Published online Jun 15, 1997. doi: 10.3748/wjg.v3.i2.101
Revised: January 31, 1997
Accepted: March 1, 1997
Published online: June 15, 1997
AIM: To investigate the expression levels of proliferating cell nuclear antigen (PCNA) and its correlation with the mitotic count, histological grade, and metastasis of human hepatocellular carcinoma (HCC).
METHODS: PCNA expression was detected immunohistochemically with PC10 monoclonal antibody for 80 cases of HCC. The PCNA labeling index (LI) was assessed by counting the positively-stained nuclei per 500 cells.
RESULTS: PCNA LI ranged from 1.8% to 91.4% (mean = 33.9%) in cancerous tissues, and was significantly related to the tumor size, histological grade, mitotic count and the metastases found in HCC samples.
CONCLUSION: HCC Proliferation activity, as defined by PCNA immunohistochemical analysis, is strongly correlated with tumor invasiveness. These findings hold potential prognostic value for HCC patients.
- Citation: Wang D, Shi JQ, Liu FX. Immunohistochemical detection of proliferating cell nuclear antigen in hepatocellular carcinoma. World J Gastroenterol 1997; 3(2): 101-103
- URL: https://www.wjgnet.com/1007-9327/full/v3/i2/101.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i2.101
Proliferating cell nuclear antigen (PCNA), also known as cyclin, is a 36 KDa nuclear protein and has been recognized as an auxiliary protein for DNA polymerase delta[1]. PCNA is believed to play an essential role in regulating both DNA synthesis and cell proliferation[2]. PCNA expression peaks at G1/S of interphase, and then drops during mitosis to a level that is too low to be detected using the immunohistochemical method. Thus, PCNA serves as an endogenous histologic marker for the G1/S phases of the cell cycle. In recent years, studies have revealed a significant relationship between PCNA expression and both the mitotic activity and grading in solid tumors[3]. Proliferation activity, defined by PCNA immunohistochemistry, is a useful signature in a variety of malignant tumors, including gastric carcinomas[4], gastrointestinal lymphomas[5] and colorectal cancers[6]. The purpose of this study is to measure the PCNA expression levels in 80 cases of human hepatocellular carcinoma (HCC) to determine whether there is a correlation between PCNA expression and HCC histological grade, mitotic count and metastasis.
We obtained cancerous and juxta cancerous tissues, which were surgically resected from 80 HCC patients at the Southwest Hospital of our university from 1988 and 1993. All specimens were fixed in 10% formaldehyde solution and embedded in paraffin. Sections 5 μm thick stained with H and E and antibodies. Tumors were divided into three groups according to size: (1) 11 cases of single tumors with a diameter ≤ 3 cm; (2) 19 cases of single tumors with a diameter between 3 cm-5 cm; and (3) 50 cases of single tumors with a diameter ≥ 5 cm or with multiple tumors. Intrahepatic and extrahepatic metastases were discovered during surgery in 29 cases and one case, respectively. According to the WHO classification, 23 cases were of the trabecular type, 31 of the pseudoglandular type, 22 of the compact type and four of the sclerosing type. The differentiation degree was categorized into four groups according to Edmondson and Steiner’s grading: Grade I in four cases (5.0%), grade II in 33 (41.3%), grade III in 27 (33.8%) and grade IV in 16 (20.0%). The number of mitotic cancer or liver cells in ten high power fields (lens objective, × 40) were randomly counted three times. Among them, the mitotic numbers for the most mitotically active areas was referred to as the mitotic count (MC). The result was divided into three groups: 0-4, 5-9, and 10 and over.
The PC10 monoclonal antibody to PCNA, a donation from Dr. D.P. Lane, Dundee, United Kingdom, was used at a dilution of 1:100 and immunohistochemical staining was performed using the ABC method. Negative control sections were incubated with PBS instead of the primary antibody.
PCNA staining was confined to the nuclei. Although the nuclear staining varied in intensity, all identifiable staining was considered positive. Sections were counted at high magnification (× 400), and 5.8 fields were randomly assessed for tumor or nontumor liver cells. Five hundred cell nuclei of each cell type were found and the PCNA labeling index (LI, %) was determined.
The significant differences between the distinct PCNA LI groups was assessed using the PDA-2 statistical package provided by our university. P < 0.05 was considered statistically significant.
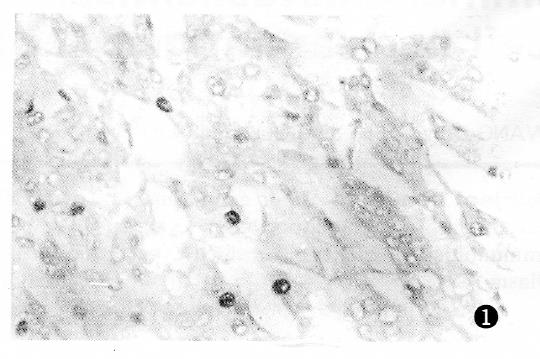
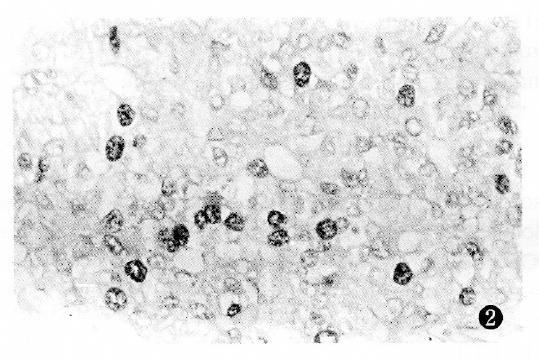
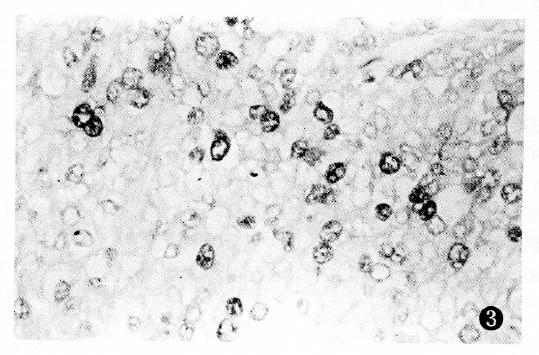
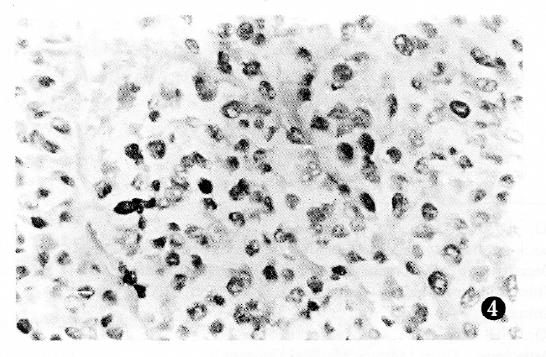
PCNA antigen was detected exclusively in the nuclei of liver and cancer cells, however no staining was detected in the cytoplasm. In non-cancerous tissues, including chronic hepatitis and nodular cirrhosis, a few PCNA-positive cells were also found and distributed in a single or focal pattern, however no reaction was observed in the normal liver tissue adjacent to the tumor. The PCNA LI was 0%-12.3% in the surrounding cancer tissues, and 1.8%-91.4% (mean 33.9%) in the cancerous tissues. The distribution of PCNA immunohistochemical reactivity within tumors appeared scattered, patchy, and diffuse. The number of PCNA-positive cells varied with the degree of HCC, with a higher tumor grading correlating with a higher PCNA LI (Figure 1, Figure 2, Figure 3 and Figure 4). Meanwhile, PCNA-positive nuclei were numerous in the tumor cells adjacent to the surrounding cancer tissues, tumor thrombi and areas of extracapsular tumor growth.
The relationship between PCNA LI and the pathological tumor features of PCNA LI was 22.5% in HCC with a diameter < 3 cm, 30.9% in HCC with diameters between 3 cm and 5 cm, and 37.4% in HCC with a diameter > 5 cm. There was significant difference between PCNA LI in HCC with a diameter < 3 cm and PCNA LI in HCC with a diameter > 5 cm. The histologic grading of HCC in PCNA LI is shown in Table 1. The differences between PCNA positivity and tumor histological grade were statistically significant.
| Histologic grading of hepatocellular carcinoma | Number of cases | Positive (¯x ± s) | Range (%) |
| Edmondson I | 4 | 4.3 ± 2.6 | 1.8-8.0 |
| Edmondson II | 33 | 20.4 ± 10.2 | 10.7-48.3 |
| Edmondson III | 27 | 42.1 ± 14.6 | 18.4-63.7 |
| Edmondson IV | 16 | 55.1 ± 17.3 | 34.6-91.9 |
PCNA LI was 18.6%, 42.4% and 55.1% in the three MC groups, respectively, and the differences were significant (P < 0.01). This indicated that there was a close relationship between PCNA LI and MC.
PCNA LI was 44.8% in 30 cases of metastatic HCC, and 29.4% in 50 cases of HCC without metastasis (P < 0.05). There was a significant correlation between PCNA LI and the rate of tumor metastasis, while no significant correlation was found between PCNA LI and HBV infection, nodular cirrhosis, or tumor classification.
The proliferative activity of tumor cells depends primarily on the malignant severity of the host tumor. We employed several in situ methods, including morphometric image analysis and immunohistochemical staining for both BrdU and argyrophil to determine cell proliferation levels, which is the “histologic gold standard”. Heratake et al[7] studied 140 HCC cases with hepatic resection, and noted that, among various clinicopathologic factors, the mitotic indices were well correlated with prognosis. This suggested that the mitotic count was accurate and reliable in predicting prognosis. Unfortunately, mitotic count can be affected by tissue fixation, the microscopy field size as well as other factors, and can therefore lead to irreproducibility and inaccurate conclusions.
Compared with the mitotic counts, PCNA immunohistochemical staining has proven to be more effective and reliable. In general, cell proliferation significantly contributed to the degree of malignancy where the less differentiated cells showed a stronger ability to proliferate, thus enhancing tumor malignancy. These studies reveal that PCNA expression is significantly related to the degree of HCC differentiation and that numerous PCNA-positive nuclei were particularly observed in tumor thrombi and areas of extracapsular tumor growth. A significant correlation was also established between PCNA LI and tumor size, histological grade, tumor metastasis, and mitotic count, which is consistent with results previously reported by Taniai et al[8]. Additionally, Ng et al[9] studied 72 patients with surgically resected HCC and found that those with a PCNA less than or equal to 200 showed significantly greater disease-free survival rates than those with scores greater than 200, therefore confirming the important role of PCNA immunohistochemical staining in predicting HCC prognosis. In conclusion, these data suggest that PCNA staining is a reliable metric of HCC tumor proliferation. This, in turn, establishes a very valuable method for not only evaluating tumor invasion and metastasis, but also predicting HCC patient prognosis.
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Liu WX
| 1. | Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987;326:515-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1328] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 2. | Takasaki Y, Deng JS, Tan EM. A nuclear antigen associated with cell proliferation and blast transformation. J Exp Med. 1981;154:1899-1909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 283] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Robbins BA, de la Vega D, Ogata K, Tan EM, Nakamura RM. Immunohistochemical detection of proliferating cell nuclear antigen in solid human malignancies. Arch Pathol Lab Med. 1987;111:841-845. [PubMed] |
| 4. | Jain S, Filipe MI, Hall PA, Waseem N, Lane DP, Levison DA. Prognostic value of proliferating cell nuclear antigen in gastric carcinoma. J Clin Pathol. 1991;44:655-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 145] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Woods AL, Hall PA, Shepherd NA, Hanby AM, Waseem NH, Lane DP, Levison DA. The assessment of proliferating cell nuclear antigen (PCNA) immunostaining in primary gastrointestinal lymphomas and its relationship to histological grade, S+G2+M phase fraction (flow cytometric analysis) and prognosis. Histopathology. 1991;19:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 168] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | al-Sheneber IF, Shibata HR, Sampalis J, Jothy S. Prognostic significance of proliferating cell nuclear antigen expression in colorectal cancer. Cancer. 1993;71:1954-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Haratake J, Takeda S, Kasai T, Nakano S, Tokui N. Predictable factors for estimating prognosis of patients after resection of hepatocellular carcinoma. Cancer. 1993;72:1178-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Taniai M, Tomimatsu M, Okuda H, Saito A, Obata H. Immunohistochemical detection of proliferating cell nuclear antigen in hepatocellular carcinoma: relationship to histological grade. J Gastroenterol Hepatol. 1993;8:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Ng IO, Lai EC, Fan ST, Ng M, Chan AS, So MK. Prognostic significance of proliferating cell nuclear antigen expression in hepatocellular carcinoma. Cancer. 1994;73:2268-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |