or systemic recurrence occurred in two-thirds of patients, with mOS after recurrence of 13.2 months and 10.6 months, respectively. There is no consensus based on high-quality evidence on which intervention is most appropriate for patients with postoperative recurrence. A phase II trial evaluated the efficacy of radiotherapy plus chemotherapy or targeted immunotherapy in patients with locally recurrent PC with KRAS mutations and PD-L1 immunohistochemistry positivity, with a mOS of 14.9 months in the SBRT plus pembrolizumab and trametinib group and 12.8 months in the SBRT plus gemcitabine group[26]. Another ongoing randomized controlled trial is evaluating the efficacy of additional SBRT in patients with locally recurrent disease compared with the current standard of care alone (NCT04881487)[27]. In general, distant recurrent disease is treated the same as primary metastatic disease. The NCCN guidelines recommend that if distant recurrence occurs during the 1st 6 months of AT, an alternative chemotherapy regimen that is different from the original regimen is administered. Otherwise, repeating systemic therapy as previously administered or switching to any other systemic regimen is recommended[14]. These are consistent with our findings that multimodal combination therapy significantly prolonged survival in patients with postoperative recurrence compared to supportive care. For patients with isolated regional recurrence, localized treatments such as radiotherapy demonstrated a trend toward prolonged survival. In general, supportive treatment and active home care for patients can effectively improve quality of life and reduce the burden on patients and families[28].
Our series demonstrated that CA19-9 Level, pathological grade, T-stage, N-stage, and resection were independent prognostic factors in patients with non-metastatic EOPC. The serum CA19-9 level is the primary serologic marker for PC diagnosis and follow-up[29]. We found that EOPC patients with baseline serum CA19-9 < 150 U/mL had significantly longer survival (hazard ratio: 0.67, 95%CI: 0.48-0.95). Pathology grades of moderately and poorly differentiated tumors were found in 49.5% and 45.0% of patients, respectively, which is consistent with other findings that concluded that EOPC is more aggressive[30].
Several studies showed that EOPC also affects prognosis through molecular genetic features. A study from the Memorial Sloan Kettering Cancer Center found that EOPC patients had a higher proportion of KRAS wildtype (15.9% vs 5.4%)[11]. Both KRAS wildtype and pathogenic germline variants were associated with better clinical outcomes in PC patients. Our study did not find that targeted therapy and immunotherapy improved survival in non-metastatic EOPC. However, a retrospective analysis of the Know Your Tumor programme showed that 26% of PC had actionable mutations and that patients with matched targeted therapy had a significantly better prognosis than patients who receive nonspecific treatment[31]. Therefore, extensive genetic testing in patients with EOPC is beneficial in identifying patients with actionable mutations and for guiding targeted therapy.
However, the limitations of this study need to be recognized. First, the data were extracted from a single tertiary referral center. This limited the diversity of the patient groups included, which may have led to bias. Second, this was a retrospective study with no available family history or molecular genetic information. Additionally, due to the diversity of chemotherapy regimens and radiotherapy parameters, the prognostic impact of different treatment details remains to be clarified in further prospective studies.
In this series, the survival outcomes of patients with non-metastatic EOPC receiving multimodal therapy were satisfactory. AT significantly improved postoperative survival in patients with localized EOPC. RCT and surgery after NAT are the preferred therapeutic options for patients with locally advanced disease. Patients with postoperative recurrence undergoing multimodal therapy can achieve good outcomes; however, the role of radiotherapy needs to be further confirmed in randomized controlled trials. As an important subgroup of PC, our findings supported an aggressive multimodal therapeutic strategy for these unique patients and emphasized the need to make treatment recommendations for PC based on age.
The incidence of early-onset pancreatic cancer (EOPC) is showing an increasing trend worldwide. Pancreatic cancer (PC) is insensitive to monotherapy and has a poor prognosis.
There are few studies on EOPC. The role of combination therapies, including surgery, radiotherapy, and chemotherapy, in non-metastatic EOPC is unclear.
To explore the survival outcomes of combination therapy in patients with non-metastatic PC.
A total of 277 patients with non-metastatic EOPC who received antitumor therapy in a tertiary care hospital were retrospectively collected. Survival curves were plotted using the Kaplan-Meier method. Univariate and multivariate analyses using Cox proportional hazards modeling were performed to determine prognostic factors.
With a median follow-up time of 34.6 months, the 1-year, 2-year, and 3-year overall survival (OS) rates for the cohort were 84.3%, 51.5%, and 27.6%, respectively. The median OS of patients with localized disease who received surgery alone and adjuvant therapy (AT) was 21.2 months and 28.8 months, respectively (P = 0.007). The median OS of patients with locally advanced disease who received radiotherapy-based combination therapy (RCT), surgery after neoadjuvant therapy (NAT), and chemotherapy was 28.5 months, 25.6 months, and 14.0 months, respectively (P = 0.002). The median OS after regional recurrence was 16.0 months, 13.4 months, and 8.9 months in the RCT, chemotherapy, and supportive therapy groups, respectively (P = 0.035). Multivariate analysis demonstrated that carbohydrate antigen 19-9 Level, pathological grade, T-stage, N-stage, and resection were independent prognostic factors for non-metastatic EOPC.
