Published online Mar 28, 2024. doi: 10.3748/wjg.v30.i12.1714
Peer-review started: November 4, 2023
First decision: December 7, 2023
Revised: December 26, 2023
Accepted: March 11, 2024
Article in press: March 11, 2024
Published online: March 28, 2024
Processing time: 145 Days and 3.1 Hours
Previous studies have reported that low hematocrit levels indicate poor survival in patients with ovarian cancer and cervical cancer, the prognostic value of hematocrit for colorectal cancer (CRC) patients has not been determined. The prognostic value of red blood cell distribution width (RDW) for CRC patients was controversial.
To investigate the impact of RDW and hematocrit on the short-term outcomes and long-term prognosis of CRC patients who underwent radical surgery.
Patients who were diagnosed with CRC and underwent radical CRC resection between January 2011 and January 2020 at a single clinical center were included. The short-term outcomes, overall survival (OS) and disease-free survival (DFS) were compared among the different groups. Cox analysis was also conducted to identify independent risk factors for OS and DFS.
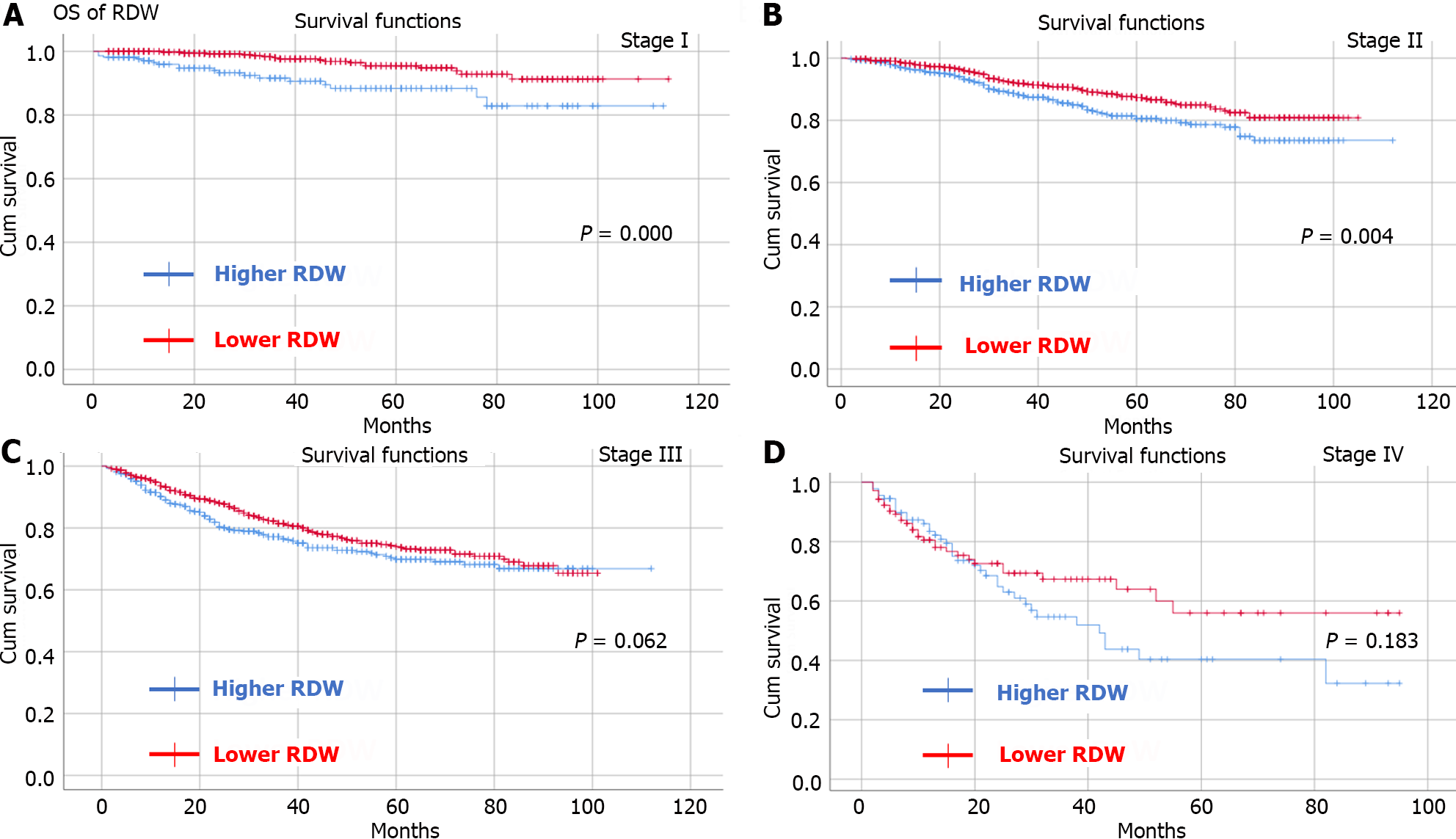
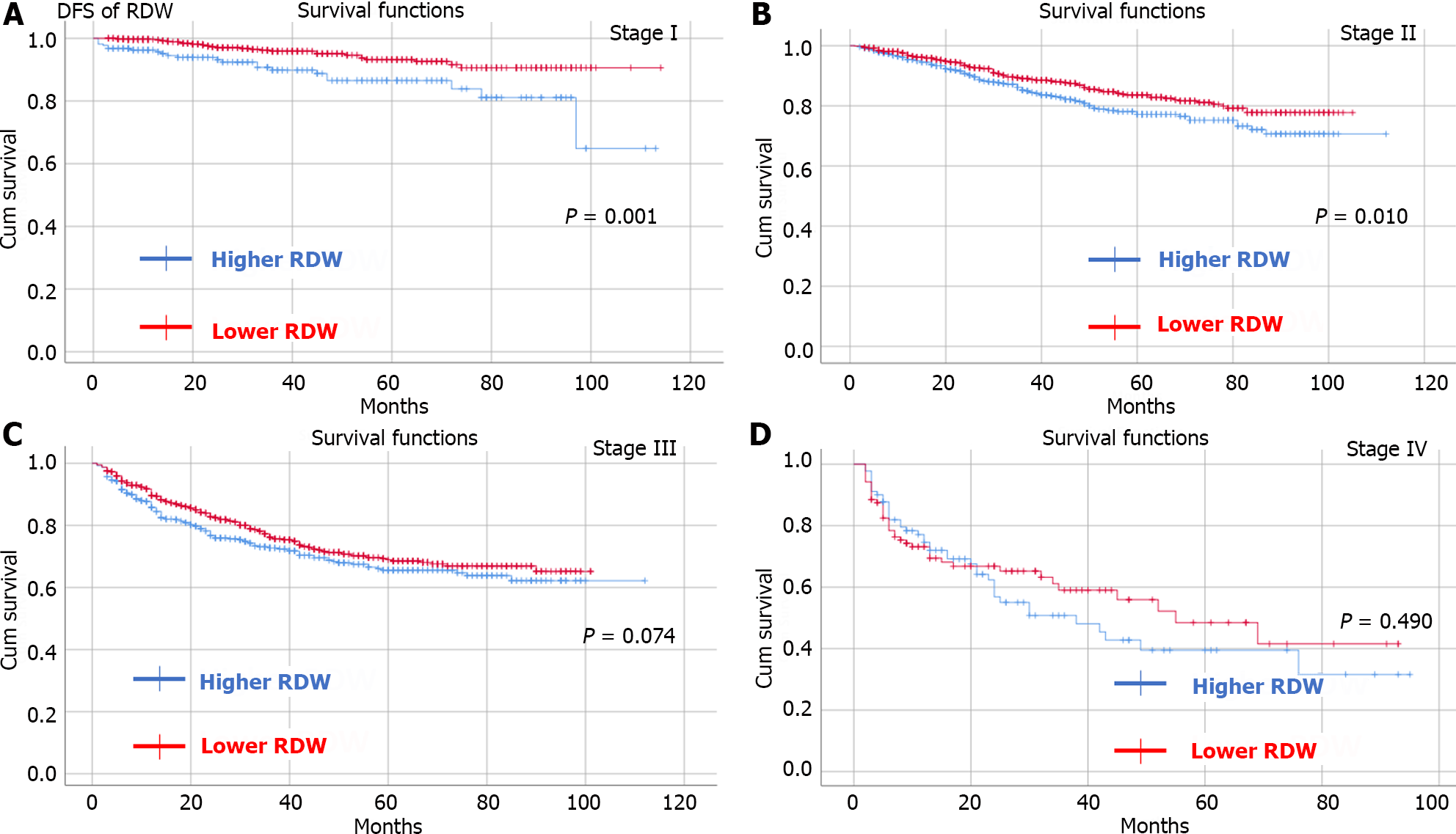
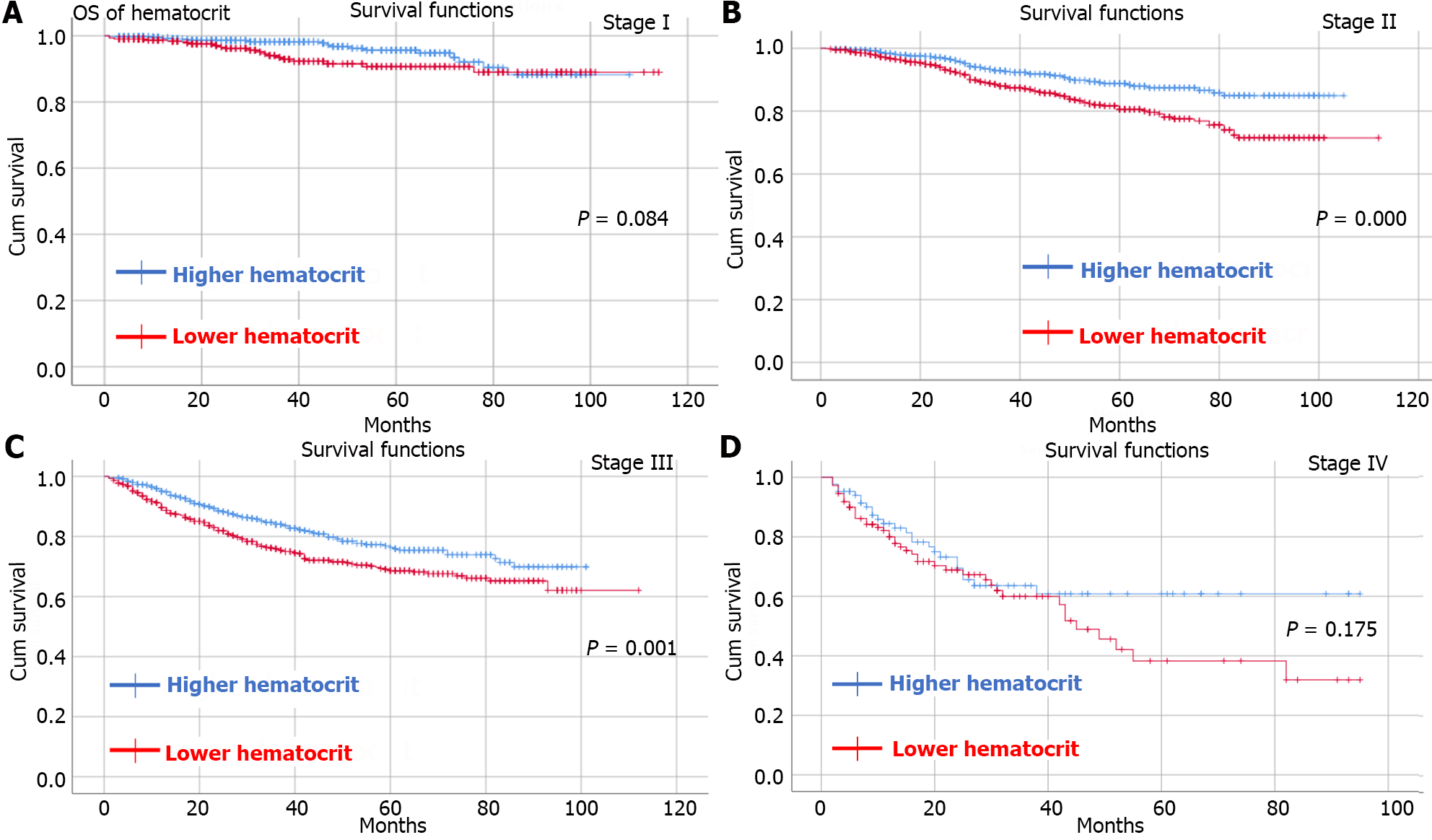
There were 4258 CRC patients who underwent radical surgery included in our study. A total of 1573 patients were in the lower RDW group and 2685 patients were in the higher RDW group. There were 2166 and 2092 patients in the higher hematocrit group and lower hematocrit group, respectively. Patients in the higher RDW group had more intraoperative blood loss (P < 0.01) and more overall complications (P < 0.01) than did those in the lower RDW group. Similarly, patients in the lower hematocrit group had more intraoperative blood loss (P = 0.012), longer hospital stay (P = 0.016) and overall complications (P < 0.01) than did those in the higher hematocrit group. The higher RDW group had a worse OS and DFS than did the lower RDW group for tumor node metastasis (TNM) stage I (OS, P < 0.05; DFS, P = 0.001) and stage II (OS, P = 0.004; DFS, P = 0.01) than the lower RDW group; the lower hematocrit group had worse OS and DFS for TNM stage II (OS, P < 0.05; DFS, P = 0.001) and stage III (OS, P = 0.001; DFS, P = 0.001) than did the higher hematocrit group. Preoperative hematocrit was an independent risk factor for OS [P = 0.017, hazard ratio (HR) = 1.256, 95% confidence interval (CI): 1.041-1.515] and DFS (P = 0.035, HR = 1.194, 95%CI: 1.013-1.408).
A higher preoperative RDW and lower hematocrit were associated with more postoperative complications. However, only hematocrit was an independent risk factor for OS and DFS in CRC patients who underwent radical surgery, while RDW was not.
Core Tip: This was the first study to show that low hematocrit could predict worse overall survival and disease-free survival in colorectal cancer (CRC) patients who underwent radical surgery. This study investigated the association between red blood cell distribution width (RDW) or hematocrit and short-term outcomes in CRC patients, which has rarely been reported previously. In conclusion, a preoperative higher RDW and lower hematocrit were associated with more postoperative complications.
- Citation: Peng D, Li ZW, Liu F, Liu XR, Wang CY. Predictive value of red blood cell distribution width and hematocrit for short-term outcomes and prognosis in colorectal cancer patients undergoing radical surgery. World J Gastroenterol 2024; 30(12): 1714-1726
- URL: https://www.wjgnet.com/1007-9327/full/v30/i12/1714.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i12.1714
According to global cancer statistics[1], there were approximately 1.93 million new cases and 0.94 million deaths from colorectal cancer (CRC) worldwide in 2020, and the incidence of this disease was estimated to increase in the next decade[2,3]; additionally, CRC will impose a heavy burden on the economy[4]. Radical surgery is the most important treatment for CRC patients[5-7]; however, many patients suffer from postoperative complications and reoccurrence after surgery. To improve the prognosis of CRC patients after surgery, various risk factors for postoperative complications and long-term prognosis have been were identified[8,9]. Many hematological indicators, such as hemoglobin[10,11], platelet counts[12,13], and the neutrophil-to-lymphocyte ratio[14,15], have also been identified because of the convenience of easy access and low cost.
The red blood cell distribution width (RDW) reflects the degree of variation in erythrocyte volume and is usually used to identify different types of anemia[16,17]. Several studies have demonstrated that RDW plays a diagnostic role in CRC patients[18-20]; moreover, many scholars have found that a high RDW is a negative predictor of overall survival (OS) and disease-free survival (DFS) independently[21-25]. The underlying mechanisms are mainly associated with tumor-related chronic inflammation and malnutrition, which accelerate tumor progression while affecting iron metabolism and suppressing the production of red blood cells, further leading to a high RDW. However, a previous study showed the opposite results: RDW was not an independent risk factor[26]. As a result, the prognostic value of RDW for CRC patients is controversial. In addition, few studies have focused on the impact of RDW on the short-term outcomes in CRC patients after radical surgery[27].
Since tumor-related chronic inflammation could lead to high RDWs, we suspected that hematocrit, another indicator of anemia, might also be related to the prognosis of CRC patients. Although previous studies have reported that low hematocrit levels indicate poor survival in patients with ovarian cancer[28] and cervical cancer[29], the prognostic value of hematocrit for CRC patients has not been determined. Therefore, this study was to explore the effect of RDW and hematocrit on the outcomes of CRC patients who underwent radical surgery.
Patients who were diagnosed with CRC and underwent radical CRC resection were included from January 2011 to January 2020 in our single clinical center. The study was approved by the ethics committee of the First Affiliated Hospital of Chongqing Medical University (2022-K205), and all patients signed informed consent forms. This study was conducted in accordance with the World Medical Association Declaration of Helsinki as well.
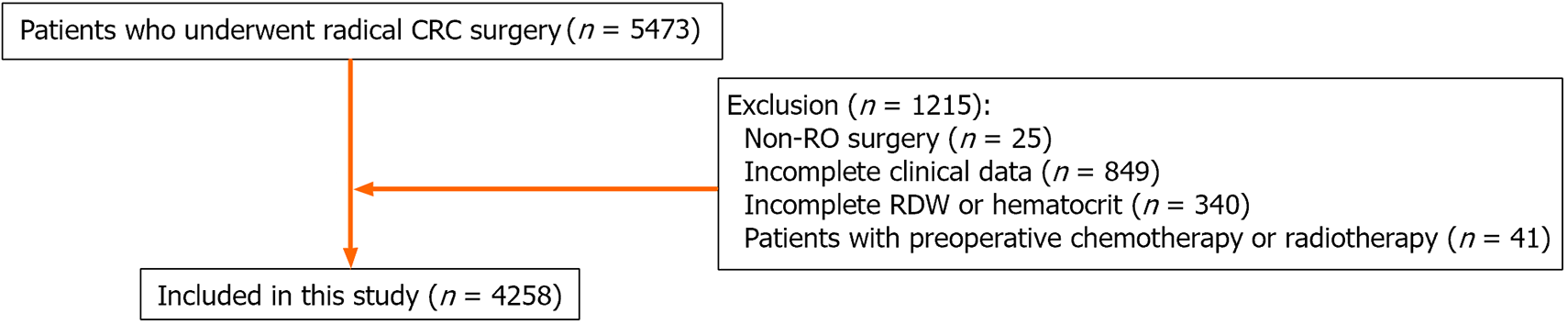
CRC patients who underwent radical CRC surgery were included (n = 5473). The exclusion criteria were as follows: (1) Non-R0 surgery (n = 25); (2) Incomplete clinical data (n = 849); (3) Incomplete RDW or hematocrit (n = 340); and (4) Patients with preoperative chemotherapy or radiotherapy (n = 41). Finally, a total of 4258 CRC patients were included in this study (Figure 1).
The baseline characteristics collected were as follows: Age, sex, body mass index (BMI), smoking, drinking, hypertension, type 2 diabetes mellitus (T2DM), coronary heart disease (CHD), surgical method, tumor location, tumor node metastasis (TNM) stage and tumor size. The short-term outcomes included operation time, intra-operative blood loss, postoperative hospital stay, overall complications and major complications. The long-term prognosis was estimated by OS and DFS. All the data were collected from electronic medical record system, outpatient visit and telephone interviews.
Based on the AJCC 8th Edition, we identified the TNM stage[30]. The postoperative complications were classified on the basis of the Clavien-Dindo[31] classification and major complications were ≥ grade III. OS was defined as the time from surgery to death or lost follow-up and DFS was calculated from the date of surgery to the date of recurrence or death.
All patients underwent radical surgery according to standard principles and R0 resection was confirmed by pathology. Patients were regularly followed up every six months in the first three years and every year in the next years.
The RDW and hematocrit were tested within a week before surgery. The value of RDW and hematocrit was expressed as a percentage. We used X-tile software to identify the optimal cut-off[32]. The optimal cut-off values for RDW and hematocrit were 14.4 and 37.7, respectively. Accordingly, patients were divided into the higher RDW group (RDW > 14.4) and the lower RDW group (RDW ≤ 14.4) as well as the higher hematocrit group (hematocrit > 37.7) and the lower hematocrit group (hematocrit ≤ 37.7).
An independent-sample t-test was used to compare the difference continuous variables that were expressed as the mean ± SD. χ2 tests or Fisher’s exact tests were used for categorical variables that were expressed as absolute values and percentages. Based on the Kaplan-Meier method, we estimated OS and DFS. In order to compare the OS and DFS between the different groups at different tumor stages, we used log-rank test. To determine independent risk factors for overall complications, logistic regression analysis was conducted. Analysis of Cox regression was conducted to identify independent risk factors for OS and DFS. Statistical significance was determined by a bilateral P value less than 0.05 using SPSS (version 22.0).
There were 4258 CRC patients who underwent radical surgery included in our study. Patients were divided into different groups according to the optimal cutoff values for RDW and hematocrit. There were 1573 patients in the lower RDW group and 2685 patients in the higher RDW group. The higher RDW group was older (P < 0.01), more likely to be female (P < 0.01), had a lower BMI (P < 0.01), a lower percentage of alcohol consumption (P < 0.01), a higher incidence of T2DM (P = 0.035) and CHD (P < 0.01), and a greater incidence of open surgery (P < 0.01), colon cancer (P < 0.01), TNM stage II (P < 0.01), TNM stage IV (P < 0.01), and tumor size ≥ 5 cm (P < 0.01) (Table 1).
| Characteristics | Higher RDW (n = 1573) | Lower RDW (n = 2685) | P value |
| RDW | 17.3 ± 3.3 | 13.0 ± 0.6 | < 0.01a |
| Age, yr | 64.1 ± 12.8 | 62.2 ± 11.7 | < 0.01a |
| Sex | < 0.01a | ||
| Male | 873 (55.5) | 1640 (61.1) | |
| Female | 700 (44.5) | 1045 (38.9) | |
| BMI, kg/m2 | 22.3 ± 3.3 | 22.9 ± 3.1 | < 0.01a |
| Smoking | 570 (36.2) | 1047 (39.0) | 0.074 |
| Drinking | 434 (27.6) | 881 (32.8) | < 0.01a |
| Hypertension | 434 (27.6) | 679 (25.3) | 0.099 |
| T2DM | 222 (14.1) | 319 (11.9) | 0.035a |
| CHD | 90 (5.7) | 91 (3.4) | < 0.01a |
| Open surgery | 281 (17.9) | 282 (10.5) | < 0.01a |
| Tumor location | < 0.01a | ||
| Colon | 940 (59.8) | 1068 (39.8) | |
| Rectum | 633 (40.2) | 1617 (60.2) | |
| TNM stage | < 0.01a | ||
| I | 218 (13.9) | 585 (21.8) | |
| II | 727 (46.2) | 1020 (38.0) | |
| III | 538 (34.2) | 976 (36.4) | |
| IV | 90 (5.7) | 104 (3.8) | |
| Tumor size | < 0.01a | ||
| < 5 cm | 739 (47.0) | 1716 (63.9) | |
| ≥ 5 cm | 834 (53.0) | 969 (36.1) | |
| Operation time (min) | 230.3 ± 81.9 | 225.8 ± 84.1 | 0.087 |
| Blood loss (mL) | 116.4 ± 171.8 | 93.3 ± 131.0 | < 0.01a |
| Hospital stay (d) | 11.5 ± 7.7 | 11.2 ± 9.2 | 0.283 |
| Overall complications | 400 (25.4) | 538 (20.0) | < 0.01a |
| Major complications | 42 (2.7) | 60 (2.2) | 0.370 |
After grouping patients according to hematocrit, there were respectively 2166 and 2092 patients in the higher hematocrit group and lower hematocrit group, respectively. The lower hematocrit group was older (P < 0.01); was more likely to be female (P < 0.01); had a lower BMI (P < 0.01); had a lower rate of smoking (P < 0.01) and drinking (P < 0.01), and had a higher incidence of T2DM (P < 0.01), CHD (P < 0.01), open surgery (P < 0.01), colon cancer (P < 0.01), or TNM stage II-IV (P < 0.01) (Table 2).
| Characteristics | Higher hematocrit (n = 2166) | Lower hematocrit (n = 2092) | P value |
| Hematocrit | 42.1 ± 3.0 | 32.2 ± 4.4 | < 0.01a |
| Age, yr | 61.2 ± 11.3 | 64.6 ± 12.7 | < 0.01a |
| Sex | < 0.01a | ||
| Male | 1580 (72.9) | 933 (44.6) | |
| Female | 586 (27.1) | 1159 (55.4) | |
| BMI, kg/m2 | 23.2 ± 3.1 | 22.2 ± 3.2 | < 0.01a |
| Smoking | 1023 (47.2) | 594 (28.4) | < 0.01a |
| Drinking | 839 (38.7) | 476 (22.8) | < 0.01a |
| Hypertension | 540 (24.9) | 573 (27.4) | 0.068 |
| T2DM | 220 (10.2) | 321 (15.3) | < 0.01a |
| CHD | 68 (3.1) | 113 (5.4) | < 0.01a |
| Open surgery | 222 (10.2) | 341 (16.3) | < 0.01a |
| Tumor location | < 0.01a | ||
| Colon | 782 (36.1) | 1226 (58.6) | |
| Rectum | 1384 (63.9) | 866 (41.4) | |
| TNM stage | < 0.01a | ||
| I | 485 (22.4) | 318 (15.2) | |
| II | 847 (39.1) | 900 (43.0) | |
| III | 750 (34.6) | 764 (36.5) | |
| IV | 84 (3.9) | 110 (5.3) | |
| Tumor size | < 0.01a | ||
| < 5 cm | 1415 (65.3) | 1040 (49.7) | |
| ≥ 5 cm | 751 (34.7) | 1052 (50.3) | |
| Operation time (min) | 228.1 ± 85.4 | 226.8 ± 81.1 | 0.593 |
| Blood loss (mL) | 96.3 ± 138.0 | 107.6 ± 157.1 | 0.012a |
| Hospital stay (d) | 11.0 ± 7.5 | 11.6 ± 9.8 | 0.016a |
| Overall complications | 419 (19.3) | 519 (24.8) | < 0.01a |
| Major complications | 53 (2.4) | 49 (2.3) | 0.823 |
Patients in the higher RDW group had greater intraoperative blood loss (P < 0.01) and more overall complications (P < 0.01) than did those in the lower RDW group. Similarly, patients in the lower hematocrit group had more intraoperative blood loss (P = 0.012), longer hospital stays (P = 0.016) and more overall complications (P < 0.01) than did those in the higher hematocrit group (Tables 1 and 2).
Multivariate logistic regression analysis of the overall complications showed that age [P < 0.01, odds ratio (OR) = 1.018, 95% confidence interval (CI): 1.011-1.025], T2DM (P = 0.018, OR = 1.297, 95%CI: 1.045-1.610), smoking (P = 0.004, OR = 1.255, 95%CI: 1.075-1.464), and open surgery (P < 0.01, OR = 2.056, 95%CI: 1.691-2.500) were independent risk factors. However, RDW (P > 0.05) and hematocrit (P > 0.05) were not identified as independent indicators of overall complications (Table 3).
| Risk factors | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age, yr | 1.024 (1.018-1.030) | < 0.01a | 1.018 (1.011-1.025) | < 0.01a |
| Sex (male/female) | 0.901 (0.777-1.045) | 0.166 | ||
| BMI, kg/m2 | 0.973 (0.951-0.995) | 0.018a | 0.983 (0.959-1.007) | 0.160 |
| Hypertension (yes/no) | 1.358 (1.158-1.592) | < 0.01a | 1.128 (0.944-1.347) | 0.186 |
| T2DM (yes/no) | 1.553 (1.270-1.900) | < 0.01a | 1.297 (1.045-1.610) | 0.018a |
| Tumor location (colon/rectum) | 0.966 (0.835-1.117) | 0.638 | ||
| Tumor stage (IV/III/II/I) | 1.053 (0.963-1.151) | 0.257 | ||
| Smoking (yes/no) | 1.173 (1.012-1.360) | 0.034a | 1.255 (1.075-1.464) | 0.004a |
| Drinking (yes/no) | 0.989 (0.845-1.157) | 0.893 | ||
| CHD (yes/no) | 1.807 (1.314-2.484) | < 0.01a | 1.365 (0.977-1.908) | 0.069 |
| Tumor size (≥ 5/< 5), cm | 1.214 (1.049-1.404) | 0.009a | 1.059 (0.910-1.234) | 0.459 |
| Surgical methods (open/laparoscopic) | 2.250 (1.860-2.721) | < 0.01a | 2.056 (1.691-2.500) | < 0.01a |
| RDW (lower/higher) | 0.735 (0.634-0.852) | < 0.01a | 0.887 (0.749-1.050) | 0.163 |
| Hematocrit (lower/higher) | 1.376 (1.189-1.591) | < 0.01a | 1.165 (0.981-1.383) | 0.082 |
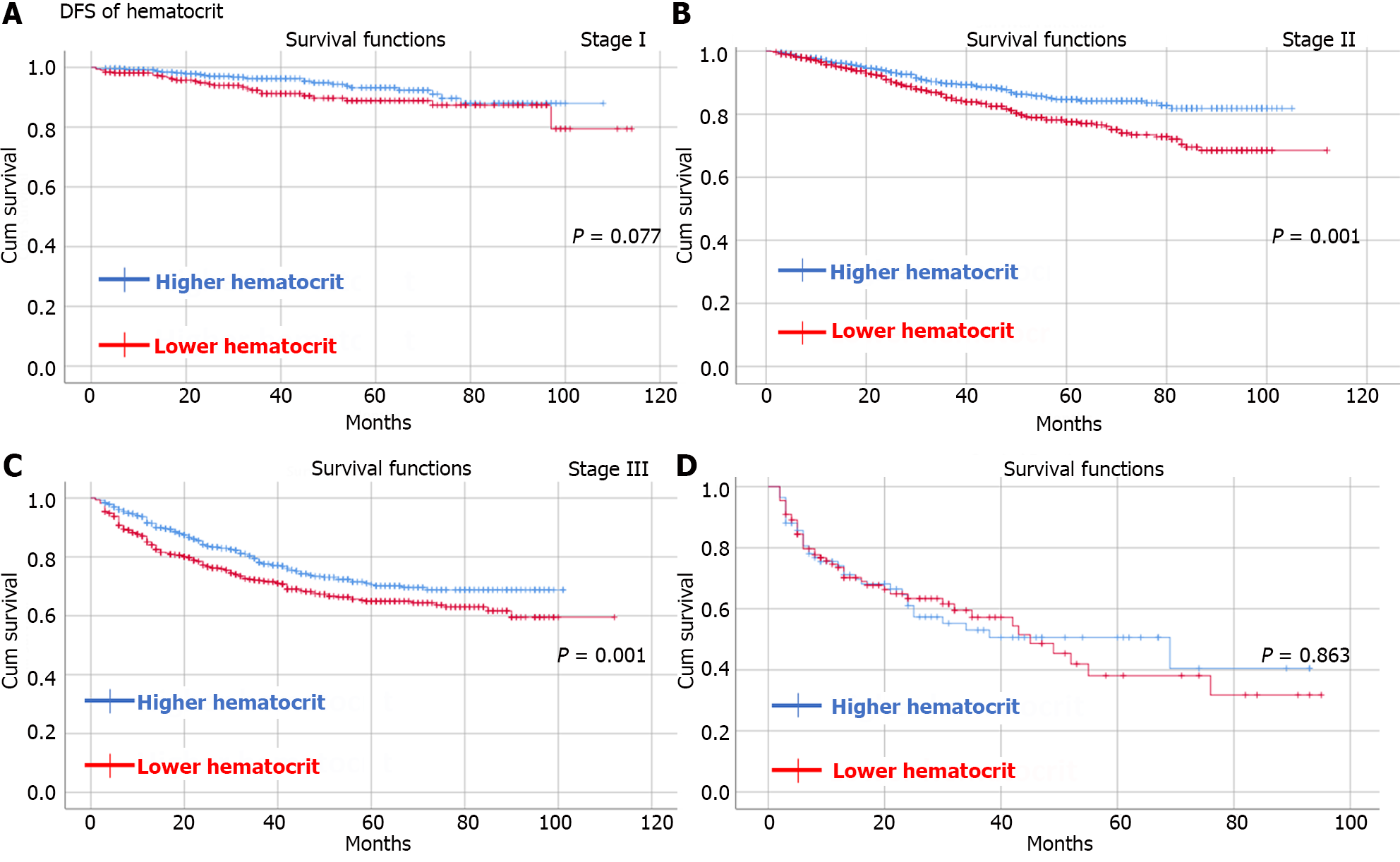
By observing OS and DFS, the median follow-up time was 35 (1-114) months. Comparing OS and DFS between the higher RDW group and the lower RDW group as well as between the higher hematocrit group and the lower hematocrit group at different TNM stages. The results showed that the higher RDW group had worse OS (Figure 2) and DFS (Figure 3) than did the lower RDW group for TNM stage I (OS, P < 0.05; DFS, P = 0.001) and stage II (OS, P = 0.004; DFS, P = 0.01); moreover, the lower RDW group; the lower hematocrit group had worse OS (Figure 4) and DFS (Figure 5) for TNM stage II (OS, P < 0.05; DFS, P = 0.001) and stage III (OS, P = 0.001; DFS, P = 0.001) than did the higher hematocrit group.
To determine independent risk factors for OS and DFS, Cox analysis was conducted. Independent risk factors for OS included age [P < 0.01, hazard ratio (HR) = 1.038, 95%CI: 1.030-1.046], tumor stage (P < 0.01, HR = 2.167, 95%CI: 1.943-2.416), tumor size (P = 0.014, HR = 1.235, 95%CI: 1.044-1.459), preoperative hematocrit (P = 0.017, HR = 1.256, 95%CI: 1.041-1.515) and overall complications (P < 0.01, HR = 1.608, 95%CI: 1.357-1.904) ; Independent risk factors for DFS included age (P < 0.01, HR = 1.027, 95%CI: 1.020-1.033), tumor stage (P < 0.01, HR = 2.093, 95%CI: 1.900-2.307), preoperative hematocrit (P = 0.035, HR = 1.194, 95%CI: 1.013-1.408) and overall complications (P < 0.01, HR = 1.510, 95%CI: 1.293-1.763). However, RDW was not an independent risk factor for OS (P = 0.396) or DFS (P = 0.308) (Tables 4 and 5).
| Risk factors | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (yr) | 1.044 (1.036-1.051) | < 0.01a | 1.038 (1.030-1.046) | < 0.01a |
| Sex (female/male) | 0.861 (0.730-1.015) | 0.074 | ||
| BMI (kg/m2) | 0.959 (0.935-0.984) | 0.001a | 0.997 (0.972-1.023) | 0.840 |
| T2DM (yes/no) | 1.303 (1.038-1.636) | 0.022a | 0.941 (0.745-1.189) | 0.610 |
| Tumor site (colon/ rectum) | 1.422 (1.091-1.853) | 0.009a | 0.984 (0.831-1.165) | 0.851 |
| Tumor stage (IV/III/II/I) | 2.202 (1.979-2.452) | < 0.01a | 2.167 (1.943-2.416) | < 0.01a |
| Smoking (yes/no) | 1.081 (0.918-1.273) | 0.351 | ||
| Drinking (yes/no) | 1.070 (0.901-1.271) | 0.438 | ||
| Hypertension (yes/no) | 1.055 (0.879-1.266) | 0.564 | ||
| CHD (yes/no) | 1.355 (0.939-1.957) | 0.105 | ||
| Tumor size (≥ 5 cm/< 5 cm) | 1.532 (1.305-1.799) | < 0.01a | 1.235 (1.044-1.459) | 0.014a |
| RDW (lower/higher) | 0.664 (0.565-0.780) | < 0.01a | 0.925 (0.774-1.107) | 0.396 |
| Hematocrit (lower/higher) | 1.693 (1.437-1.995) | < 0.01a | 1.256 (1.041-1.515) | 0.017a |
| Overall complications (yes/no) | 1.903 (1.611-2.248) | < 0.01a | 1.608 (1.357-1.904) | < 0.01a |
| Risk factors | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (yr) | 1.031 (1.025-1.038) | < 0.01a | 1.027 (1.020-1.033) | < 0.01a |
| Sex (female/male) | 0.861 (0.730-1.015) | 0.058 | ||
| BMI (kg/m2) | 0.975 (0.953-0.997) | 0.029a | 1.003 (0.981-1.026) | 0.784 |
| T2DM (yes/no) | 1.127 (0.911-1.396) | 0.271 | ||
| Tumor site (colon/ rectum) | 1.126 (0.975-1.300) | 0.107 | ||
| Tumor stage (IV/III/II/I) | 2.120 (1.926-2.334) | < 0.01a | 2.093 (1.900-2.307) | < 0.01a |
| Smoking (yes/no) | 1.093 (0.944-1.267) | 0.234 | ||
| Drinking (yes/no) | 1.104 (0.946-1.288) | 0.208 | ||
| Hypertension (yes/no) | 1.036 (0.879-1.220) | 0.676 | ||
| CHD (yes/no) | 1.281 (0.917-1.791) | 0.147 | ||
| Tumor size (≥ 5 cm/< 5 cm) | 1.363 (1.180-1.574) | < 0.01a | 1.115 (0.962-1.294) | 0.149 |
| RDW (lower/higher) | 0.713 (0.616-0.824) | < 0.01a | 0.920 (0.783-1.080) | 0.308 |
| Hematocrit (lower/higher) | 1.509 (1.304-1.747) | < 0.01a | 1.194 (1.013-1.408) | 0.035a |
| Overall complications (yes/no) | 1.705 (1.463-1.987) | < 0.01a | 1.510 (1.293-1.763) | < 0.01a |
In this retrospective study, 4258 CRC patients who underwent radical surgery were divided into different groups according to the optimal cutoff values for RDW and hematocrit. The prognostic value of the RDM and hematocrit for the short-term outcomes and prognosis (including OS and DFS) was investigated.
In terms of short-term outcomes, previous studies have reported that RDW and hematocrit could predict postoperative complications in brain surgery[33], cardiac surgery[34,35] and so on, but few studies have focused on CRC patients. In our study, we found that a higher RDW and lower hematocrit were associated with greater intraoperative blood loss and more postoperative complications. Nevertheless, neither RDW nor hematocrit was an independent risk factor for overall complications. However, further studies are needed to validate the roles of RDW and hematocrit in determining surgical complications in CRC patients.
Many studies have shown that RDW is a predictor of the long-term prognosis of CRC patients. Several researchers have conducted a propensity matching score of 5135 CRC patients and found that patients with higher RDWs had worse OS and DFS[21]. However, the study enrolled only patients with TNM stage I-II disease, and multivariate analysis was lacking. One study reported that RDW was a negative predictor of OS and DFS in patients with TNM stage I-III disease[22], and another study reported that found preoperative RDW could predict the OS[24]; however, the sample sizes of these studies were relatively small, and many confounding factors, such as T2DM and CHD, were missed, which might cause bias. Furthermore, a study of 591 patients revealed that a higher RDW was associated with worse OS in patients with TNM stage I disease; however, RDW was not an independent risk factor[25].
Most studies have attributed the prognostic value of RDW to tumor-related chronic inflammation[25,36,37]. Tumor development of tumor, reoccurrence, and metastasis have been shown to interact with the systemic inflammatory response[38], and the latter is associated with overproduction of cytokines such as interleukins and tumor necrosis factor, which might influence iron metabolism and suppress the production of red blood cells[39]. Thus, RDW might represent systemic inflammation and tumor burden. In our study, although the higher RDW group had significantly worse OS and DFS than did the lower RDW group for TNM stage I and stage II disease, RDW could not predict the OS or DFS independently, which was similar to the conclusion conclusions of previous studies[26].
In contrast, we found that low hematocrit could predict worse OS and DFS in CRC patients after radical surgery. In our study, patients with stage II-III disease were more likely to have worse OS and DFS. The predictive value of hematocrit has been reported for gynaecological tumours[28,29] and renal carcinoma[40], but the underlying mechanism has remained unclear. The hematocrit was used to measure the volume of red blood cells in whole blood and estimate the oxygen-carrying ability of blood, and a lower hematocrit often indicated anemia. It was also demonstrated that anemia was associated with worse survival in CRC patients after surgery[41,42] because anemia could cause hypoxia, which might promote tumor development[43]. Moreover, the relationship between anemia and tumor-related chronic inflammation, as mentioned above, might further explain the prognostic value of hematocrit.
Interestingly, although both RDW and hematocrit are hematocrit were erythrocyte-related parameters and reflect the status of anemia, we found that hematocrit was an independent risk factor for OS and DFS, while RDW was not. In addition to the bias caused by our research design, there might be several unknown mechanisms that need to be further investigated.
To our knowledge, this was the first study to show that low hematocrit could predict worse OS and DFS in CRC patients after radical surgery. A relatively large sample size of 4258 patients were included in this study. Moreover, we investigated the association between RDW or hematocrit and short-term outcomes in CRC patients, which has rarely been reported previously. This study has several limitations. First, the retrospective nature of this single-center study might cause inaccurate baseline information and bias. Second, chemotherapy information was lacking for TNM III-IV patients, which might affect the analysis of the survival. Therefore, there is a need for multicenter prospective studies to further examine the prognostic role of RDW and hematocrit.
In conclusion, a preoperative higher RDW and lower hematocrit were associated with more postoperative complications. However, only hematocrit was an independent risk factor for OS and DFS in CRC patients who underwent radical surgery, while RDW was not.
The prognostic value of red blood cell distribution width (RDW) for colorectal cancer (CRC) patients is controversial and the prognostic value of hematocrit for CRC patients has not been determined.
This was the first study to show that low hematocrit could predict worse overall survival (OS) and disease-free survival (DFS) in CRC patients after radical surgery.
The objective of this study was to explore the effect of RDW and hematocrit on the outcomes of CRC patients who underwent radical surgery.
Patients who were diagnosed with CRC and underwent radical CRC resection between January 2011 and January 2020 at a single clinical center were included. An independent-sample t-test was used to compare the difference continuous variables that were expressed as the mean ± SD. χ2 tests or Fisher’s exact tests were used for categorical variables that were expressed as absolute values and percentages. The short-term outcomes, OS and DFS were compared among the different groups. To determine independent risk factors for overall complications, logistic regression analysis was conducted. Analysis of Cox regression was conducted to identify independent risk factors for OS and DFS.
There were 4258 CRC patients who underwent radical surgery included in our study. The higher RDW group had a worse OS and DFS than did the lower RDW group for tumor node metastasis (TNM) stage I (OS, P < 0.05; DFS, P = 0.001) and stage II (OS, P = 0.004; DFS, P = 0.01) than the lower RDW group; the lower hematocrit group had worse OS and DFS for TNM stage II (OS, P < 0.05; DFS, P = 0.001) and stage III (OS, P = 0.001; DFS, P = 0.001) than did the higher hematocrit group. RDW (P > 0.05) and hematocrit (P > 0.05) were not identified as independent indicators of overall complications. Preoperative hematocrit was an independent risk factor for OS [P = 0.017, hazard ratio (HR) = 1.256, 95% confidence interval (CI): 1.041-1.515] and DFS (P = 0.035, HR = 1.194, 95%CI: 1.013-1.408). However, RDW was not an independent risk factor for OS (P = 0.396) or DFS (P = 0.308).
This was the first study to show that low hematocrit could predict worse OS and DFS in CRC patients after radical surgery. A preoperative higher RDW and lower hematocrit were associated with more postoperative complications. However, only hematocrit was an independent risk factor for OS and DFS in CRC patients who underwent radical surgery, while RDW was not.
Further multicenter prospective studies are needed to investigate the prognostic role of RDW and hematocrit.
We acknowledge all the authors whose publications are referred in our article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bordonaro M, United States S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64681] [Article Influence: 16170.3] [Reference Citation Analysis (177)] |
| 2. | Hossain MS, Karuniawati H, Jairoun AA, Urbi Z, Ooi J, John A, Lim YC, Kibria KMK, Mohiuddin AKM, Ming LC, Goh KW, Hadi MA. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 417] [Article Influence: 139.0] [Reference Citation Analysis (1)] |
| 3. | Cheng YX, Tao W, Liu XY, Yuan C, Zhang B, Wei ZQ, Peng D. Hypertension Remission after Colorectal Cancer Surgery: A Single-Center Retrospective Study. Nutr Cancer. 2022;74:2789-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Yang Y, Han Z, Li X, Huang A, Shi J, Gu J. Epidemiology and risk factors of colorectal cancer in China. Chin J Cancer Res. 2020;32:729-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 5. | Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1024] [Cited by in RCA: 1311] [Article Influence: 262.2] [Reference Citation Analysis (1)] |
| 6. | Peng D, Liu XY, Cheng YX, Tao W, Cheng Y. Improvement of Diabetes Mellitus After Colorectal Cancer Surgery: A Retrospective Study of Predictive Factors For Type 2 Diabetes Mellitus Remission and Overall Survival. Front Oncol. 2021;11:694997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Grosek J, Ales Kosir J, Sever P, Erculj V, Tomazic A. Robotic versus laparoscopic surgery for colorectal cancer: a case-control study. Radiol Oncol. 2021;55:433-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Peng D, Cheng YX, Cheng Y. Improved Overall Survival of Colorectal Cancer under Multidisciplinary Team: A Meta-Analysis. Biomed Res Int. 2021;2021:5541613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Cheng YX, Tao W, Zhang H, Peng D, Wei ZQ. Does liver cirrhosis affect the surgical outcome of primary colorectal cancer surgery? A meta-analysis. World J Surg Oncol. 2021;19:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Dagmura H, Daldal E, Okan I. The Efficacy of Hemoglobin, Albumin, Lymphocytes, and Platelets as a Prognostic Marker for Survival in Octogenarians and Nonagenarians Undergoing Colorectal Cancer Surgery. Cancer Biother Radiopharm. 2022;37:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Jiang H, Li H, Li A, Tang E, Xu D, Chen Y, Zhang Y, Tang M, Zhang Z, Deng X, Lin M. Preoperative combined hemoglobin, albumin, lymphocyte and platelet levels predict survival in patients with locally advanced colorectal cancer. Oncotarget. 2016;7:72076-72083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 12. | Wallace K, Li H, Brazeal JG, Lewin DN, Sun S, Ba A, Paulos CM, Rachidi S, Li Z, Alekseyenko AV. Platelet and hemoglobin count at diagnosis are associated with survival in African American and Caucasian patients with colorectal cancer. Cancer Epidemiol. 2020;67:101746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Erstad DJ, Taylor MS, Qadan M, Axtell AL, Fuchs BC, Berger DL, Clancy TE, Tanabe KK, Chang DC, Ferrone CR. Platelet and neutrophil to lymphocyte ratios predict survival in patients with resectable colorectal liver metastases. Am J Surg. 2020;220:1579-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Li Y, Xu T, Wang X, Jia X, Ren M. The prognostic utility of preoperative neutrophil-to-lymphocyte ratio (NLR) in patients with colorectal liver metastasis: a systematic review and meta-analysis. Cancer Cell Int. 2023;23:39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 15. | Cui M, Xu R, Yan B. A persistent high neutrophil-to-lymphocyte ratio predicts poor prognosis in patients with colorectal cancer undergoing resection. Mol Clin Oncol. 2020;13:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Wang PF, Song SY, Guo H, Wang TJ, Liu N, Yan CX. Prognostic role of pretreatment red blood cell distribution width in patients with cancer: A meta-analysis of 49 studies. J Cancer. 2019;10:4305-4317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Saito H, Shimizu S, Shishido Y, Miyatani K, Matsunaga T, Fujiwara Y. Prognostic significance of the combination of preoperative red cell distribution width and platelet distribution width in patients with gastric cancer. BMC Cancer. 2021;21:1317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 18. | Yang D, Quan W, Wu J, Ji X, Dai Y, Xiao W, Chew H, Sun Z, Li D. The value of red blood cell distribution width in diagnosis of patients with colorectal cancer. Clin Chim Acta. 2018;479:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Shi C, Xie M, Li L, Li K, Hu BL. The association and diagnostic value of red blood cell distribution width in colorectal cancer. Medicine (Baltimore). 2019;98:e15560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Huang J, Zhao Y, Liao L, Liu S, Lu S, Wu C, Wei C, Xu S, Zhong H, Liu J, Guo Y, Zhang S, Gao F, Tang W. Evaluation of Red Cell Distribution Width to Lymphocyte Ratio as Potential Biomarker for Detection of Colorectal Cancer. Biomed Res Int. 2019;2019:9852782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Cheng KC, Lin YM, Liu CC, Wu KL, Lee KC. High Red Cell Distribution Width Is Associated with Worse Prognosis in Early Colorectal Cancer after Curative Resection: A Propensity-Matched Analysis. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Zhang X, Wu Q, Hu T, Gu C, Bi L, Wang Z. Elevated red blood cell distribution width contributes to poor prognosis in patients undergoing resection for nonmetastatic rectal cancer. Medicine (Baltimore). 2018;97:e9641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Virdee PS, Marian IR, Mansouri A, Elhussein L, Kirtley S, Holt T, Birks J. The Full Blood Count Blood Test for Colorectal Cancer Detection: A Systematic Review, Meta-Analysis, and Critical Appraisal. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Kust D, Lucijanic M, Urch K, Samija I, Celap I, Kruljac I, Prpic M, Lucijanic I, Matesa N, Bolanca A. Clinical and prognostic significance of anisocytosis measured as a red cell distribution width in patients with colorectal cancer. QJM. 2017;110:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Li Y, Xing C, Wei M, Wu H, Hu X, Li S, Sun G, Zhang G, Wu B, Zhang F, Li Z. Combining Red Blood Cell Distribution Width (RDW-CV) and CEA Predict Poor Prognosis for Survival Outcomes in Colorectal Cancer. J Cancer. 2019;10:1162-1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Pedrazzani C, Tripepi M, Turri G, Fernandes E, Scotton G, Conci S, Campagnaro T, Ruzzenente A, Guglielmi A. Prognostic value of red cell distribution width (RDW) in colorectal cancer. Results from a single-center cohort on 591 patients. Sci Rep. 2020;10:1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Pilling LC, Atkins JL, Kuchel GA, Ferrucci L, Melzer D. Red cell distribution width and common disease onsets in 240,477 healthy volunteers followed for up to 9 years. PLoS One. 2018;13:e0203504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Chen J, Li Y, Cui H. Preoperative low hematocrit is an adverse prognostic biomarker in ovarian cancer. Arch Gynecol Obstet. 2021;303:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Deng Q, Long Q, Liu Y, Yang Z, Du Y, Chen X. Prognostic value of preoperative peripheral blood mean platelet volume/platelet count ratio (MPV/PC) in patients with resectable cervical cancer. BMC Cancer. 2021;21:1282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Weiser MR. AJCC 8th Edition: Colorectal Cancer. Ann Surg Oncol. 2018;25:1454-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 651] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 31. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8636] [Article Influence: 539.8] [Reference Citation Analysis (0)] |
| 32. | Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252-7259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1947] [Cited by in RCA: 2940] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 33. | Flanagan LS, Choi CB, Lemdani MS, Shah A, Parray A, Sukyte-Raube D, Fang CH, Baredes S, Eloy JA. Complication Risk in Ventral Skull Base Surgery Based on Preoperative Hematocrit. Laryngoscope. 2022;132:1707-1713. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Liao Y, Zhang R, Shi S, Lin X, Wang Y, Chen W, Zhao Y, Bao K, Zhang K, Chen L, Fang Y. Red blood cell distribution width predicts gastrointestinal bleeding after coronary artery bypass grafting. BMC Cardiovasc Disord. 2022;22:436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 35. | Lee SI, Lee SY, Choi CH, Park CH, Park KY, Son KH. Relation between changes in red blood cell distribution width after coronary artery bypass grafting and early postoperative morbidity. J Thorac Dis. 2018;10:4244-4254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Chen JL, Wu JN, Lv XD, Yang QC, Chen JR, Zhang DM. The value of red blood cell distribution width, neutrophil-to-lymphocyte ratio, and hemoglobin-to-red blood cell distribution width ratio in the progression of non-small cell lung cancer. PLoS One. 2020;15:e0237947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Ide S, Toiyama Y, Okugawa Y, Omura Y, Kitajima T, Fujikawa H, Hiro J, Ohi M, Kusunoki M. Clinical significance of an increased red blood cell distribution width in patients with rectal cancer undergoing chemoradiotherapy followed by surgery. Surg Today. 2020;50:551-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | McSorley ST, Johnstone M, Steele CW, Roxburgh CSD, Horgan PG, McMillan DC, Mansouri D. Normocytic anaemia is associated with systemic inflammation and poorer survival in patients with colorectal cancer treated with curative intent. Int J Colorectal Dis. 2019;34:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Xanthopoulos A, Giamouzis G, Dimos A, Skoularigki E, Starling RC, Skoularigis J, Triposkiadis F. Red Blood Cell Distribution Width in Heart Failure: Pathophysiology, Prognostic Role, Controversies and Dilemmas. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 40. | Cao C, Shou J, Shi H, Jiang W, Kang X, Xie R, Shang B, Bi X, Zhang J, Zheng S, Zhou A, Li C, Ma J. Novel cut-off values of time from diagnosis to systematic therapy predict the overall survival and the efficacy of targeted therapy in renal cell carcinoma: A long-term, follow-up, retrospective study. Int J Urol. 2022;29:212-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Wilson MJ, van Haaren M, Harlaar JJ, Park HC, Bonjer HJ, Jeekel J, Zwaginga JJ, Schipperus M. Long-term prognostic value of preoperative anemia in patients with colorectal cancer: A systematic review and meta-analysis. Surg Oncol. 2017;26:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 42. | Kwon YH, Lim HK, Kim MJ, Park JW, Ryoo SB, Jeong SY, Park KJ. Impacts of anemia and transfusion on oncologic outcomes in patients undergoing surgery for colorectal cancer. Int J Colorectal Dis. 2020;35:1311-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Väyrynen JP, Tuomisto A, Väyrynen SA, Klintrup K, Karhu T, Mäkelä J, Herzig KH, Karttunen TJ, Mäkinen MJ. Preoperative anemia in colorectal cancer: relationships with tumor characteristics, systemic inflammation, and survival. Sci Rep. 2018;8:1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |