Published online Jun 15, 1997. doi: 10.3748/wjg.v3.i2.87
Revised: January 31, 1997
Accepted: March 1, 1997
Published online: June 15, 1997
AIM: To study the correlation between changes in p53 and Waf1p21 expression and cell proliferation, determined by proliferating cell nuclear antigen (PCNA), at different stages of human esophageal carcinogenesis.
METHODS: Biopsied and resected esophageal tissues from a high risk population of esophageal cancer in northern China were used in this study. All specimens were fixed in 85% alcohol and processed for routine histology. The avidin biotin peroxidase complex (ABC) method was used to detect p53, Waf1p21 and PCNA.
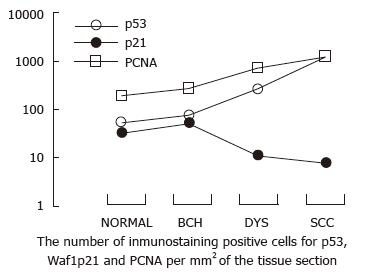
RESULTS: Strong nuclear staining of p53, Waf1p21 and PCNA was observed in normal esophageal epithelium and epithelia with different lesion severities. As the lesions progressed to dysplasia (DYS) and to esophageal squamous cell carcinoma (SCC), the Waf1p21 immunoreactivity percentage decreased. The number of Waf1p21-positive cells slightly increased from normal to basal cell hyperplasia (BCH), but did not further increase in DYS and SCC. The total number of Waf1p21-positive cells was lower than the number of p53-positive cells in normal and BCH esophageal epithelia and much lower in DYS and SCC. Waf1p21-positive cells were located in the third and fourth cell layers in half of the samples examined, which was 2-4 cell layers higher than the cells expressing PCNA and p53 in the same histological categories of normal, BCH and DYS.
CONCLUSION: Low Waf1p21 levels at the DYS stage may be related to a functional loss of p53. Other mechanisms may also be responsible for the decreased Waf1p21 expression in DYS and SCC.
- Citation: Wang LD, Yang WC, Zhou Q, Xing Y, Jia YY, Zhao X. Changes in p53 and Waf1p21 expression and cell proliferation in esophageal carcinogenesis. World J Gastroenterol 1997; 3(2): 87-89
- URL: https://www.wjgnet.com/1007-9327/full/v3/i2/87.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i2.87
Esophageal carcinoma (EC) is a widely occurring disease in Huixian and Linxian of the Henan Province in northern China and remains a leading cause of cancer-related deaths[1]. The development of human esophageal squamous cell carcinoma (SCC) is a progressive multistage process[2-5]. An early indicator of abnormality in patients predisposed to EC is increased esophageal epithelial cell proliferation, which is morphologically manifested as basal cell hyperplasia (BCH), dysplasia (DYS) or carcinoma in situ (CIS), which are considered precancerous EC lesions. Changes in cell proliferation and cell death may be key factors that contribute to the rate of neoplastic progression and tumor growth. Although previous studies on subjects from these high incidence areas have suggested the fundamental importance of epithelial cell hyperproliferation in human esophageal carcinogenesis, little information is available on the molecular basis of cell proliferation in this disease.
The tumor suppressor transcription factor p53 has been shown to function in the cellular DNA damage response, resulting in either G1 arrest or apoptosis[6]. The Waf1/CIP1 gene encodes protein p21, which is transcriptionally regulated by p53[7]. Upon DNA damage, p53 upregulates Waf1p21 expression to induce G1 arrest to allow time for damaged DNA to be repaired, or it triggers apoptosis to eliminate genetically damaged cells[8]. As Waf1p21 is upregulated by wild type P53, the levels of Waf1p21 protein may reflect the functional status of p53 in carcinogenesis.
Our previous studies indicated that P53 protein accumulated in early human esophagus lesions and even in histopathologically normal epithelium[9,10]; p53 gene mutations were observed in some of these samples[11-13]. The results suggested that P53 protein accumulation and gene mutation were early events in esophageal carcinogenesis.
Quantitative analysis of both p53 and Waf1p21 expression can provide important insights into cancer development, but such analysis has not been performed at different stages of human esophageal carcinogenesis. In this study, we investigated the expression of p53, Waf1p21 and PCNA in human esophageal epithelia with different severities of precancerous and cancerous lesions from subjects in Henan, China.
Esophageal tissues were biopsied from 241 symptom-free subjects, and surgically resected EC specimens were collected from 38 patients in Huixian County and Linxian County, China. Of these 279 subjects, 160 were males (20-71 years of age with a mean ± SD of 48 ± 14 years) and 119 females (20-79 years of age with a mean ± SD of 47 ± 16 years). None of the cancer patients received chemotherapy or radiotherapy treatment before the operation. All specimens were fixed in 85% alcohol, embedded in paraffin, and serially sectioned at 5 μm. The sections were mounted onto histostick-coated slides. Three or four adjacent ribbons were collected for histopathological analysis (hematoxylin and eosin stain) and immunohistochemical staining.
Histopathological diagnoses for esophageal epithelia were made according to cellular morphological changes and tissue architecture using previously established criteria[14]. In brief, the normal esophageal epithelium contained 1.3 proliferating basal cell layers, and the papillae were confined to the lower half of the epithelium. In BCH, the proliferating basal cells increased to more than three cell layers and less than half of the entire epithelial thickness. DYS was characterized by partial loss of cell polarity and nuclear atypia. SCC was characterized by confluent and invasive sheets of cohesive, polymorphous cells with hyperchromatic nuclei.
The avidin biotin peroxidase complex (ABC) method was used for p53, Waf1p21 and PCNA antigen detection (Ongogene Science, Inc., Manhasset, NY, United States). After dewaxing, inactivating endogenous peroxidase activity, and blocking crossreactivity with normal serum, the sections were incubated overnight at 4 ˚C in a diluted solution of primary antibodies (1:1000 for p53, 1:20 for Waf1p21 and 1:200 for PCNA). Primary antibody detection was achieved by subsequent application of a biotinylated anti primary antibody, an avidin biotin complex conjugated to horseradish peroxidase, and diaminobenzidine (Vectastain Elite Kit; Vector Laboratories, Burlingame, CA, United States). Normal serum and absence of primary antibody were used as negative controls.
Quantitative analysis of nuclear immunostaining results were recorded as the number of positive cells per mm2 of tissue section as described previously[10]. All positively stained cells were counted in the entire tissue section under a microscope at × 400 magnification. A total of 24 fields were typically counted for biopsied tissues and 50 fields for surgically resected specimens.
The mean ± SE of the p53-, Waf1p21- and PCNA-positive cell number/mm2 in the esophageal biopsy and surgically resected EC samples in each histologic category were calculated by using univariate analysis, and comparisons were made using the Wilcoxon rank sum test for unpaired data and correlation by using Pearson′s correlations. The χ2 test was used for the statistical analysis of the percentage of positively stained samples (p < 0.05 was considered significant).
We observed intense p53 protein and PCNA nuclear immunostaining of tissues with different lesions, which was consistent with our previous observations. In brief, we observed similar immunostaining patterns for PCNA and p53, but the number of PCNA-positive cells was higher than that of p53 in the same histological category of normal, BCH and DYS (3-4 fold, Table 1). As the esophageal tissue progressed from BCH, DYS to SCC, the number of PCNA- and p53-positive cells significantly increased and expanded upwards in the epithelium.
| Histology | p53 immunostaining | Waf1p21 immunostaining | Proliferating Cell Nuclear Antigen immunostaining | |||||
| Positive | Positive cells/mm2 | Positive | Positive cells/mm2 | Samples examined | Positive cells/mm2 | |||
| % | (n/n) | % | (n/n) | |||||
| Normal | 74.2 | (23/31) | 53 ± 78 | 39.3 | (11/28) | 34 ± 72 | 31 | 200 ± 113 |
| BCH | 87.2 | (102/117) | 74 ± 66 | 37.8 | (14/37) | 53 ± 10 | 106 | 286 ± 150 |
| DYSb | 100 | (31/31) | 262 ± 341 | 27.3 | (3/11) | 11 ± 20.2 | 31 | 719 ± 389 |
| SCC | 88.5 | (23/26) | 1297 ± 1110 | 14.3 | (1/7) | 8 ± 22 | 26 | 1261 ± 545 |
The percentage of samples with Waf1p21-positive immunostaining was 39 and 38 in normal and BCH esophageal epithelia, respectively. Waf1p21 immunostaining was confined to the cell nucleus. As the lesions progressed to DYS and SCC, the percentage of Waf1p21 immunoreactivity decreased. The number of Waf1p21-positive cells slightly increased from normal to BCH, but there was no further increase in DYS and SCC. The total number of Waf1p21-positive cells was lower than that of p53 in normal and BCH esophageal epithelia, much lower in DYS (24-fold) and markedly lower in SCC (162-fold) (Table 1, Figure 1). Waf1p21-positive cells localized to the third and fourth cell layers in half of the samples, which was 2-4 cell layers higher than PCNA-positive and p53-positive cells in the same histological categories of normal, BCH and DYS (Figure 2).
Interestingly, we found that the distribution of Waf1p21-positive cells was distinct from that of p53- and PCNA-positive cells such that Waf1p21-positive cells localized to the more differentiated middle third of the epithelium, which was approximately 2-4 layers of basal cells higher than p53- and PCNA-positive cells, suggesting that Waf1p21 expression may be related to cell differentiation. The small number of Waf1p21-positive cells may be due to functional loss of p53 by mutations.
Dysplasia has been considered a severe precancerous lesion of esophageal squamous cell carcinoma. The current study indicated that the levels of p53 and Waf1p21 was similar in normal and BCH lesions, but there was a clear segregation of high p53 and low Waf1p21 expression, from dysplasia to SCC, suggesting that dysplasia may be an important target for molecular insult in esophageal carcinogenesis. The molecular mechanism for the development of dysplasia remains to be further characterized.
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Liu WX
| 1. | Yang CS. Research on esophageal cancer in China: a review. Cancer Res. 1980;40:2633-2644. [PubMed] |
| 3. | Mundle SD, Gao XZ, Khan S, Gregory SA, Preisler HD, Raza A. Two in situ labeling techniques reveal different patterns of DNA fragmentation during spontaneous apoptosis in vivo and induced apoptosis in vitro. Anticancer Res. 1995;15:1895-1904. [PubMed] |
| 4. | Qiu SL, Yang GR. Precursor lesions of esophageal cancer in high-risk populations in Henan Province, China. Cancer. 1988;62:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Wang LD, Lipkin M, Qui SL, Yang GR, Yang CS, Newmark HL. Labeling index and labeling distribution of cells in esophageal epithelium of individuals at increased risk for esophageal cancer in Huixian, China. Cancer Res. 1990;50:2651-2653. [PubMed] |
| 6. | Shimamura A, Fisher DE. p53 in life and death. Clin Cancer Res. 1996;2:435-440. [PubMed] |
| 7. | Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1744] [Cited by in RCA: 1732] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 8. | Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci United States. 1992;89:7491-7495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1145] [Cited by in RCA: 1196] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 9. | Wang LD, Hong JY, Qiu SL, Gao H, Yang CS. Accumulation of p53 protein in human esophageal precancerous lesions: a possible early biomarker for carcinogenesis. Cancer Res. 1993;53:1783-1787. [PubMed] |
| 10. | Wang LD, Shi ST, Zhou Q, Goldstein S, Hong JY, Shao P, Qiu SL, Yang CS. Changes in p53 and cyclin D1 protein levels and cell proliferation in different stages of human esophageal and gastric-cardia carcinogenesis. Int J Cancer. 1994;59:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Gao H, Wang LD, Zhou Q, Hong JY, Huang TY, Yang CS. p53 tumor suppressor gene mutation in early esophageal precancerous lesions and carcinoma among high-risk populations in Henan, China. Cancer Res. 1994;54:4342-4346. [PubMed] |
| 12. | Wang LD, Zhou Q, Hong JY, Qiu SL, Yang CS. p53 protein accumulation and gene mutations in multifocal esophageal precancerous lesions from symptom free subjects in a high incidence area for esophageal carcinoma in Henan, China. Cancer. 1996;77:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Shi ST, Feng B, Yang GY, Wang LD, Yang CS. Immunohistoselective sequencing (IHSS) of p53 tumor suppressor gene in human oesophageal precancerous lesions. Carcinogenesis. 1996;17:2131-2136. [PubMed] |
| 14. | Wang LD, Qiu SL, Yang GR, Lipkin M, Newmark HL, Yang CS. A randomized double-blind intervention study on the effect of calcium supplementation on esophageal precancerous lesions in a high-risk population in China. Cancer Epidemiol Biomarkers Prev. 1993;2:71-78. [PubMed] |