AT improves postoperative survival in localized patients. NAT after surgery and RCT are the preferred treatment options for patients with locally advanced EOPC.
This study proposed that patients with EOPC should be treated with aggressive multimodal therapy. However, multicenter randomized controlled studies are needed to further understand this subject.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vardhana S, United States S-Editor: Lin C L-Editor: A P-Editor: Chen YX
| 1. | National Cancer Institute. Cancer Stat Facts: Pancreatic Cancer. 2023. [cited 29 November 2023]. Available from: https://seer.cancer.gov/statfacts/html/pancreas.html. |
| 2. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5140] [Article Influence: 467.3] [Reference Citation Analysis (0)] |
| 3. | Ugai T, Sasamoto N, Lee HY, Ando M, Song M, Tamimi RM, Kawachi I, Campbell PT, Giovannucci EL, Weiderpass E, Rebbeck TR, Ogino S. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat Rev Clin Oncol. 2022;19:656-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 283] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 4. | Gupta S, Harper A, Ruan Y, Barr R, Frazier AL, Ferlay J, Steliarova-Foucher E, Fidler-Benaoudia MM. International Trends in the Incidence of Cancer Among Adolescents and Young Adults. J Natl Cancer Inst. 2020;112:1105-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 5. | The Lancet Gastroenterology Hepatology. Cause for concern: the rising incidence of early-onset pancreatic cancer. Lancet Gastroenterol Hepatol. 2023;8:287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 6. | Raimondi S, Maisonneuve P, Löhr JM, Lowenfels AB. Early onset pancreatic cancer: evidence of a major role for smoking and genetic factors. Cancer Epidemiol Biomarkers Prev. 2007;16:1894-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 695] [Article Influence: 173.8] [Reference Citation Analysis (0)] |
| 8. | Lüttges J, Stigge C, Pacena M, Klöppel G. Rare ductal adenocarcinoma of the pancreas in patients younger than age 40 years. Cancer. 2004;100:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Ulanja MB, Moody AE, Beutler BD, Antwi-Amoabeng D, Rahman GA, Alese OB. Early-onset pancreatic cancer: a review of molecular mechanisms, management, and survival. Oncotarget. 2022;13:828-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Ben-Aharon I, Elkabets M, Pelossof R, Yu KH, Iacubuzio-Donahue CA, Leach SD, Lowery MA, Goodman KA, O'Reilly EM. Genomic Landscape of Pancreatic Adenocarcinoma in Younger vs Older Patients: Does Age Matter? Clin Cancer Res. 2019;25:2185-2193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Varghese AM, Singh I, Singh R, Kunte S, Chou JF, Capanu M, Wong W, Lowery MA, Stadler ZK, Salo-Mullen E, Saadat LV, Wei AC, Reyngold M, Basturk O, Benayed R, Mandelker D, Iacobuzio-Donahue CA, Kelsen DP, Park W, Yu KH, O'Reilly EM. Early-Onset Pancreas Cancer: Clinical Descriptors, Genomics, and Outcomes. J Natl Cancer Inst. 2021;113:1194-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Saadat LV, Chou JF, Gonen M, Soares KC, Kingham TP, Varghese AM, Jarnagin WR, D'Angelica MI, Drebin JA, O'Reilly EM, Wei AC. Treatment patterns and survival in patients with early-onset pancreatic cancer. Cancer. 2021;127:3566-3578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | LaPelusa M, Shen C, Arhin ND, Cardin D, Tan M, Idrees K, Geevarghese S, Chakravarthy B, Berlin J, Eng C. Trends in the Incidence and Treatment of Early-Onset Pancreatic Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | National Comprehensive Cancer Network. Pancreatic adenocarcinoma (version 2.2023). 2023. [cited 29 November 2023]. Available from: http://www.nccn.org/professionals/physician_gls/default.aspx. |
| 15. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21625] [Article Influence: 1351.6] [Reference Citation Analysis (1)] |
| 16. | de Jong EJM, van der Geest LG, Besselink MG, Bouwense SAW, Buijsen J, Dejong CHC, Koerkamp BG, Heij LR, de Hingh IHJT, Hoge C, Kazemier G, van Laarhoven HWM, de Meijer VE, Stommel MWJ, Tjan-Heijnen VCG, Valkenburg-van Iersel LBJ, Wilmink JW, Geurts SME, de Vos-Geelen J; Dutch Pancreatic Cancer Group. Treatment and overall survival of four types of non-metastatic periampullary cancer: nationwide population-based cohort study. HPB (Oxford). 2022;24:1433-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1361] [Article Influence: 113.4] [Reference Citation Analysis (0)] |
