Published online Mar 28, 2024. doi: 10.3748/wjg.v30.i12.1706
Peer-review started: November 26, 2023
First decision: December 14, 2023
Revised: January 22, 2024
Accepted: March 15, 2024
Article in press: March 28, 2024
Published online: March 28, 2024
Processing time: 122 Days and 16.4 Hours
Endoscopic resection (ER) of colorectal polyps has become a daily practice in most endoscopic units providing a colorectal cancer screening program and requires the availability of local experts and high-end endoscopic devices. ER procedures have evolved over the past few years from endoscopic mucosal resection (EMR) to more advanced techniques, such as endoscopic submucosal dissection and endo-scopic full-thickness resection. Complete resection and disease eradication are the ultimate goals of ER-based techniques, and novel devices have been developed to achieve these goals. The EndoRotor® Endoscopic Powered Resection System (Interscope Medical, Inc., Northbridge, Massachusetts, United States) is one such device. The EndoRotor is a powered resection tool for the removal of alimentary tract mucosa, including post-EMR persistent lesions with scarring, and has both CE Mark and FDA clearance. This review covers available published evidence documenting the usefulness of EndoRotor for the management of recurrent colorectal polyps.
Core Tip: Colorectal cancer (CRC) screening was initiated and implemented over the last two decades with a widespread variability in strategies and coverage in different parts of Europe even after the first appearance of the European guidelines for CRC screening. Recurrent or previously manipulated lesions are usually fibrotic with tethering to the muscularis and display the non-lifting sign, making subsequent resection challenging. The EndoRotor® Endoscopic Powered Resection System is a powered debridement device for the removal of alimentary tract mucosa, including post-endoscopic mucosal resection persistent lesions with scarring, and has both CE Mark and FDA clearance. This review covers available published evidence documenting the usefulness of EndoRotor for the management of recurrent scarred colorectal polyps.
- Citation: Zaghloul M, Rehman H, Sansone S, Argyriou K, Parra-Blanco A. Endoscopic treatment of scarred polyps with a non-thermal device (Endorotor): A review of the literature. World J Gastroenterol 2024; 30(12): 1706-1713
- URL: https://www.wjgnet.com/1007-9327/full/v30/i12/1706.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i12.1706
Colorectal cancer (CRC) places an enormous burden on health care services worldwide. Globally, CRC is ranked third in incidence and second in mortality[1]. Despite its increasing trends, more favourable outcomes are observed in many high-incidence countries due to increased colonoscopy screening programs and early removal of any precursor lesions[2,3].
CRC screening has been initiated and implemented over the last two decades, during which there has been widespread variability in strategies and coverage in different parts of Europe even after the first appearance of the European Guidelines for CRC screening[2,4]. These guidelines emphasize detailed quality requirements for the endoscopic unit infrastructure as well as certain competency levels of both the endoscopist and assisting staff[5]. Early detection and complete excision of adenomas are pivotal for minimizing the risk of CRC. Choosing the optimal resection technique is critical in achieving complete excision and is dependent on many factors, including the lesion morphology, size, location, estimated risk of submucosal invasion, and degree of expertise of the endoscopist. Endoscopic resection (ER) choices are explicitly indicated in different guidelines of the Gastroenterological and Endoscopic Societies[6,7]. Endoscopic Mucosal Resection (EMR) is a well-established technique for removing colorectal adenomas[8]. This approach is the standard treatment for sessile polyps 10-20 mm in diameter. For larger polyps, en bloc resection is rarely achievable, and piecemeal resection is used. In a large European study, the recurrence rate after EMR of lesions > 20 mm reached 31.7% after 3–6 months[9]. Another report from Australia showed early recurrence rates of 16.0%[8].
With the implementation of CRC screening, an interval cancer problem has emerged, which has been attributed to incomplete resection of the primary lesion in approximately 27% of patients when large colon polyps were first resected[8,10,11]. Recurrent or previously manipulated lesions are usually fibrotic when tethered to the muscularis and display the non lifting sign, making subsequent resection challenging. There are no standards or approved guidelines for ER for these fibrotic and/or recurrent polyps, but there are many techniques reported in the literature, including endoscopic submucosal dissection (ESD), endoscopic full-thickness resection (EFTR), argon plasma coagulation (APC), and endoscopic powered resection (EPR) systems. Although effective, ESD results in an increased risk of perforation due to thermal injury and difficulty in planar identification caused by fibrosis. EFTR is a hybrid surgical approach indicated for lesions < 30 mm where the full thickness of the lumen wall is resected. Recent studies have shown the procedure to be technically successful in approximately 90% of patients; however, the complete resection rate for lesions > 20 mm was significantly lower than that for lesions ≤ 20 mm (58% vs 81%, P = 0.0038), and serious adverse events such as perforation, appendicitis, and small bowel fistula have been reported[12]. APC can result in thermal injury leading to perforation and is associated with a high recurrence rate[12].
The EndoRotor® EPR System (Interscope Medical, Inc., Northbridge, Massachusetts, United States) is a powered debridement device for the removal of alimentary tract mucosa, including post-EMR persistent lesions with scarring, and has both CE Mark and FDA clearance. This review covers available published evidence documenting the usefulness of EndoRotor for the management of recurrent colorectal polyps.
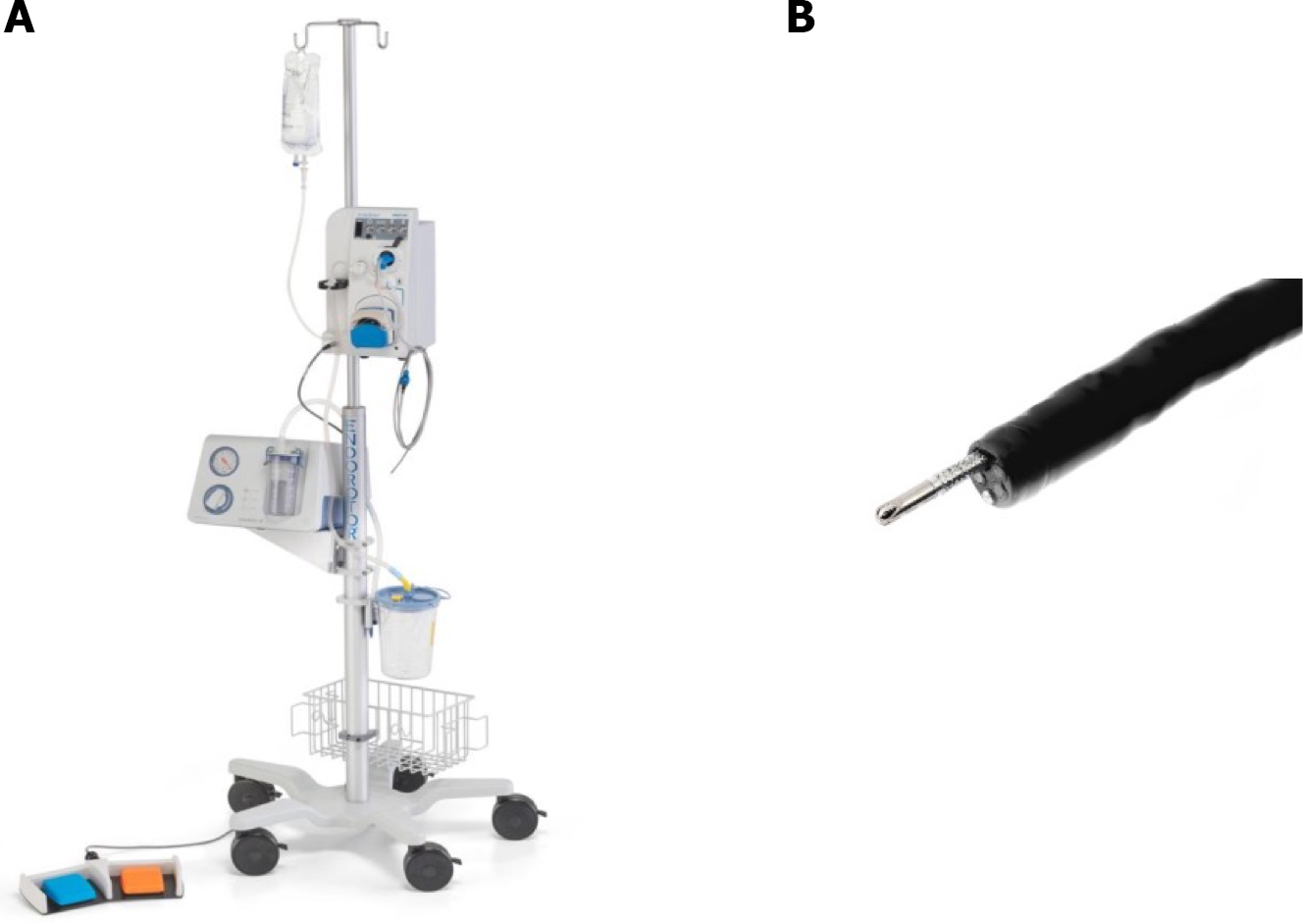
The EndoRotor EPR System consists of a power console, a beveled tip single-use catheter, foot control pedals, and a specimen trap. The power console contains a motor drive for catheter blade rotation, a peristaltic pump for irrigation, and a dedicated vacuum system. A single-use catheter with an outer diameter of 3.1 mm connects to the power console and is compatible with endoscopes that have a minimum working channel of 3.2 mm. The catheter had demarcation lines to assist with cutting window positioning during the procedure. The console is set to high (1750 rotations/min) or low (1000 rotations/min) speed, and the vacuum is set between 50 and 200 mmHg of negative pressure. The catheter is controlled by the endoscopist using two foot pedals, and the tissue is resected by aspiration into a cutting window with a rotating blade. Resected tissue is simultaneously suctioned through the inner lumen of the catheter and into a specimen trap with a preloaded micron filter that can be sent for histopathological analysis (Figure 1).
EndoRotor was first evaluated in vivo by Hollerbach et al[13], who performed several successful gastric (15 Lesions) and colonic (10 Lesions) mucosal resections in German Landrace pigs under general endotracheal anaesthesia with resected areas ranging from 15-70 mm2. This initial experiment showed the high potential of the EndoRotor EPR System for facilitating safe and effective ER in relatively large mucosal areas in various parts of the gastrointestinal (GI) tract.
Following this animal study, multiple human case reports were published (Table 1). Tillinger[14] successfully removed a scarred recurrent rectal adenoma in two sessions with only minimal bleeding that was endoscopically controlled. Surkunalingam et al[15] reported successful resection of recurrent scars on Paris 0-IIa+c lesions over a fold. Despite initial trials of removal by ESD followed by standard EMR, the lesion’s location and shape made resection unsuccessful, mandating the use of an innovative approach in which the investigators selected EndoRotor to facilitate resection. Additionally, Stadler et al[16] successfully removed a large recurrent laterally spreading granular tumour (LST-G) rectal adenoma via both ESD for the non scarred region and EndoRotor for the tough, fibrosed, adherent region. Furthermore, Pellegatta et al[17] reported the complete removal of scarred polyps in the rectum, a LST-G of 40 mm, occupying half the circumference with the Kudo IIIL pit pattern in a single setting despite multiple previous EMR and APC trials.
| Ref. | Type | Resected lesions | Comments/reported complications |
| Hollerbach et al[14], 2016 | In vivo animal study | Gastric (15 lesions; 4 oesophagus, 9 stomach and 2 duodenum) and colonic (10 lesions) | Minimal bleeding (blood loss did not exceed more than 10 – 15 cc of blood); No perforations occurred during “normal” resections. EndoRotor® was significantly faster than current standard techniques, 2 perforations occurred (these serious adverse events only occurred during experiments that were performed deliberately to test the limits of the gastrointestinal wall when excessive force was applied) |
| Tillinger et al[15], 2016 | Case report | Circumferential rectal recurrent scarred LST-G mixed lesion 3 cm in length | The whole lesion could be removed in two sessions. Minor bleeding was controlled by means of adrenalin-injection and coagulation with argon plasma. No further complications occurred |
| Surkunalingam et al[16], 2018 | Case report | 20 m, Paris IIa+c | Prolonged time; The inability to obtain an en-bloc specimen |
| Emmanuel et al[20], 2021 | Retrospective study | 41 lesions post EMR or pEMR. Anorectum/rectum 8 (19.5), left-sided colon segment 10 (24.4), right-sided colon segment 13 (31.7) Caecum 10 (24.4) | Only one case of postpolypectomy syndrome 1 (2.4) |
| Kandiah et al[19], 2019 | Prospective pilot study | 19 flat scared polyps (Paris 0-IIa/0-IIb/0-Is) from 7 to 70 mm in the rectum and sigmoid | Cured maximally in 2 sessions. Minor intraprocedural bleeding in 2 cases. There were no perforations, delayed bleeding, postpolypectomy syndrome or complications requiring surgery |
| Stadler et al[17], 2019 | Case report | Recurrent rectal adenoma LST-G | No reported complication successful removal of the scarred part in combination with ESD |
| Pellegatta et al[18], 2020 | Case report | Scarred polyp in the rectum, a LST-G of 40 mm, hemicirconferential with adenomatous pit pattern (Kudo IIIL) | No recurrence was endoscopically revealed at 6 months’ follow-up. Complete removal in one session |
| Kaul et al[21], 2021 | Retrospective study | 41 lesions; Oesophagus 8 (23.5); Duodenum 5 (14.7); Colon18 (52.9); Rectum 3 (8.8) | Technical success (the ability to complete PED using the EndoRotor device without the use of additional resection modalities) was achieved in 97.6% of lesions (n = 40). Clinical success (no endoscopic and/or histologic evidence of residual/recurrent lesion on follow-up examination) was achieved in 79.2% of patients. Adverse events were reported in 3 patients (8.8%). postprocedural chest pain in one patient with oesophageal lesion. Two patients had delayed bleeding with colonic lesions. Intraprocedural bleeding was observed in 10 patients (29.4%; 4 colon, 5 oesophageal, and 1 duodenum) |
Kandiah et al[18], in a prospective pilot study of 19 patients with recurrent scarred colorectal lesions, reported that EndoRotor was a safe and effective technique for the management of these challenging scarred polyps. Investigation of 84% of patients who underwent 1–2 EndoRotor procedures revealed disease eradication, and no serious adverse events occurred.
Emmanuel et al[19] used EndoRotor to acquire histological samples from the margins of LST-G lesions post EMR with visually apparently normal lateral margins to evaluate evidence of microscopic adenoma as a possible mechanism of recurrence after ER, especially after piecemeal EMR (pEMR). These authors showed that microscopic residual adenoma in the mucosal bridges of lesion bases could be missed by traditional pEMR, as proven via histological examinations of tissue samples retrieved from the EndoRotor. This case series emphasized the potential complementary use of EndoRotor after pEMR to remove any remnants at the base as well as resect the margins.
The largest published clinical human case series with EndoRotor was reported in a retrospective multicentre study from the United States where EndoRotor was evaluated in 40 upper and lower GI lesions, with a previous trial of ER in 85.4% of them. There were 28 colorectal polyps, 25 of which had previously undergone therapeutic intervention. Resection was complete with the Endorotor in all 28 patients. Seventeen (60.7%) patients underwent follow-up endoscopy at least 2 months later, and there was no residual adenomatous tissue in 15 (88.2%) patients[20]. However, this study is subject to selection bias because it was retrospective.
Notably, EndoRotor was evaluated in only a single-arm manner in all the studies, with no comparison to other ER methods, such as EMR, ESD, or ablation, used for the management of difficult adherent GI lesions.
There are several technical advantages to using this cold automated mechanical suction and resection device. First, the absence of heat or ablation of mucosa leads to less scarring, delayed bleeding, or perforation, as expected with other thermal methods[17,18]. Second, there are no concerns about patients with pacemakers because no electric current is used. However, although a single tool was used in most of the reported cases, the Endorotor could also be applied after removing nodular parts of the polyp via traditional resection techniques such as EMR or ESD; in that case, the advantages of cold resection would be lost, at least in part. Third, the alternative to Endorotor for recurrent polyps could be a significantly more invasive technique, mainly full-thickness resection and ESD. Fourth, due to the simultaneous suction and catheter cutting rotation, it is expected that the entire mucosal surface could be removed because of its specific viscoelastic properties against the more fibrous muscularis layer, making it potentially safe for preserving the muscle layer, as no perforations were reported during its use. Fifth, the beveled inner sheath hole is approximately 3 mm2 in area, allowing the user to resect 2 - 4 mm of tissue per second with each rotation of the inner cannula (1000-1700 rpm), ensuing a very rapid resection. Sixth, the EndoRotor catheters are provided in variable lengths suitable for the scope used by gastroscopy (1240 mm and 1270 mm) or colonoscopy (1890 mm and 1540 mm) to shorten the length of the extraendoscopic catheter for more effective transmission of rotational power. Seventh, the console is easily controlled by 2-foot pedals, one blue to activate rotation of the inner cutting blade of the catheter and then continuous pressing of the orange pedal to activate the suction force throughout the entire cutting process. Once the orange pedal is released, the cutting canula automatically stops for safety reasons. The device has a short learning curve for experienced endoscopists performing EMR and/or ESD. Pathologic tissue samples are simultaneously collected during the cutting operation and automatically transported back to the collection trap, providing a single step of cutting and collecting tissue, thus reducing the resection time.
The EndoRotor EPR System allows adjustment of the suction force (mmHg) and cutting blade speed (revolutions per minute) depending on the nature of the lesion and the preference of the endoscopist. This approach could be helpful for the management of lesions with different degrees of fibrosis, and it would also reduce the need for milder resection parameters at the beginning of the learning curve or in locations with an increased risk of perforation. The manufacturer recommends vacuum levels of 50 to 200 mmHg for resecting endoluminal lesions. In Kandiah et al[18] scarred lesions were treated from 100 to 300 mmHg and in Emmanuel et al[19] 50 to 100 mmHg when completing EMR margin resections. Studies comparing different settings are lacking. Only one study mentioned the detailed settings used and recommended starting ablation at a high speed (1750 rpm) with 150–200 mmHg suction[21].
The main limitations of the EndoRotor system for the treatment of scarred polyps include the following: (1) The system requires a working channel of 3.2 mm or more; thus, it is impossible to use this system with endoscopes with a reduced channel size. However, this should not be a problem in the management of colorectal polyps, as even paediatric colonoscopes have 3.2 mm channels. However, for its application in upper endoscopy, regular gastroscopy with 2.8 mm channels is not suitable[15]; (2) EndoRotor catheters are relatively stiff, and certain angles of inclination, such as 90°, can be challenging for the operator and the system, while small tilts (30° to 40°) are more convenient[13]. Furthermore, retroflexing the endoscope with the resection catheter inside the working channel is impossible due to the reduced flexion ability of the endoscope with the catheter occupying the whole channel. Therefore, therapy cannot be administered in retroflexion, although this could be solved by lifting just beyond the target lesion to push it into view[19]; (3) Despite the ease of collecting tissue resected, the quality of the collected tissue being similar to that obtained from traditional biopsy forceps does not enable the depth of invasion to be judged. These samples could only confirm the histopathological type and presence, absence, and degree of dysplasia. This leads to consideration of proper patient selection, e.g., previous EMR confirmed a benign lesion that recurred due to persistence. Therefore, EPR is not appropriate in principle for the treatment of suspicious lesions; and (4) EPR is not a one-time procedure, as opposed to ESD/EFTR, but rather requires follow-up every 3-6 months until complete eradication of the polyp can be confirmed, which is typically achieved within 2-3 sessions. Even if the lesion seems to have been entirely treated with an EPR +/- EMR session, it is usually impossible to completely rule out minute residual polyp tissue.
The EndoRotor ER system is a relatively expensive tool compared to conventional EMR devices or APC, and retreatment with Endorotor can be required in follow-up procedures. However, the cost of this approach is less than that of EFTR or colectomy for the treatment of polyps. Moreover, being an outpatient, well tolerated, and safe procedure would argue against the primary cost of the EndoRotor EPR System compared to other modalities and taking possible hospital stays into consideration.
Cost is certainly a factor when treating patients, but it must be weighed against patient quality of life and procedural effectiveness. Reimbursement for the endoscopic and surgical management of colorectal polyps varies widely in different settings. Cost-economic studies are required to compare different endoscopic and surgical techniques for the management of residual/recurrent polyps.
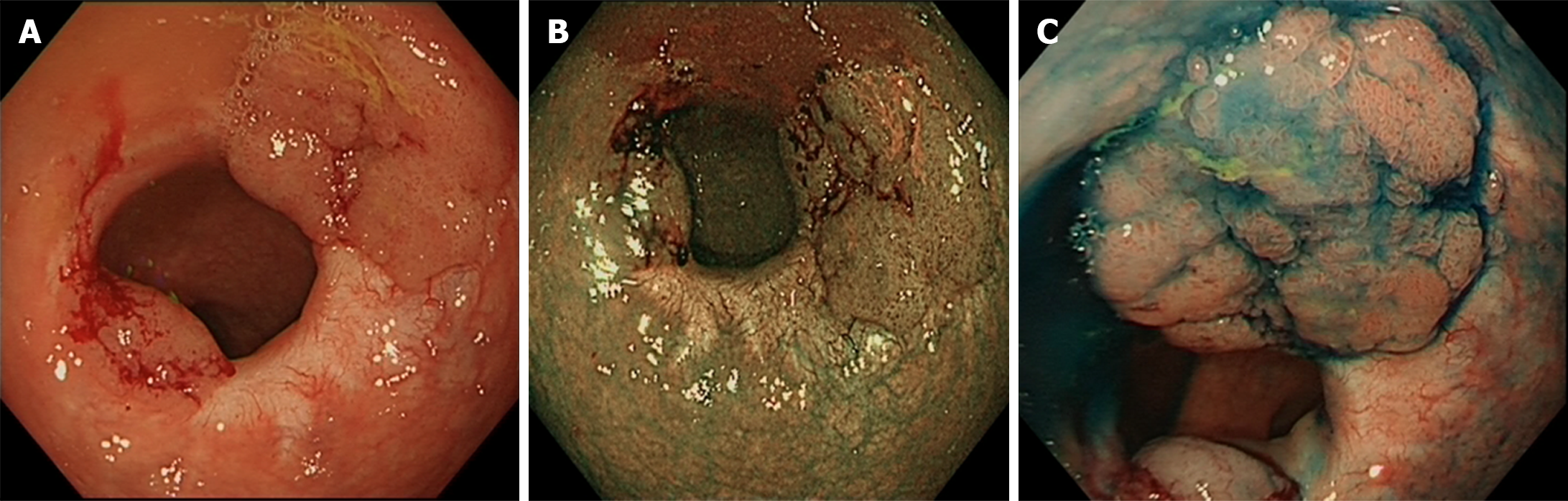
As we are currently actively applying this technique for the treatment of recurrent colorectal polyps, we would like to report a case in which two recurrent rectal polyps were found on a previous polypectomy site in an 85-year-old male who underwent transanal endoscopic operation and two EMRs for a large LSTGM polyp in the rectum over a period of 3 years. Both recurrent polyps were laterally spreading tumour-nongranular and were separated by scar tissue. Histology confirmed low-grade dysplasia and tubulovillous adenoma of both polyps.
In the initial session, the patient underwent exclusive EndoRotor treatment (Figure 2).
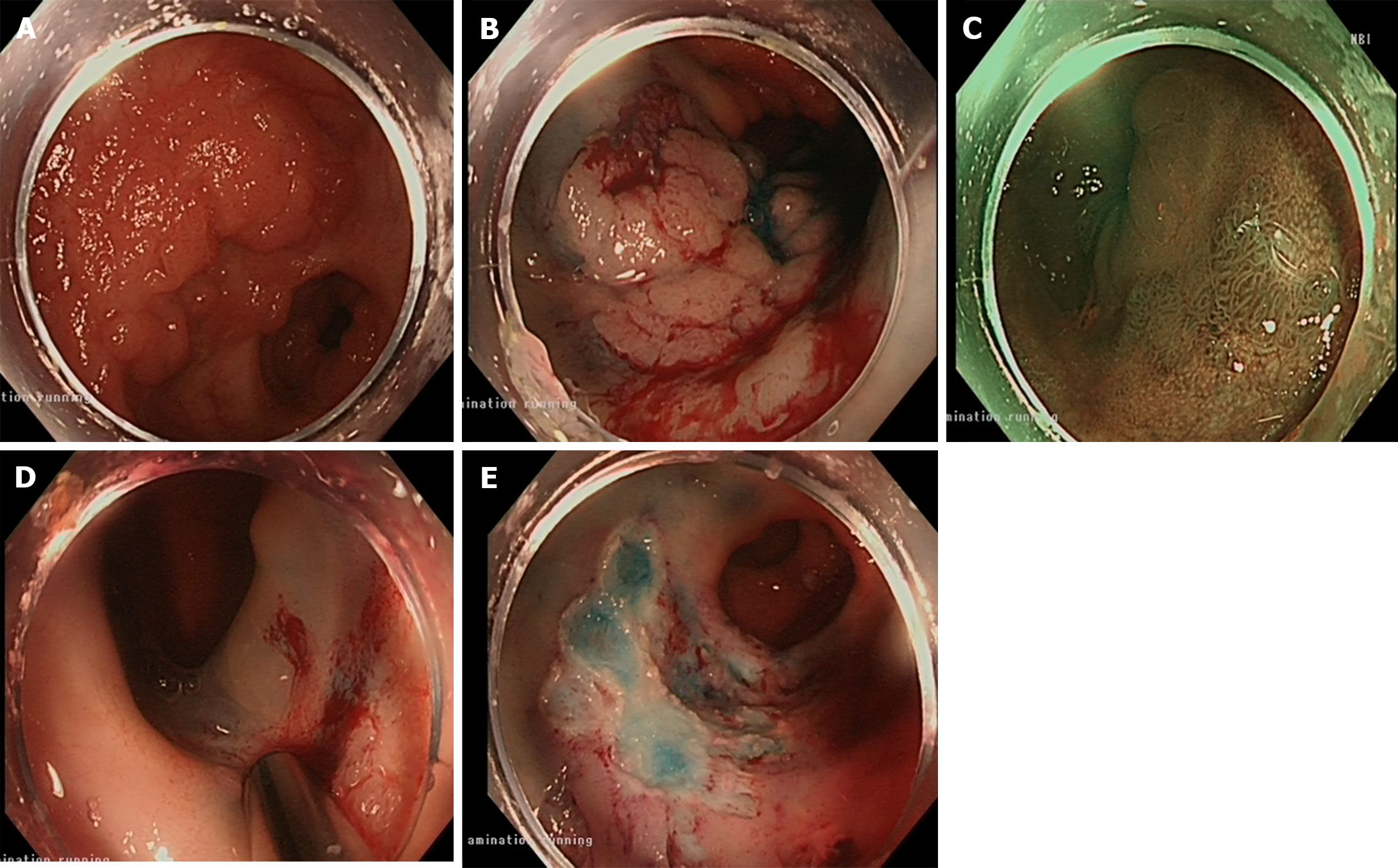
An elevated (IIa) lesion was subsequently resected via the EMR and EndoRotor (Figure 3).
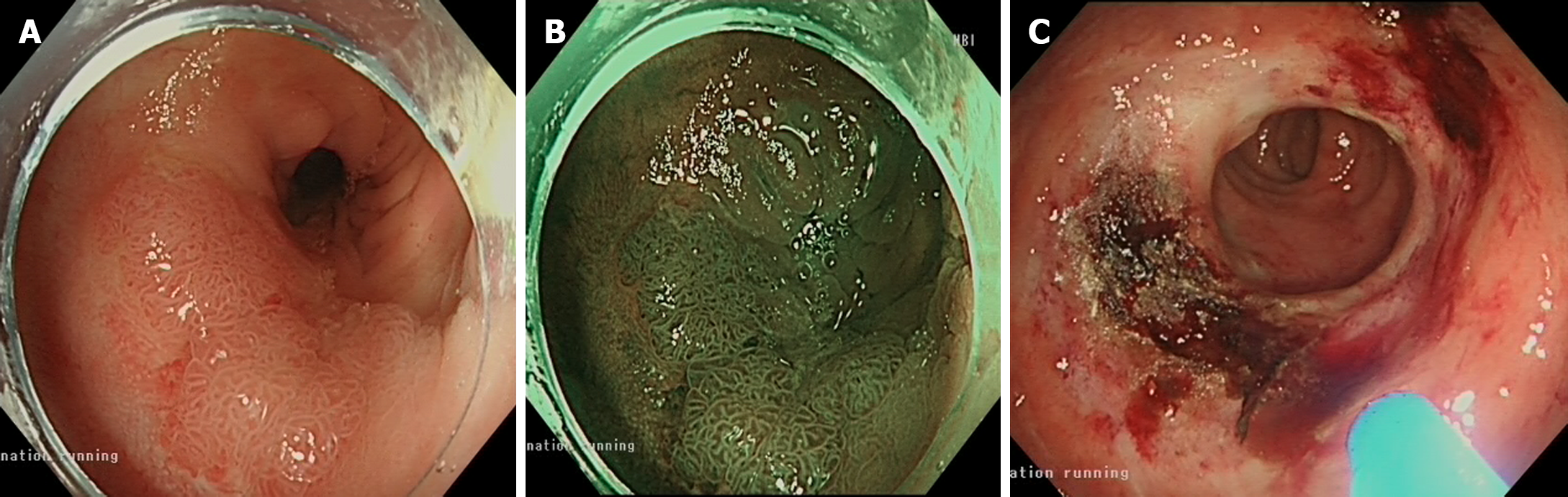
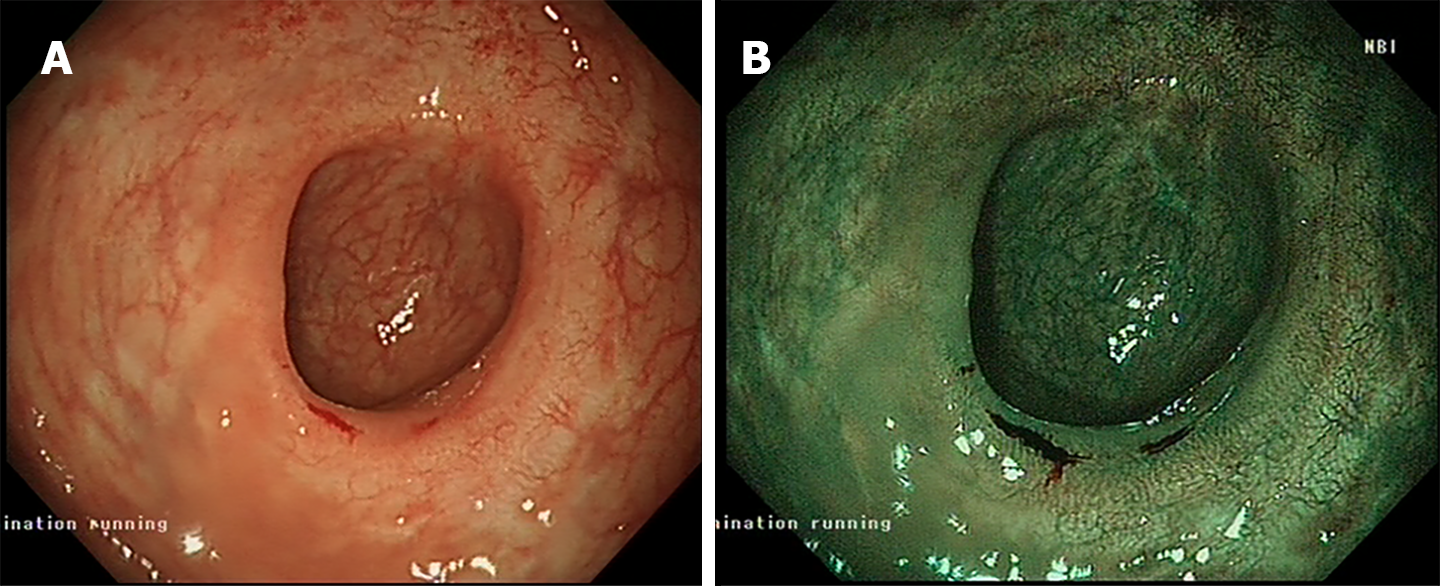
At the second follow-up, which was conducted six months after the initial presentation, 75% in the size of the original polyp was observed, prompting its resection via EndoRotor and Pulsed Argon Plasma Coagulation was used (Figure 4). During the follow-up 6 months after the last intervention, the patient exhibited a healthy scar under both white light and narrow-band imaging assessments (Figure 5) which was confirmed to be histologically normal tissue.
EndoRotor is a novel tool for endoscopists performing EMR and/or ESD and should be considered a treatment option for patients with recurrent colorectal lesions. Where the FTRD offers a solution for small adherent recurrent lesions, larger flat lesions could be problematic and only amenable to ablation, ESD or EndoRotor resection. Ablative therapy does not allow the collection of tissue samples. ER (ESD) is a highly technically demanding procedure with a long learning curve. EndoRotor use should be discussed in multidisciplinary MDT meetings where all options (endoscopic and surgical) are considered, as well as through discussion with the patient. Despite the paucity of evidence, EndoRotor showed high safety and efficacy in the resection of recurrent scarred lesions with previously known pathology. Moreover, this technique could be a valuable addition to tertiary referral endoscopy centres for difficult cases.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fu Z, China S-Editor: Lin C L-Editor: A P-Editor: Chen YX
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64536] [Article Influence: 16134.0] [Reference Citation Analysis (176)] |
| 2. | Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, Kuipers EJ. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 913] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 3. | Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1578] [Article Influence: 263.0] [Reference Citation Analysis (2)] |
| 4. | von Karsa L, Patnick J, Segnan N. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Executive summary. Endoscopy. 2012;44 Suppl 3:SE1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Valori R, Rey JF, Atkin WS, Bretthauer M, Senore C, Hoff G, Kuipers EJ, Altenhofen L, Lambert R, Minoli G; International Agency for Research on Cancer. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Quality assurance in endoscopy in colorectal cancer screening and diagnosis. Endoscopy. 2012; 44 Suppl 3: SE88-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 765] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 7. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Saitoh Y, Tsuruta O, Sugihara KI, Igarashi M, Toyonaga T, Ajioka Y, Kusunoki M, Koike K, Fujimoto K, Tajiri H. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2020;32:219-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 8. | Moss A, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Burgess NG, Sonson R, Byth K, Bourke MJ. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015;64:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 367] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 9. | Knabe M, Pohl J, Gerges C, Ell C, Neuhaus H, Schumacher B. Standardized long-term follow-up after endoscopic resection of large, nonpedunculated colorectal lesions: a prospective two-center study. Am J Gastroenterol. 2014;109:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, Levy LC, Toor A, Mackenzie TA, Rosch T, Robertson DJ. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74-80.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 553] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 11. | Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy. 2014;46:388-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 275] [Article Influence: 25.0] [Reference Citation Analysis (2)] |
| 12. | ASGE Technology Committee; Aslanian HR, Sethi A, Bhutani MS, Goodman AJ, Krishnan K, Lichtenstein DR, Melson J, Navaneethan U, Pannala R, Parsi MA, Schulman AR, Sullivan SA, Thosani N, Trikudanathan G, Trindade AJ, Watson RR, Maple JT. ASGE guideline for endoscopic full-thickness resection and submucosal tunnel endoscopic resection. VideoGIE. 2019;4:343-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 13. | Hollerbach S, Wellmann A, Meier P, Ryan J, Franco R, Koehler P. The EndoRotor(®): endoscopic mucosal resection system for non-thermal and rapid removal of esophageal, gastric, and colonic lesions: initial experience in live animals. Endosc Int Open. 2016;4:E475-E479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Tillinger W. Der Endorotor®–Ein neues endoskopisches Resektionssystem: Fallbericht//The EndoRotor®–a novel endoscopic device for mucosal resection. A case report. Z Gastroenterol. 2016;54:32. [DOI] [Full Text] |
| 15. | Surkunalingam N, Das A, Khosravi F, Sachdev M. Sigmoid colon polyp EMR with novel endoscopic morcellator. VideoGIE. 2018;3:191-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Stadler A, Knabe M, May A. [Endoscopic resection of a scarred rectal adenoma using EndoRotor]. Z Gastroenterol. 2019;57:1226-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Pellegatta G, Mangiavillano B, Maselli R, Bhandari P, Di Leo M, Badalamenti M, Repici A. Cutting-edge effective endoscopic technique to remove scarred polyps. Endoscopy. 2020;52:E362-E363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 18. | Kandiah K, Subramaniam S, Chedgy F, Thayalasekaran S, Venetz D, Aepli P, Bhandari P. A novel non-thermal resection tool in endoscopic management of scarred polyps. Endosc Int Open. 2019;7:E974-E978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Emmanuel A, Williams S, Gulati S, Ortenzi M, Gunasingam N, Burt M, Ratcliff S, Hayee B, Haji A. Incidence of microscopic residual adenoma after complete wide-field endoscopic resection of large colorectal lesions: evidence for a mechanism of recurrence. Gastrointest Endosc. 2021;94:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Kaul V, Diehl D, Enslin S, Infantolino A, Tofani C, Bittner K, Tariq R, Aslam R, Ayub K. Safety and efficacy of a novel powered endoscopic debridement tissue resection device for management of difficult colon and foregut lesions: first multicenter U.S. experience. Gastrointest Endosc. 2021;93:640-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Knabe M, Blößer S, Wetzka J, Ell C, May A. Non-thermal ablation of non-neoplastic Barrett's esophagus with the novel EndoRotor® resection device. United European Gastroenterol J. 2018;6:678-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |