Published online Jan 28, 2023. doi: 10.3748/wjg.v29.i4.731
Peer-review started: October 16, 2022
First decision: November 17, 2022
Revised: November 27, 2022
Accepted: January 9, 2023
Article in press: January 9, 2023
Published online: January 28, 2023
Processing time: 96 Days and 3.8 Hours
Large or transmural defects induced by gastrointestinal endoscopic manipulations are difficult to close, although complete closure is recommended for better recovery. Endoscopic purse-string assisted suturing (EPSS) has been used in clinical practice and has proven to be an effective and safe technique for the closure of large mucosal defects. However, details regarding the efficacy of endoscopic pre-purse-string suture (P-EPSS) are unknown, especially that it offers several advantages over conventional EPSS (C-EPSS).
To elucidate the outcomes of EPSS-assisted closure in different clinical situations, and evaluate the efficacy of P-EPSS.
This retrospective observational study included a total of 180 patients who underwent closure assisted by P-EPSS (n = 63) or C-EPSS (n = 117) between July 2014 and June 2020. The P-EPSS and C-EPSS groups were compared and the intergroup differences in aspects such as the lesion size, location, and mor-phology, incidence of complete closure, intraoperative perforation, and delayed adverse events were evaluated. Data on the features and clinical course of cases with adverse events were collected for further analysis.
Patients with lesion size larger than 3 cm, lesions located at the fundus of stomach, or submucosal tumors originating from the deep mucosa were more likely to undergo P-EPSS-assisted closure. The P-EPSS group showed a sign-ificantly higher proportion of intraoperative perforation (56% vs 17%) and a much shorter procedure time (9.06 ± 6.14 min vs 14.84 ± 7.25 min). Among adverse events, the incidence of delayed perforation (5% vs 4%; P = 0.82) and delayed bleeding (3% vs 4%; P = 0.96) did not differ significantly between the groups. Multivariate analysis revealed that lesions with incomplete closure [odds ratio (OR) = 21.33; 95% confidence interval (CI): 5.45-83.45; P < 0.01] or size greater than 3 cm (OR = 3.14; 95%CI: 1.08-9.18; P = 0.039) showed a statistical tendency to result in an increase in delayed adverse events.
The present study revealed that EPSS could achieve secure complete closure of mucosal defect. P-EPSS could shorten the procedure and yield complete closure of mucosal defects. Rather than closure-type selection, incomplete closure or lesion size larger than 3 cm were associated with worse outcomes.
Core Tip: Endoscopic purse-string assisted suturing (EPSS) has proven to be an effective and safe technique for the closure of large mucosal defects. Endoscopic pre-purse-string suture (P-EPSS) is recently introduced and offers several advantages over conventional endoscopic purse-string suture (C-EPSS). We found that the novel method could offer several advantages over C-EPSS. This retrospective observational study included a total of 180 patients who underwent P-EPSS (n = 63) or C-EPSS (n = 117), and evaluate the feasibility and efficacy of P-EPSS-assisted closure in different clinical situations. In conclusion, EPSS could achieve secure complete closure of mucosal defect. P-EPSS could shorten the procedure and yield complete closure of mucosal defects rather than the C-EPSS closure-type.
- Citation: Li MM, Zhang Y, Sun F, Huai MX, Zhang FY, Qu CY, Shen F, Li ZH, Xu LM. Feasibility and efficacy of endoscopic purse-string suture-assisted closure for mucosal defects induced by endoscopic manipulations. World J Gastroenterol 2023; 29(4): 731-743
- URL: https://www.wjgnet.com/1007-9327/full/v29/i4/731.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i4.731
The endoscopic purse-string assisted suturing (EPSS) method has been used in clinical practice for nearly 20 years and has proven to be an effective and safe technique for the closure of large mucosal defects induced by endoscopic manipulations[1-5]. Inspired by the surgical purse-string suture strategy, an endoloop and repositionable clips have been successfully combined to manage intraluminal wounds via an endoscope[6-10]. However, some defects are difficult to resect and are expected to induce perforation during endoscopic manipulations, necessitating the application of an endoscopic pre-purse-string suture (P-EPSS) procedure. This novel suture method was first described by Wu et al[11] and was usually applied in cases involving exposed endoscopic full-thickness resections (EFTRs). At our medical center, we expanded the indications for P-EPSS and attempted to use it in more procedures such as endoscopic submucosal dissection (ESD) and endoscopic submucosal excavation (ESE) of big defects, potentially simplifying the process of closure and avoiding the possibility of postoperative adverse events.
To our knowledge, no previous reports have described the advantages of P-EPSS over conventional EPSS (C-EPSS). Furthermore, previous clinical studies on the application of EPSS usually had small sample sizes and mainly focused on providing detailed descriptions of endoscopic procedures rather than assessing the feasibility and efficacy of the technique[12-15]. Therefore, we conducted a large-scale analysis of case series focusing on the effectiveness and safety of EPSS. We compared the differences between the P-EPSS and C-EPSS group in many aspects and tried to illustrate the considerations involved in choosing the appropriate closure type according to the defect’s clinical characteristics.
This was a retrospective observational study performed in accordance with the Helsinki Declaration. It was approved by the Ethics Committee at Xinhua Hospital (approval number: XHEC-C-2018-109), Shanghai Jiaotong University School of Medicine. Written informed consent for the procedures was obtained from all patients.
