Published online Jun 15, 1997. doi: 10.3748/wjg.v3.i2.104
Revised: January 31, 1997
Accepted: March 1, 1997
Published online: June 15, 1997
AIM: To study the effects of hepatic arterial infusion chemotherapy (HAI) or embolization (HAE) in the treatment of primary hepatic carcinoma (PHC) and the factors influencing these effects.
METHODS: A retrospective analysis of 188 patients (166 males and 22 females) with PHC treated with HAI (n = 82) or HAE (n = 106) was conducted.
RESULTS: In the group as a whole, the percentage of patients experiencing therapeutic outcomes was as follows: Symptomatic relief (59.6%); Tumor shrinkage (55%); A decrease in blood alpha fetoprotein (AFP) (37.8%) and overall survival rates at 0.5, 1.0, 2.0, and 3.0 years of 75.4%, 46%, 23.5% and 14.7%, respectively. The mean survival time in the entire group was 12.2 mo, and the longest survival period was 50 mo. In the HAI group the survival rates at 0.5, 1.0. 2.0 and 3.0 years of follow-up were 61.0%, 25.4%, 4.5% and 0%, respectively, with a mean survival time of 7.7 mo. In the HAE group the corresponding survival rates were 86.8%, 61.7%, 37.8% and 26.1%, respectively, with a mean survival time of 15.7 mo. Eighteen patients received secondary surgery. Factors that had a favorable effect on therapeutic outcomes were presence of no or only mild liver cirrhosis, presence of stage I or Stage II disease, presence of only a single tumor, a tumor size less than 10 cm in diameter, an absence cancerous thrombus within the portal vein or hepatic arterio venous fistula, and HAE treatment.
CONCLUSION: This study helped identify the effects of HAI and HAE in patients with PHC, and identified some important factors which influence such treatment.
- Citation: Zheng CS, Feng GS, Zhou RM, Liang B, Liang HM, Zhen J, Yu JM, Liu H. Hepatic arterial infusion chemotherapy and embolization in the treatment of primary hepatic carcinoma. World J Gastroenterol 1997; 3(2): 104-107
- URL: https://www.wjgnet.com/1007-9327/full/v3/i2/104.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i2.104
Treatment options for patients with primary hepatic carcinoma (PHC) include hepatic arterial infusion chemotherapy (HAI) or embolization (HAE). In this study, we investigated outcomes in PHC patients treated with HAI and/or HAE. We also investigated factors that influence the efficacy of HAI and HAI in the treatment PHC patients.
Between May 1990 and February 1994, we recruited 388 patients with PHC who were not fit for surgical resection and we instead treated with HAI and HAE. Of these 388 patients, follow-up information was available for 188 patients, and these 188 patients formed the patient group for this study. One hundred and eighty eight patients with PHC were clinically diagnosed. They included 166 males and 22 females. Their ages ranged from 21 to 70 years with a mean age of 47 years. The sizes of the tumors ranged from 4 to 25 cm in diameter with a mean diameter of 11.2 cm. 170 patients had liver cirrhosis. After interventional treatment, 18 patients obtained the chance to receive the secondary surgery, the resected specimens were confirmed to be hepatocellular carcinoma pathologically. At the first visit to a doctor, no patient had indication for surgical operation and they were treated with HAI or HAE.
After puncturing the femoral artery percutaneously by Seldinger′s method, celiac or common hepatic arteriography was initially performed in each patient. After angiography, the catheter was superselectively inserted into the blood supplying artery of the tumor for HAI or HAE (the success rate was 87%). Common chemotherapeutic drugs that were infused included 500 mg of carboplatin (CAP), 1000 mg of 5-Fu and 10-20 mg of mitomycin C (MMC). The emulsion of 10-20 mL 40% Lipiodol (Lip) mixed with MMC was used for peripheral angioembolization. Gelatin sponges (GS) or Bletilla Striata (BS) powders (in sizes of 1-2 mm3), which were mixed with 5 mL of 60% Meglumini Diatrizoatis composita (a contrast medium) before injection, was used for proximal angioembolization. The BS powders were a self prepared embolizing agent[1].
After HAI or HAE treatment, changes in symptoms and signs, hepatic and renal function, and blood alpha fetoprotein (AFP) were observed. Liver ultrasonography (USG), computed tomography (CT), angiography, and other necessary examinations were performed regularly during follow-up.
Symptomatic relief was assessed in terms of improvement in appetite and body weight lasting for more than 2 mo. Tumor shrinkage was defined as a reduction in tumor size of more than 25% that lasted for at least two months, as measured by USG, CT, or angiography. A decrease on AFP decrease was defined as a reduction by more than 25%, or a normal AFP level, which lasted more than two months. After treatment, the survival rates at 0.5, 1, 2 and 3 years, as well as the mean survival time, were calculated according to the life table method[2]. Factors potentially influencing HAI and HAE treatment effects were assessed with Mann Whitney U test or Chi square test[3].
Of the 188 patients included in the study, 166 were males and 22 were females. Their ages ranged from 21 to 70 years with a mean age of 47 years. Tumors size ranged from 4 to 25 cm in diameter with a mean diameter of 11.2 cm. A total of 170 patients had liver cirrhosis. After HAI or HAE treatment, 18 patients obtained the chance to receive the secondary surgery, the resected specimens were confirmed to be hepatocellular carcinoma pathologically.
The findings of the angiography for PHC are shown in Table 1. The majority of PHC cases showed a considerable number of tumor vessels and a substantial degree of tumor stain. The nodulous type of PHC primarily showed increased tumor vessels and stain, while the massive type of PHC was marked by increased tumor vessels, displacement of the arteries, and vascular encasement. The diffusive type of PHC primarily showed tumor stain, pool or lake-like collections of contrast medium, and only rarely showed displacement of the arteries.
| Findings | Cases | % |
| Tumor stain | 186 | 98.9 |
| Tumor vessels | 180 | 95.7 |
| Displacement and distortion of arteries | 128 | 68.1 |
| Pool or lake-like collections | 114 | 60.6 |
| Vascular encasement | 106 | 56.4 |
| Tumor thrombus within portal vein | 96 | 51.1 |
| Extrahepatic metastases | 32 | 17.0 |
| Arteriovenous fistula | 24 | 12.8 |
In the entire patient group, 33.5% had tumor shrinking rates of 75% or more, 44% of the patients had a tumor shrinking rate of 50% or more, 55% of patients had a shrinkage rate of 25% or more, and six patients showed no evidence of In 12 patients, tumor size did not change after treatment, and tumor size increased in eight patients post-treatment. The tumor shrinking rate was significantly higher in the HAE group than in the HAI group (Table 2) (P < 0.01).
| Group | n | Tumor shrinking rate | No change | Enlargement | ||
| ≥ 50% | 25%-50% | < 25% | ||||
| HAI | 80 | 2 | 8 | 59 | 7 | 4 |
| HAE | 102 | 78 | 12 | 3 | 5 | 4 |
After treatment, there was symptomatic relief in 59.6% of the patients) and a decrease in blood AFP in 37.8% of patients. The survival rates at 0.5, 1, 2, and 3 years were 75.4%, 46%, 23.5% and 14.7%, respectively, with a mean survival time of 12.2 mo and a maximum survival period of 50 mo.
The survival rates were higher in the HAE group than those in the HAI group (Table 3). The mean survival time was also longer in the HAE group, of which the HAE-Lp-BS group showed better effects than the HAE-Lp-GS group.
| Methods of treatment | n | Survival rates (%) | Mean survival time (mo) | |||
| 6 mo | 12 mo | 24 mo | 36 mo | |||
| HAI | 82 | 61.0 | 25.4 | 4.5 | 0 | 7.7 |
| HAE | 106 | 86.8a | 61.7 | 37.8 | 26.1 | 15.7 |
| HAE-Lp-GS | 50 | 80.0 | 48.9 | 31.1 | 0 | 11.1 |
| HAE-Lp-BS | 56 | 92.9b | 81.9 | 44.9c | 33.6 | 19.8 |
Survival rates according to various factors are shown in Table 4. Survival rates in patients with stage 1 PHC were high. There were no significant differences in survival rates between the nodulous and the massive types of PHC (P > 0.05). However, among patients with the nodulous type of PHC, survival rates in patients with multinodulous type PHC were lower compared to survival rates for patients with massive type of PHC (P < 0.01).
| Factors | n | Survival rate (%) | |||
| 6 mo | 12 mo | 24 mo | 36 mo | ||
| Clinical stages | |||||
| II | 112 | 88 | 60 | 33 | 19 |
| III | 68 | 53 | 17 | 0 | |
| Pathological types | |||||
| Nodulous | 28 | 83 | 53 | 24 | 19 |
| Massive | 128 | 77 | 48 | 27 | 15 |
| Diffusive | 32 | 56 | 23 | 0 | |
| Mass diameter (cm) | |||||
| < 5 | 10 | 100 | 100 | 62 | 54 |
| 5-10 | 78 | 85 | 61 | 32 | 18 |
| ≥ 10 | 100 | 66 | 29 | 11 | 0 |
| Treatment time | |||||
| 1 | 58 | 45 | 0 | ||
| 2 | 48 | 79 | 48 | 23 | 0 |
| > 2 | 82 | 95 | 78 | 39 | 26 |
| Tumor thrombi | |||||
| (+) | 96 | 65 | 28 | 3 | 0 |
| (-) | 92 | 87 | 65 | 39 | 23 |
| Liver cirrhosis | |||||
| None or mild | 109 | 87 | 68 | 39 | 24 |
| Moderate or severe | 79 | 39 | 18 | 4 | 0 |
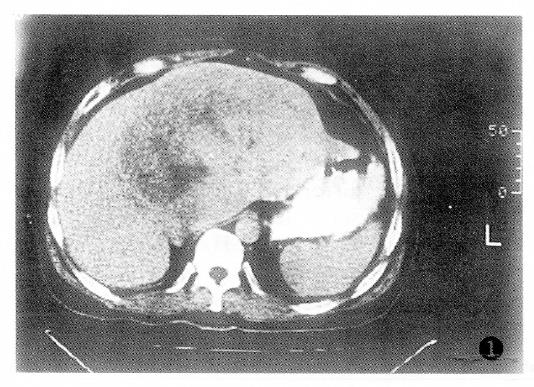
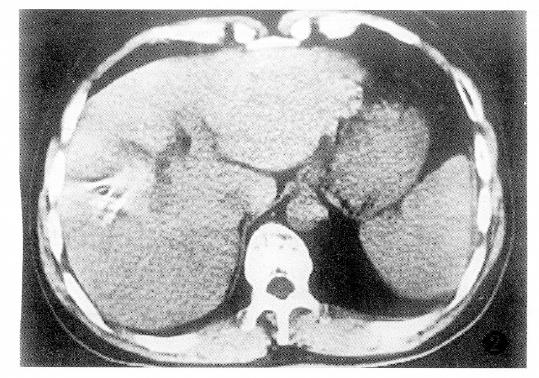
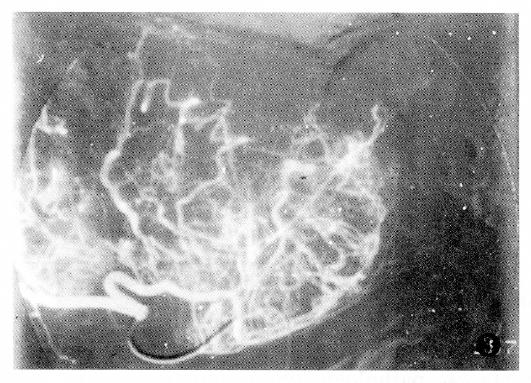
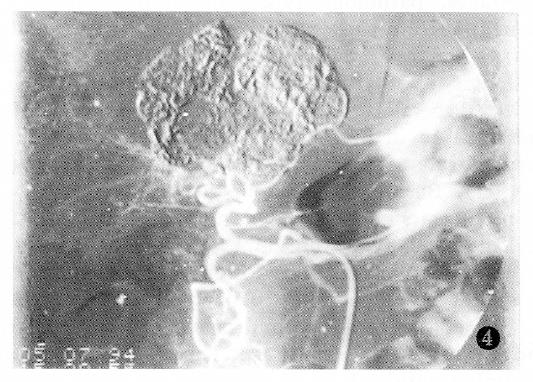
Additionally, PHC with tumor thrombus within the portal vein or the PHC associated with liver cirrhosis had poor prognosis (Table 4).The factor having the biggest effect on outcomes was treatment method (Figure 1, Figure 2, Figure 3 and Figure 4).
After HAE, 18 patients with obvious tumor shrinkage and favorable physical conditions were eligible for secondary surgical resections. Among them, 15 patients are still alive today. Pathologically, most of the resected specimens were defined clearly, with integrated encapsulation in 12 tumors. In the areas of the tumors, the tumor cells had complete or partial coagulative necrosis, with a few inflammatory cells and some extent of proliferation of fibrous tissue, and some amount of remaining tumor cells at the surrounding tumor sites.
After HAI or HAE, common symptoms were nausea, vomiting, liver site pain, and fever. The majority of patients developed fever of 38-39 °C, and a few patients had fever of 39.5-40 °C. The fever lasted 5-20 d and patients recovered in a mean period of two weeks. Other symptoms disappeared gradually, 3-7 d after treatment. Complications include the occurrence of hematoma at the punctured site (5 cases), gastric ulcer (2 cases) and acute failure of liver function (2 cases).
Generally, the natural survival time of patients with PHC is 1-4 mo and the majority of PHC patients may not be fit for surgical resection[4]. HAI and HAE may prolong the survival of patients with PHC and make it possible for some patients to become fit for secondary surgical resection.[5]. The effect of HAI is short, but HAE can produce a more substantial necrosis of tumors, thereby leading to a a more favorable outcome. Nishufu and Soge reported that one, two and three years survival rates in 523 PHC patients treated with HAE-Lp-GS were 60.4%, 42.9% and 28%, respectively[6], whereas in the Okuda and Tang′s study the corresponding rates for PHC patients treated with HAI were 8.8%-22%, 0.0%-8.9% and 0%, respectively[7].
The reason why the efficacy was better in this study may have been that many patients were in early or intermediate stage PHC (63.8%) and were treated with the superselective embolization of the hepatic segmental arteries. Additionally, in this study, the indications for treatment of HAI and HAE were strict. The following conditions are considered as contraindications. (1) serum AST and/or ALT levels more than twice of the normal upper limit value, with total serum bilirubin more than 25.7 μmol. (2) serum AST and/or ALT levels greater than four times of the normal upper limit value. (3) Total serum bilirubin levels more than 51.3 μmol. (4) A complete or partial blockage of the, but HAI might be still performed. And (5) the presence of obvious ascites.
We found several factors influenced the effects of treatment. First the prognoses of the single nodulous and the massive types of PHC were better than those of the multi nodulous and the diffusive types. This might be due to the fact that the former two types of PHC had plenty of tumor vessels, encapsulations, less diffusion and obvious tumor necrosis after HAI or HAE. In contrast, the latter two types of PHC were often complicated with serious liver cirrhosis and poor liver function, had tumor thrombus within the portal vein, often had extrahepatic metastasis,, and were not suitable to be treated with HAE[8].Second we also found that stages I and II PHCs had better treatment effects than stage III PHCs, especially with respect to long-term effects. Recently, it was believed that in stage I PHCs, surgical resection might be the first choice of treatment, for the interventional treatment could hardly raise the survival rate of stage I PHC in view of the fact that HAI and HAE could injure the normal liver parenchyma and thereby counteract their anticarcinogenic effects[7]. Stages II and III PHCs were considered fit for interventional treatment, but most patients with stage III-PHC were not sufficiently fit for HAE treatment and thus, the therapeutic effects were poor. Third, the effects of PHCs with no or mild liver cirrhosis were better than those with moderate or serious liver cirrhosis. PHC was often complicated with liver cirrhosis, which gradually worsened along with PHC progression. Because serious liver cirrhosis influenced surgical resection, HAI, and HAE, the PHCs with serious liver cirrhosis after any of the treatments only yielded low survival rate s. Liver transplantation[7] might better suit these patients.
Fourth, tumor thrombus within the portal vein or arterio venous fistula unfavorably influenced the prognosis of PHC. On the one hand, the tumor thrombus easily produced intrahepatic diffusion and/or extrahepatic metastasis; on the other hand, it blocked the portal vein and produced portal hypertension which in the long run could cause the patient′s death from a massive hemorrhage of the upper digestive tract. When the tumor thrombus blocks the portal vein by by more than 60%, or a large arterio venous fistula occurs, HAE should not be performed in order to avoid liver failure and pulmonary infarction[8].
Currently, three methods of HAI and HAE are commonly used: (1) single HAI, (2) HAI combined with HAE-Lp or HAE-GS, and (3) HAI combined with HAE-Lp-GS. Prior literature and the results of this study reveal that among the three above-mentioned methods, HAI combined with HAE-Lp-GS yields the best effect in treatment of PHC. This method not only kept a local higher concentration of anticarcinogen to kill the tumor cells in the tumor area, but also blocked the peripheral and proximal blood supply of the arteries of the tumor to promote its ischemic necrosis[5]. In the HAE group in this study, treatment with HAE-Lp-BS in 5-6 patients showed the best therapeutic effects, in which the Bletilla Striata powder, a traditional Chinese medicine, was used. This is a new type of drug prepared by us for intravascular embolization. It has coagulant, antibiotic, and antineoplastic function, which can accomplish a persistent embolization of the main trunk of the arterial blood supply of the tumor, so as to cause long term ischemic necrosis of the tumor and less collateral circulation[9]. This prolongs the intermission of the patient′s treatment, and lessened symptoms and complications of the patients. As such, in patients with PHC, treatment with HAE-Lp-BS may be preferable to treatment with HAE-Lp-GS.
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Liu WX
| 1. | Feng GS, Yan XQ, Wang LY. Animal experiment and clinical application of Bletilla Striata as an embolizing agent. Zhonghua Fangshe Yixue Zazhi. 1985;19:193-196. |
| 2. | Geng GY. Epidemiology. 2 nd Editor. Beijing: The People's Health Publishing House 1984; 203-206. |
| 3. | Lin QF, Liu XX, Liang HC. Environmental medical statistics. 1st Editor. Beijing: The People's Health Publishing House 1989; 147-153. |
| 4. | Bartolozzi C, Lencioni R, Caramella D, Vignali C, Cioni R, Mazzeo S, Carrai M, Maltinti G, Capria A, Conte PF. Treatment of large HCC: transcatheter arterial chemoembolization combined with percutaneous ethanol injection versus repeated transcatheter arterial chemoembolization. Radiology. 1995;197:812-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 127] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Chang JM, Tzeng WS, Pan HB, Yang CF, Lai KH. Transcatheter arterial embolization with or without cisplatin treatment of hepatocellular carcinoma. A randomized controlled study. Cancer. 1994;74:2449-2453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Nishufu U, Soge Y. Recent status of interventional radiology. J Clin Surg (in Japanese). 1987;42:1623-1625. |
| 7. | Okuda K, Tang ZY. Neoplasms of the liver. 1st Editor. Tokyo: Springer Pub. Co 1987; 32-34, 327-329. [DOI] [Full Text] |
| 8. | Chung JW, Park JH, Han JK, Choi BI, Han MC, Lee HS, Kim CY. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology. 1996;198:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 259] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Zheng C, Feng G, Zhou R. [New use of Bletilla striata as embolizing agent in the intervention treatment of hepatic carcinoma]. Zhonghua Zhongliu Zazhi. 1996;18:305-307. [PubMed] |