| 18. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1945] [Article Influence: 277.9] [Reference Citation Analysis (0)] |
| 19. | Versteijne E, van Dam JL, Suker M, Janssen QP, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, Buijsen J, Busch OR, Creemers GM, van Dam RM, Eskens FALM, Festen S, de Groot JWB, Groot Koerkamp B, de Hingh IH, Homs MYV, van Hooft JE, Kerver ED, Luelmo SAC, Neelis KJ, Nuyttens J, Paardekooper GMRM, Patijn GA, van der Sangen MJC, de Vos-Geelen J, Wilmink JW, Zwinderman AH, Punt CJ, van Tienhoven G, van Eijck CHJ; Dutch Pancreatic Cancer Group. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J Clin Oncol. 2022;40:1220-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 417] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 20. | Cao B, Zhang L, Wu C, Liu X, Wang Q, Tong F, Yang W, Wang J. Survival Outcomes and Failure Patterns in Patients with Inoperable Non-Metastatic Pancreatic Cancer Treated with Definitive Radiotherapy. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Petrelli F, Comito T, Ghidini A, Torri V, Scorsetti M, Barni S. Stereotactic Body Radiation Therapy for Locally Advanced Pancreatic Cancer: A Systematic Review and Pooled Analysis of 19 Trials. Int J Radiat Oncol Biol Phys. 2017;97:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 22. | Leonhardt CS, Kinny-Köster B, Hank T, Habib JR, Shoucair S, Klaiber U, Cameron JL, Hackert T, Wolfgang CL, Büchler MW, He J, Strobel O. Resected Early-Onset Pancreatic Cancer: Practices and Outcomes in an International Dual-Center Study. Ann Surg Oncol. 2023;30:2433-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX vs gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5640] [Article Influence: 402.9] [Reference Citation Analysis (1)] |
| 24. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4889] [Article Influence: 407.4] [Reference Citation Analysis (0)] |
| 25. | Shi Y, Zhang S, Han Q, Li J, Yan H, Lv Y, Shi H, Liu R, Dai G. Nab-paclitaxel plus S-1 in advanced pancreatic adenocarcinoma (NPSPAC): a single arm, single center, phase II trial. Oncotarget. 2017;8:92401-92410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Zhu X, Cao Y, Liu W, Ju X, Zhao X, Jiang L, Ye Y, Jin G, Zhang H. Stereotactic body radiotherapy plus pembrolizumab and trametinib vs stereotactic body radiotherapy plus gemcitabine for locally recurrent pancreatic cancer after surgical resection: an open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2022;23:e105-e115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 27. | van Goor IWJM, Daamen LA, Besselink MG, Bruynzeel AME, Busch OR, Cirkel GA, Groot Koerkamp B, Haj Mohammed N, Heerkens HD, van Laarhoven HWM, Meijer GJ, Nuyttens J, van Santvoort HC, van Tienhoven G, Verkooijen HM, Wilmink JW, Molenaar IQ, Intven MPW; Dutch Pancreatic Cancer Group. A nationwide randomized controlled trial on additional treatment for isolated local pancreatic cancer recurrence using stereotactic body radiation therapy (ARCADE). Trials. 2022;23:913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Li CY, Song YJ, Zhao L, Deng MH, Li RX, Zhang XL, Li QX, Shi Y, Luan HY, Sun YY, Hu Y, Sai XY. Insomnia Burden among Informal Caregivers of Hospitalized Lung Cancer Patients and Its Influencing Factors. Biomed Environ Sci. 2023;36:715-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Fahrmann JF, Schmidt CM, Mao X, Irajizad E, Loftus M, Zhang J, Patel N, Vykoukal J, Dennison JB, Long JP, Do KA, Chabot JA, Kluger MD, Kastrinos F, Brais L, Babic A, Jajoo K, Lee LS, Clancy TE, Ng K, Bullock A, Genkinger J, Yip-Schneider MT, Maitra A, Wolpin BM, Hanash S. Lead-Time Trajectory of CA19-9 as an Anchor Marker for Pancreatic Cancer Early Detection. Gastroenterology. 2021;160:1373-1383.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 30. | Beeghly-Fadiel A, Luu HN, Du L, Shi C, McGavic DP, Parikh AA, Raskin L. Early onset pancreatic malignancies: Clinical characteristics and survival associations. Int J Cancer. 2016;139:2169-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Pishvaian MJ, Blais EM, Brody JR, Lyons E, DeArbeloa P, Hendifar A, Mikhail S, Chung V, Sahai V, Sohal DPS, Bellakbira S, Thach D, Rahib L, Madhavan S, Matrisian LM, Petricoin EF 3rd. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020;21:508-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 366] [Article Influence: 73.2] [Reference Citation Analysis (0)] |