A total of 180 consecutive patients with large mucosal defects who underwent C-EPSS or P-EPSS at our institute from July 2014 to June 2020 were included in the study. We compared the findings between the P-EPSS group (n = 63) and C-EPSS group (n = 117) for further analysis. All patients had undergone different endoscopic manipulations (ESD, ESE, or exposed EFTR), and the endoscopic suture method was applied to close large mucosal defects with or without transmural defects. All the procedures were completed by four chief physicians who had more than 10 years of experience in performing endoscopic operations. The patient database included data pertaining to patient demographics, location and size of the mucosal defects after resection, procedure time, successful closure rate, delayed adverse events, duration of hospitalization, and total cost of hospitalization.
The endoscopic manipulation always started with the mucosa marking procedure, in which markings were placed 3 to 5 mm outside the circumference of the lesion. After injection of epinephrine diluted in saline solution (1:100000) into the submucosal layer to raise the submucosa, endoscopists performed the resection procedure. The C-EPSS procedure is a common method used for closure of defects. After dissection of precancerous lesions or tumor specimens, an endoloop and clips were inserted simultaneously into the location of defect through the therapeutic endoscope for complete closure. Single-channel or double-channel endoscopy was used according to the specific defect status. The endoloop was anchored onto the full thickness of the defect’s distal margin with the clip, followed by insertion of three to six additional clips to anchor the endoloop at different sides of the margin. Finally, the endoloop was tightened by slightly pulling all the edges together. Other clips were used if any clip was not accurately positioned or the purse-string suture was not tight.
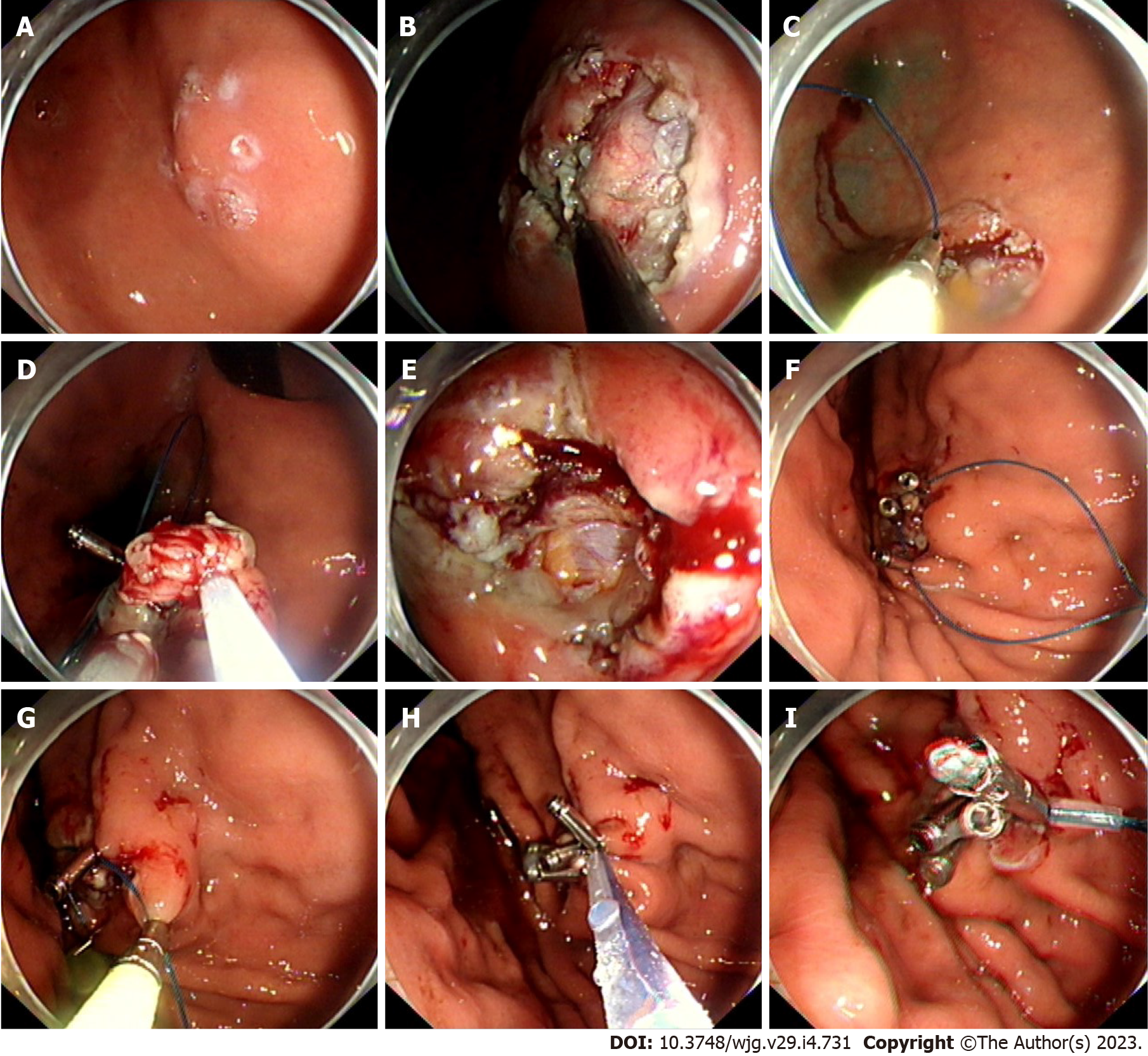
The P-EPSS procedure was always used when defects were difficult to resect and expected to induce perforation. In these cases, to reduce difficulties in manipulation in the suturing process, decrease the entry of gas into the abdominal cavity, and avoid postoperative adverse events, the endoloop and clips were advanced around the defect before the end of the dissection phase; this procedure was routinely performed with one or two clips. The remaining clips were anchored around the defects when the lesions were resected. Tightening of the endoloop was immediately performed after placement of the clips. This procedure was named P-EPSS. An example of the closure procedure is shown in Figures 1A-I and the Video(Supplementary material).
The procedure time of the P-EPSS-assisted closure was defined as the time from the usage of the first clip in the operation to completion of the closure, but the time required for the resection procedure was excluded (as shown in Figures 1C and 1F-I). Exclusion of the time required for the resection procedure was considered appropriate due to the differences in the difficulty levels in the operation.
After the manipulations, the patients received drug therapy and were fasted for 2-4 d; body temperature was monitored daily, and postoperative adverse events were recorded as required. After evaluating the results of blood examinations and abdominal radiographs, the patients were allowed to drink, which was followed by a liquid diet. Proton pump inhibitors (rabeprazole 20 mg/d, lansoprazole 30 mg/d, or esomeprazole 20 mg/d) were administered for 1 mo if the patients had undergone upper gast-rointestinal (GI) tract surgeries. Prophylactic antibiotics were routinely used in patients who showed intraoperative perforation and in patients with elevated white blood cell counts. If the patients showed good outcomes, they were discharged after 3-5 d. The first clinical follow-up was performed 3-6 mo after the manipulation, and then annually after the first year. The follow-up assessments included evaluation of digestive symptoms and gastro/enteroscopy.
The success rate of complete closure, incidence of adverse events (delayed perforation and bleeding), procedure time, duration of hospitalization, and total costs were analysed as outcomes of EPSS-assisted closure of mucosal defects and compared them between the P-EPSS and C-EPSS groups. The definition of perforation required elucidation in advance and included intraoperative and delayed perforation. The former was defined as a perforation observed before the end of the endoscopic manipulation, indicating the formation of a transmural defect during the operation; the latter was defined as any perforation found thereafter. Delayed bleeding was defined as hematemesis or melena requiring endoscopic hemostasis after the procedure. We did not treat adverse events occurring during the endoscopic procedure, such as intraoperative perforation in exposed EFTR, as events; only delayed adverse events such as perforations or bleeding appearing after the end of closure were treated as events. We collected data on the features and clinical course of cases with adverse events, and analyzed associations between the unsatisfactory outcomes and defect features (complete closure or not, location, size, morphology, and closure type).
Categorical variables were analyzed using Pearson’s chi-squared test or Fisher’s exact test, as appropriate. For instance, as listed in Table 1, we used Pearson’s chi-squared test to analyze the predictors when using the P-EPSS method. Continuous variables were analyzed using the student t test. The data for duration and cost of hospitalization were analyzed and summarized in Table 2. P values < 0.05 were considered statistically significant. All data were analyzed using the SPSS for Windows statistical software package (version 22.0; SPSS, Chicago, IL, United States).
| Outcomes | Total | P-EPSS | C-EPSS | P value |
| Complete closure, n (%) | 169 (94) | 59 (94) | 110 (94) | 0.80 |
| Intraoperative perforation, n (%) | 55 (31) | 35 (56) | 20 (17) | < 0.011 |
| Delayed adverse events, n (%) | ||||
| Delayed perforation | 8 (4) | 3 (5) | 5 (4) | 0.82 |
| Delayed bleeding | 7 (4) | 2 (3) | 5 (4) | 0.96 |
| Operation time (min), mean ± SD | 12.82 ± 7.83 | 9.06 ± 6.14 | 14.84 ± 7.25 | < 0.011 |
| Fasting period (d), median (range) | 2 (1-5) | 2 (1-5) | 2 (1-5) | 0.88 |
| Hospital stay (d), median (range) | 6 (4-11) | 6 (4-10) | 6 (4-11) | 0.87 |
| Total cost (dollars), mean ± SD | 2481 ± 445 | 2448 ± 365 | 2498 ± 418 | 0.47 |
Patient details are described in Table 3. During the study period, 117 patients underwent C-EPSS, while 63 patients underwent P-EPSS for closure of defects induced by endoscopic manipulations. The ratio of different endoscopic manipulations (exposed EFTR:ESE:ESD) used in the study was approximately 1:1:3 (36:32:112), but this ratio showed no significant difference between the P-EPSS and C-EPSS groups (P > 0.05). In terms of the location, more than three-quarters of the defects were located in the stomach, especially in the part of the fundus (44%). The mean defect size was 2.5 ± 1.46 cm. The difference in defect size between the P-EPSS and C-EPSS groups was significant (3.6 ± 1.13 cm vs 2.5 ± 0.68 cm, P < 0.05). In terms of defect morphology, more than 70% of the defects were submucosal tumors; 42% were submucosal tumors (SMTs) located in the deep muscularis propria and 31% were superficial SMTs. The size and morphology of the defects in the two groups were different, and a detailed analysis of these characteristics is provided in the next section.
| Patients detail | Total (n = 180) | P-EPSS (n = 63) | C-EPSS (n = 117) | P value |
| Age (yr), mean (range) | 60 (38-78) | 62 (42-76) | 60 (38-78) | 0.19 |
| Sex, n (%) | 0.75 | |||
| Female | 112 (62) | 38 (60) | 74 (63) | |
| Male | 68 (38) | 25 (40) | 43 (37) | |
| Manipulation, n (%) | 0.40 | |||
| EFTR | 36 (20) | 15 (24) | 21 (18) | |
| ESE | 32 (18) | 13 (21) | 19 (16) | |
| ESD | 112 (62) | 35 (55) | 77 (66) | |
| Location, n(%) | 0.10 | |||
| Stomach | ||||
| Fundus | 79 (44) | 35 (56) | 44 (38) | |
| Body | 45 (25) | 11 (17) | 34 (29) | |
| Antrum | 22 (12) | 8 (13) | 14 (12) | |
| Colon and rectum | 34 (19) | 9 (14) | 25 (21) | |
| Defect size (cm), mean ± SD | 2.89 ± 1.46 | 3.6 ± 1.13 | 2.5 ± 0.68 | 0.021 |
| Morphology, n(%) | 0.011 | |||
| Deep SMT | 76 (42) | 36 (57) | 40 (34) | |
| Superficial SMT | 55 (31) | 15 (24) | 40 (34) | |
| Precancerous lesions | 49 (27) | 12 (19) | 37 (32) |
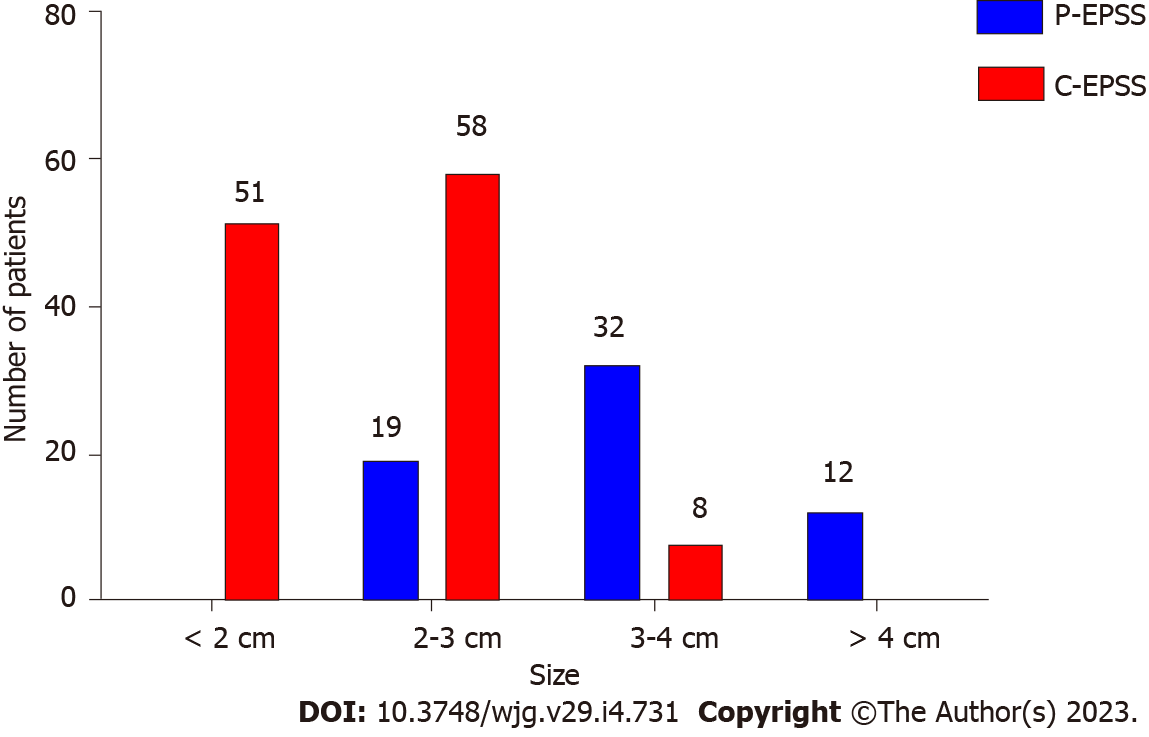
As mentioned above, many factors, including the defect’s position, size, and morphology, could indicate and predict the choice of the P-EPSS method. In terms of position, more than half (54%) of the defects in the P-EPSS group were located at the fundus of the stomach, which was much higher than the corresponding proportion in the C-EPSS group (38%). In terms of the size, the proportion of cases with defects larger than 3 cm in the P-EPSS group was significantly more than the proportion of cases with defects < 3 cm in size (P < 0.01), indicating a significant trend toward the use of P-EPSS to close large defects. We designed a histogram to illustrate the choice of closure type according to the defect size, as shown in Figure 2. In terms of the morphology, no significant increase was observed in the proportion of cases involving P-EPSS in relation to the morphology of SMTs (33% vs 41%, P > 0.05). The difference between the P-EPSS and C-EPSS groups was significant only for patients with SMTs originating from the muscularis propria (57% vs 34%, P < 0.01). In terms of differences in endoscopic manipulations, no significant increase was observed in the proportion of P-EPSS cases in the exposed EFTR group (42% vs 33%, P > 0.05).
Next, we performed multivariate analysis to identify predictors for P-EPSS. Defect location at the fundus of the stomach [odds ratio (OR) = 2.07; 95% confidence interval (CI): 1.11-3.86; P = 0.03], defect size larger than 3 cm (OR = 31.55; 95%CI: 12.86-77.40; P < 0.01), and SMTs originating from the deep mucosa (OR = 2.57; 95%CI: 1.37-4.81; P < 0.01) were significantly associated with a high rate of P-EPSS (Table 1).
Table 2 presents a comparison of treatment outcomes between the P-EPSS and C-EPSS groups. The proportion of complete closure assisted by purse-string sutures was 94% and was similar between the two groups. Patients with incomplete closure received conservative treatment and eventually recovered from the trauma. None of them required alternative surgical repair procedures. While more patients from the P-EPSS group were inclined to experience intraoperative perforation (56% vs 17%, P < 0.01), the procedure time was significantly shorter in the P-EPSS group (9.06 ± 6.14 min vs 14.84 ± 7.25 min, P < 0.01).
In the assessment of adverse events, the two groups showed no significant differences in the proportions of delayed perforation (5% vs 4%; P = 0.82) and delayed bleeding (3% vs 4%; P = 0.96). Cases of adverse events were successfully managed by endoscopic treatment and conservative therapy. After the treatment, the fasting period, duration of hospitalization, and total cost of hospitalization were similar between the two groups.
The features and clinical course of the 15 cases showing delayed adverse events are shown in Table 4, and the results of the logistic regression analysis are shown in Table 5. Multivariate analysis revealed that incomplete closure of the defect was the main independent predictor for an increased number of delayed perforation or bleeding events, and it showed approximately 95% significant increase in delayed adverse events (OR = 21.33; 95%CI: 5.45-83.45; P < 0.01). Moreover, cases with defect size more than 3 cm showed a statistical tendency toward an increase in delayed adverse events (OR = 3.14; 95%CI: 1.08-9.18; P = 0.039). However, defect position (fundus or others) or morphology (SMT or precancerous lesions) as well as the closure type selected (P-EPSS or C-EPSS) were not related to significant differences in delayed adverse events.
| Age (yr) | Sex | Manipulation | Location | Size (cm) | Morphology | Closure type | Complete closure | Intraoperative perforation | Procedure time (min) | Delayed adverse events | Discharge (d) | Total cost (dollars) |
| 60 | Male | ESD | Fundus | 3 | Superficial SMT | C-EPSS | Yes | Yes | 20 | Delayed bleeding | 10 | 2522 |
| 58 | Male | EFTR | Fundus | 4 | Deep SMT | P-EPSS | Yes | Yes | 15 | Delayed perforation | 11 | 2835 |
| 75 | Female | ESD | Rectum | 2.5 | Superficial SMT | C-EPSS | No | No | 22 | Delayed bleeding | 9 | 2710 |
| 56 | Female | ESE | Fundus | 3.5 | Deep SMT | P-EPSS | No | No | 24 | Delayed perforation | 6 | 2238 |
| 74 | Female | ESD | Antrum | 2 | Precancerous lesion | C-EPSS | Yes | No | 20 | Delayed bleeding | 7 | 2489 |
| 68 | Male | ESD | Body | 2.5 | Deep SMT | C-EPSS | No | No | 10 | Delayed perforation | 8 | 2470 |
| 58 | Male | EFTR | Fundus | 4 | Superficial SMT | P-EPSS | Yes | Yes | 15 | Delayed perforation | 7 | 2556 |
| 62 | Male | ESE | Rectum | 3.5 | Precancerous lesion | C-EPSS | No | No | 20 | Delayed perforation | 10 | 2884 |
| 63 | Female | ESD | Antrum | 3.5 | Precancerous lesion | C-EPSS | No | No | 12 | Delayed bleeding | 8 | 2690 |
| 71 | Male | ESD | Rectum | 2.5 | Precancerous lesion | C-EPSS | Yes | No | 14 | Delayed perforation | 6 | 2729 |
| 69 | Male | ESE | Colon | 2 | Precancerous lesion | C-EPSS | Yes | No | 18 | Delayed perforation | 8 | 2577 |
| 76 | Male | EFTR | Fundus | 3.5 | Deep SMT | P-EPSS | Yes | Yes | 25 | Delayed perforation | 8 | 2306 |
| 56 | Female | ESD | Fundus | 2.5 | Superficial SMT | C-EPSS | Yes | Yes | 10 | Delayed bleeding | 7 | 2423 |
| 61 | Male | EFTR | Fundus | 2.5 | Deep SMT | C-EPSS | No | Yes | 15 | Delayed perforation | 6 | 2789 |
| 70 | Female | EFTR | Body | 3.5 | Superficial SMT | P-EPSS | Yes | Yes | 20 | Delayed bleeding | 7 | 2687 |
| Factors | Total (AE) | Odds ratio | 95%CI | P value |
| Closure of defects | ||||
| Incomplete | 11 (6) | 21.33 | 5.45-83.45 | < 0.011 |
| Complete | 169 (9) | 1 | ||
| Location (%) | ||||
| Fundus | 79 (7) | 1.13 | 0.39-3.26 | 0.82 |
| Others | 101 (8) | 1 | ||
| Defect size | ||||
| ≥ 3 cm | 52 (8) | 3.14 | 1.08-9.18 | 0.0391 |
| < 3 cm | 128 (7) | 1 | ||
| Morphology | ||||
| Deep SMT | 76 (6) | 0.90 | 0.31-2.66 | 0.86 |
| Others (superficial SMT and precancerous lesion) | 104 (9) | 1 | ||
| Closure type | ||||
| P-EPSS | 63 (5) | 0.92 | 0.30-2.83 | 1.00 |
| C-EPSS | 117 (10) | 1 | ||
| Intraoperative perforation | ||||
| Yes | 55 (7) | 2.13 | 0.73-6.21 | 0.24 |
| No | 125 (8) | 1 | ||
| Dissection method | ||||
| EFTR | 36 (5) | 2.16 | 0.69-6.78 | 0.19 |
| Others (ESD and ESE) | 144 (10) | 1 |
With the development and popularization of endoscopic techniques, more GI diseases can be detected early and manipulated using minimally invasive methods[16-18]. GI endoscopy procedures such as ESD, ESE, EFTR, and submucosal tunnel endoscopic resection are widely used for the treatment of precancerous lesions or SMTs within the GI tract[19-23]. However, their increasing usage has led to the problem of achieving successful complete closure of the mucosal defects induced by different endoscopic manipulations.
Clips can be applied to effectively manage small defects, but they are too small to work well for large mucosal defects. More importantly, tissue approximation with full-thickness closure is technically impossible with clips, and clips may prematurely drop off the mucosa due to peristalsis and the radial forces of large post-EFTR defects, resulting in delayed perforation and severe complications. Furthermore, clip-closure methods appear to be strongly operator-dependent[9,24,25].
These factors have necessitated the development of new techniques. As reported in one study, various techniques have been used for closure of large defects (especially for exposed defects after EFTR), and these mainly consist of clip- and endoloop-assisted closure methods[26]. For instance, multiple studies reported that over-the-scope-clip (OTSC) application, which can close defects with serosa-to-serosa apposition, unlike mucosa-to-mucosa apposition, can successfully close defects with long-term reliability[27-30]. However, OTSCs can close GI defects only up to 2.5-3 cm in size[31,32], and the OTSC system shows limited effectiveness in some anatomic sites, such as the pylorus and the proximal esophagus. In addition, the edema and tissue folding associated with the usage of OTSCs can potentially narrow the lumen[33], and the OTSC technique is expensive, which can increase the financial burden on patients.
Purse-string assisted suturing has been widely used and is a financially feasible approach in cases with large defects induced by endoscopic manipulations. However, previous studies on the efficacy of EPSS methods evaluated a limited source of cases. Moreover, most of these studies focused on providing a detailed introduction of the procedure for purse-string sutures rather than performing a detailed analysis of its feasibility and efficacy. Wang et al[12] reported that the purse-string suture for colonic mucosal defects could be successfully completed using a single-channel endoscope. They described the detailed procedure of the EPSS method and concluded that no severe complications were recorded in all 18 cases. Kato et al[34] summarized their findings for duodenal ESD and found that complete closure of the mucosal defect by the EPSS method was relatively easy and involved a reduced delayed adverse event rate. Other related studies always introduced the use of this technique for some specific conditions, such as iatrogenic perforations in the colon or duodenum. Ryu et al[13] performed the purse-string suture technique to close iatrogenic colon perforations in eight cases and verified that EPSS can be appropriate for closing large colon perforations. In their analyses of the data obtained from 23 cases involving the closure of large iatrogenic duodenal perforations with purse-string sutures, Zhu et al[14] concluded that the EPSS method was feasible, effective, and easy for closure of perforations. Furthermore, some other studies focused on endoloop-assisted closure of exposed defects after EFTR[35-39]. In comparison with other closure methods, the adoption in combination with endoclips may allow the management of larger post-EFTR defects and may reinforce the wound closure[24,40].
To the best of our knowledge, the present study is the largest case series exploring the effectiveness and safety of the EPSS method. A total of 180 cases involving EPSS-assisted closure performed at our medical center were enrolled in this study for further analysis. More importantly, our study was the first to compare the feasibility and efficacy of different closure types (P-EPSS and C-EPSS) used in endoscopic manipulations.
P-EPSS has been employed as a novel closure method at our institution, accounting for 63 cases between 2014 and 2020. The previous study mainly used P-EPSS in the treatment of gastric tumors originating from the muscularis propria or gastric extra-luminal growth tumors[11]. In this study, we expanded the indications of P-EPSS and used this novel closure method in more situations, such as ESD or ESE cases with big defects. This approach is especially appropriate for defects characterized by a large size and specific positions or morphologies (e.g., fundus or deep SMTs). All of the above clinical characteristics point to the same tendency, i.e., intraoperative perforation. Thus, it is difficult and time-consuming to achieve complete closure, highlighting the need for careful selection of cases treated with P-EPSS-assisted closure.
In the present study, the P-EPSS group showed a significantly higher incidence of intraoperative perforation and a significantly shorter procedure time in comparison with the C-EPSS group. In this regard, the approach used to measure the procedure time of the EPSS method requires elucidation in advance. The time required for the resection procedure should be excluded for fairness, because the difficulty of endoscopic resection is different. Since the endoscopist had to take more time and effort to achieve complete closure of transmural defects, most of them would prefer to avoid the possibility of perforation in manipulations. Unexpectedly, the novel P-EPSS method could solve this problem from a different perspective by turning passive perforation to active perforation, and thereby providing sufficient time for preparation of perforation closure. Subtle and sensitive handling of the endoscope is essential for accomplishing the manipulation in EPSS. Although closure of the defects of transmural GI lesions revealed clinical effectiveness, this approach was not technically easy. The maneuverability of the endoscope was poor, especially in portions with big size and specific positions or morphologies. Further modifications are required to generalize this method in the future.
Regarding adverse events, the present study did not show that P-EPSS reduced the occurrence of adverse events, although the sample size may have been insufficient to detect differences between groups because of the retrospective design. Interestingly, the selection of P-EPSS- or C-EPSS-assisted closure showed no relationship with the adverse event rate, but defects with incomplete closure and defect size larger than 3 cm were associated with worse outcomes. Cases of adverse events were successfully managed by endoscopic treatment and conservative therapy. After the treatment, the fasting period, duration of hospitalization, and total cost were similar between the two groups. Taken together, our results suggest that the P-EPSS procedure is an effective and safe method for closing the defects of difficult targets in endoscopic operations.
Our study had several limitations, including a selection bias caused by the inclusion of more cases of large defects and intraoperative perforation in the P-EPSS group; this was primarily attributable to the single-center retrospective design and should be taken into account when interpreting the findings. However, we could not eliminate selection bias. We tried to overcome these limitations by including consecutive patients during the study period. Second, all procedures were performed by expert endoscopists at a high-volume center, limiting the generalizability of the findings. We hope to design a prospective study with a different cohort to compare the criteria for choosing between P-EPSS or C-EPSS before the endoscopic manipulation. Nevertheless, despite these limitations, our study is the largest case series on the topic of EPSS-assisted endoscopic closure and the first report to present a comparison of P-EPSS and C-EPSS.
Our findings revealed that EPSS could achieve secure complete closure of mucosal defect. P-EPSS could shorten the procedure time and achieve secure complete closure of mucosal defects in clinical practice. Selection of P-EPSS was based on the defect’s size, location, and the depth of the SMT. Defects with incomplete closure or size larger than 3 cm were associated with worse outcomes, rather than the selection of closure type.
Endoscopic purse-string assisted suturing (EPSS) has proven to be an effective and safe technique for the closure of large mucosal defects. Recently, the endoscopic pre-purse-string suture (P-EPSS) procedure was invented and was widely used in clinical.
The novel invented P-EPSS should be analysed and compared with the conventional EPSS (C-EPSS) procedure.
Elucidate the outcomes of EPSS-assisted closure in different clinical situations, and evaluate the efficacy of P-EPSS.
The study included a total of 180 patients who underwent closure assisted by EPSS between July 2014 and June 2020. The P-EPSS (n = 63) and C-EPSS (n = 117) groups were compared and the intergroup differences in aspects such as the lesion size, location, and morphology, incidence of complete closure, intraoperative perforation, and delayed adverse events were evaluated.
Patients with lesion size larger than 3 cm, lesions located at the fundus of stomach, or submucosal tumors originating from the deep mucosa were more likely to undergo P-EPSS-assisted closure. The P-EPSS group showed a significantly higher proportion of intraoperative perforation and a much shorter procedure time. But the incidence of adverse events did not differ significantly between P-EPSS and C-EPSS groups. Lesions with incomplete closure or size greater than 3 cm showed a statistical tendency to result in an increase in delayed adverse events.
EPSS could achieve secure complete closure of mucosal defect. P-EPSS could shorten the procedure and yield complete closure of mucosal defects.
To eliminate the selection bias, we would dedicate to design a new cohort prospective study to compare the criteria of deciding in advance whether to use P-EPSS or C-EPSS before endoscopic manipulations.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sulbaran MN, Brazil; Takita M, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Li Y, Wu JH, Meng Y, Zhang Q, Gong W, Liu SD. New devices and techniques for endoscopic closure of gastrointestinal perforations. World J Gastroenterol. 2016;22:7453-7462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Verlaan T, Voermans RP, van Berge Henegouwen MI, Bemelman WA, Fockens P. Endoscopic closure of acute perforations of the GI tract: a systematic review of the literature. Gastrointest Endosc. 2015;82:618-28.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Qiao Z, Ling X, Zhu J, Ying G, Xu L, Zhu H, Tang J. Therapeutic application of purse-string sutures with nylon loops and metal clips under single-channel endoscopy for repair of gastrointestinal wall defects. Exp Ther Med. 2018;15:4356-4360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Draganov PV, Wang AY, Othman MO, Fukami N. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol. 2019;17:16-25.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 318] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 5. | Matsuda T, Fujii T, Emura F, Kozu T, Saito Y, Ikematsu H, Saito D. Complete closure of a large defect after EMR of a lateral spreading colorectal tumor when using a two-channel colonoscope. Gastrointest Endosc. 2004;60:836-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Shadhu K, Ramlagun D, Wang Y, Ping X, Chen T, Zhu Y, Xu Z. Re-evaluation of purse string suture in laparoscopic appendectomy. Surg Endosc. 2020;34:779-786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Joo J, Custis T, Armstrong AW, King TH, Omlin K, Kappel ST, Eisen DB. Purse-string suture vs second intention healing: results of a randomized, blind clinical trial. JAMA Dermatol. 2015;151:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Shi D, Li R, Chen W, Zhang D, Zhang L, Guo R, Yao P, Wu X. Application of novel endoloops to close the defects resulted from endoscopic full-thickness resection with single-channel gastroscope: a multicenter study. Surg Endosc. 2017;31:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW, Liu JZ. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc. 2011;25:2926-2931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 10. | Zhang Y, Wang X, Xiong G, Qian Y, Wang H, Liu L, Miao L, Fan Z. Complete defect closure of gastric submucosal tumors with purse-string sutures. Surg Endosc. 2014;28:1844-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Wu N, Liu S, Chen M, Zeng X, Wang F, Zhang J, She Q. The prepurse-string suture technique for gastric defect after endoscopic full-thickness resection (with video). Medicine (Baltimore). 2018;97:e12118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Wang J, Zhao L, Wang X, Liu L, Wang M, Fan Z. A novel endoloop system for closure of colonic mucosal defects through a single-channel colonoscope. Endoscopy. 2017;49:803-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Ryu JY, Park BK, Kim WS, Kim K, Lee JY, Kim Y, Park JY, Kim BJ, Kim JW, Choi CH. Endoscopic closure of iatrogenic colon perforation using dual-channel endoscope with an endoloop and clips: methods and feasibility data (with videos). Surg Endosc. 2019;33:1342-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Zhu S, Lin J, Xu F, Guo S, Huang S, Wang M. Purse-string sutures using novel endoloops and repositionable clips for the closure of large iatrogenic duodenal perforations with single-channel endoscope: a multicenter study. Surg Endosc. 2019;33:1319-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Zhu H, Tang JH, Ling X, Qian JP, Shen DM. [Application of purse string suture with Harmonious Clips and Olympus endoloop in single channel endoscopy for large gastric antrum mucosa defect]. Zhonghua Yi Xue Za Zhi. 2018;98:3074-3078. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 926] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 17. | Waddingham W, Nieuwenburg SAV, Carlson S, Rodriguez-Justo M, Spaander M, Kuipers EJ, Jansen M, Graham DG, Banks M. Recent advances in the detection and management of early gastric cancer and its precursors. Frontline Gastroenterol. 2021;12:322-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Li B, Chen D, Wan X. Endoscopic purse-string suture technique: An effective remedy for a large iatrogenic rectal perforation. Dig Endosc. 2019;31:e9-e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Cai MY, Martin Carreras-Presas F, Zhou PH. Endoscopic full-thickness resection for gastrointestinal submucosal tumors. Dig Endosc. 2018;30 Suppl 1:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Mori H, Kobara H, Nishiyama N, Masaki T. Current status and future perspectives of endoscopic full-thickness resection. Dig Endosc. 2018;30 Suppl 1:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | ASGE Technology Committee; Aslanian HR, Sethi A, Bhutani MS, Goodman AJ, Krishnan K, Lichtenstein DR, Melson J, Navaneethan U, Pannala R, Parsi MA, Schulman AR, Sullivan SA, Thosani N, Trikudanathan G, Trindade AJ, Watson RR, Maple JT. ASGE guideline for endoscopic full-thickness resection and submucosal tunnel endoscopic resection. VideoGIE. 2019;4:343-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 22. | Ye L, Zeng X, Yuan X, Guo L, Zhang Y, Li Y, Hu B. Purse-string suture and double percutaneous endoscopic gastrostomies for treating a postoperative duodenal fistula. Endoscopy. 2019;51:E55-E56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Lu ZY, Zhao DY. Gastric schwannoma treated by endoscopic full-thickness resection and endoscopic purse-string suture: A case report. World J Gastroenterol. 2021;27:3940-3947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Mangiavillano B, Viaggi P, Masci E. Endoscopic closure of acute iatrogenic perforations during diagnostic and therapeutic endoscopy in the gastrointestinal tract using metallic clips: a literature review. J Dig Dis. 2010;11:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Dong HY, Wang YL, Jia XY, Li J, Li GD, Li YQ. Modified laparoscopic intragastric surgery and endoscopic full-thickness resection for gastric stromal tumor originating from the muscularis propria. Surg Endosc. 2014;28:1447-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 26. | Ma LS. What is the purpose of launching World Journal of Gastrointestinal Surgery? World J Gastrointest Surg. 2009;1:1-2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Tang SJ, Naga YM, Wu R, Zhang S. Over-the-scope clip-assisted endoscopic full thickness resection: a video-based case series. Surg Endosc. 2020;34:2780-2788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Guo JT, Zhang JJ, Wu YF, Liao Y, Wang YD, Zhang BZ, Wang S, Sun SY. Endoscopic full-thickness resection using an over-the-scope device: A prospective study. World J Gastroenterol. 2021;27:725-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Al-Bawardy B, Rajan E, Wong Kee Song LM. Over-the-scope clip-assisted endoscopic full-thickness resection of epithelial and subepithelial GI lesions. Gastrointest Endosc. 2017;85:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 30. | Tashima T, Nonaka K, Ryozawa S, Tanisaka Y. Endoscopic purse-string suturing with an over-the-scope clip for closure of a large mucosal defect after gastric ESD. Dig Liver Dis. 2018;50:1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Weiland T, Fehlker M, Gottwald T, Schurr MO. Performance of the OTSC System in the endoscopic closure of iatrogenic gastrointestinal perforations: a systematic review. Surg Endosc. 2013;27:2258-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 32. | Manta R, Manno M, Bertani H, Barbera C, Pigò F, Mirante V, Longinotti E, Bassotti G, Conigliaro R. Endoscopic treatment of gastrointestinal fistulas using an over-the-scope clip (OTSC) device: case series from a tertiary referral center. Endoscopy. 2011;43:545-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 33. | Coman RM, Yang D, Draganov PV. Endoscopic full-thickness resection with use of the over-the-scope clip: a word of caution! Gastrointest Endosc. 2017;86:749-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Kato M, Ochiai Y, Fukuhara S, Maehata T, Sasaki M, Kiguchi Y, Akimoto T, Fujimoto A, Nakayama A, Kanai T, Yahagi N. Clinical impact of closure of the mucosal defect after duodenal endoscopic submucosal dissection. Gastrointest Endosc. 2019;89:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 35. | Ye LP, Yu Z, Mao XL, Zhu LH, Zhou XB. Endoscopic full-thickness resection with defect closure using clips and an endoloop for gastric subepithelial tumors arising from the muscularis propria. Surg Endosc. 2014;28:1978-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Hu JW, Ge L, Zhou PH, Li QL, Zhang YQ, Chen WF, Chen T, Yao LQ, Xu MD, Chu Y. A novel grasp-and-loop closure method for defect closure after endoscopic full-thickness resection (with video). Surg Endosc. 2017;31:4275-4282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Li J, Meng Y, Ye S, Wang P, Liu F. Usefulness of the thread-traction method in endoscopic full-thickness resection for gastric submucosal tumor: a comparative study. Surg Endosc. 2019;33:2880-2885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Shi Q, Chen T, Zhong YS, Zhou PH, Ren Z, Xu MD, Yao LQ. Complete closure of large gastric defects after endoscopic full-thickness resection, using endoloop and metallic clip interrupted suture. Endoscopy. 2013;45:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 39. | Tang AL, Liao XQ, Shen SR, Xiao DH, Yuan YX, Wang XY. Application of clips assisted with foreign body forceps in defect closure after endoscopic full-thickness resection. Surg Endosc. 2016;30:2127-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Akimoto T, Goto O, Nishizawa T, Yahagi N. Endoscopic closure after intraluminal surgery. Dig Endosc. 2017;29:547-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |