Published online Jan 28, 2023. doi: 10.3748/wjg.v29.i4.692
Peer-review started: October 29, 2022
First decision: November 14, 2022
Revised: November 28, 2022
Accepted: January 9, 2023
Article in press: January 9, 2023
Published online: January 28, 2023
Processing time: 83 Days and 11.5 Hours
Helicobacter pylori (H. pylori) infection is a risk factor for many diseases, including peptic ulcer disease and gastric cancer. While H. pylori eradication therapy can prevent these diseases, potentially unfavorable effects of eradication therapy have also been reported in some diseases, such as gastroesophageal reflux disease (GERD), Barrett’s esophagus (BE), inflammatory bowel disease (IBD), allergic diseases, and metabolic diseases. Consequently, both positive and negative impacts should be considered when assessing the effects of H. pylori eradication therapy.
To compare the incidence of these diseases before and after H. pylori eradication and to comprehensively assess its effects.
This retrospective cohort study used a Japanese nationwide health claims database (April 2009-March 2020), developed by the Japanese Ministry of Health, Labour and Welfare. The database contained almost all health insurance claims data issued in Japan, and specific health check-up data for individuals who took the check-ups. Descriptive statistics were used for the analyses. Patients who received primary eradication therapy were defined as those prescribed medi-cation for H. pylori eradication. New diagnoses, defined as incidence of upper gastrointestinal diseases and IBD, and prevalence of allergic diseases were compared before and after eradication. The incidence and prevalence of each disease were also compared between the 3-year period before eradication (from the 4th to the 2nd year prior to the year of eradication) and the 3-year period after eradication (from the 1st to the 3rd year after the year of eradication) based on the age category and calendar year and month. Changes in body mass index and proportion of patients with metabolic syndrome (MS) were examined before and after eradication.
We identified 5219731 patients who received primary eradication therapy. The 65-69 years age group had the greatest number of patients in both sexes. There was no significant increase in the incidence of GERD after eradication when considering the effects of aging and reporting period. However, the incidence of BE was higher in the 3-year period after eradication than in the 3-year period before eradication for all age categories (0.02%-0.10% vs < 0.01%-0.05%). The incidence of IBD and prevalence of allergic disease were also higher after eradication. In contrast, the incidence of gastric and duodenal ulcers and gastritis was reduced after eradication. In patients with at least one entry of health check-up data (1701111 patients), the percentage of patients with MS showed a slight increase following eradication (11.0% in the year of eradication and 12.2% after 5 years).
The results suggest that H. pylori eradication therapy reduces peptic ulcers and gastritis; however, it is associated with increased incidence of several other chronic diseases.
Core Tip: While Helicobacter pylori (H. pylori) eradication can prevent certain diseases including peptic ulcer diseases and gastric cancer, unfavorable effects of eradication therapy have also been reported. We analyzed a Japanese nationwide health claims database containing almost all health insurance claims data to compare the incidence and prevalence of specific diseases before and after H. pylori eradication to comprehensively assess its effects. We identified 5219731 patients who received primary eradication therapy. H. pylori eradication drastically reduced peptic ulcers and gastritis but was associated with an increase in Barrett’s esophagus, inflammatory bowel disease, allergic disease, and metabolic syndrome.
- Citation: Mizukami K, Sugano K, Takeshima T, Murakami K. Disease trends after Helicobacter pylori eradication based on Japanese nationwide claims and the health check-up database. World J Gastroenterol 2023; 29(4): 692-705
- URL: https://www.wjgnet.com/1007-9327/full/v29/i4/692.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i4.692
Helicobacter pylori (H. pylori) infection is a risk factor for many diseases, including peptic ulcers[1], gastric cancer[2], gastric mucosa-associated lymphoid tissue lymphoma[3], H. pylori-associated gastritis[4], idiopathic thrombocytopenic purpura[5], and iron deficiency anemia[6]. H. pylori eradication therapy can effectively prevent these diseases. However, unfavorable effects of eradication therapy have also been reported. For instance, the relationship between H. pylori infection and gastroesophageal reflux disease (GERD) is controversial. Some studies have suggested a lower incidence of GERD and its complications, such as Barrett’s esophagus (BE), in patients with H. pylori infection[7-10]. A study reported that the prevalence of GERD increased in patients after successful H. pylori eradication and was comparable to that in patients without H. pylori[11]. However, some studies found no association between H. pylori eradication and GERD[12], while others reported that eradication is not associated with GERD development in dyspeptic patients but might pose a higher risk in patients with peptic ulcers[13]. An inverse association of H. pylori infection with some immune system diseases, including allergies[14-18] and inflammatory bowel disease (IBD)[18-22], has also been reported. Moreover, relationships between H. pylori infection and metabolic diseases and associated conditions such as obesity have also been described. H. pylori infection may increase the risk of dyslipidemia[23,24] and metabolic syndrome (MS)[25]. While favorable effects of H. pylori eradication on triglyceride and high-density lipoprotein cholesterol (HDL-C) levels[26] or dyslipidemia[27] have been reported, it has also been found that eradication therapy may increase the incidence of hyperlipidemia and obesity[28,29]. Therefore, both positive and negative impacts should be considered when assessing the effects of H. pylori eradication therapy. A comprehensive evaluation of these effects in Japan, where large-scale eradication therapy was commenced earlier than it was in other countries due to its coverage by universal healthcare, might provide useful information for clinicians worldwide.
Herein, we performed a comprehensive analysis of the effects of H. pylori eradication therapy on various diseases. This study used a Japanese nationwide health claims database, the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB), developed by the Japanese Ministry of Health, Labour and Welfare, containing almost all (≥ 95%) health insurance claims data issued in Japan[30]. In this database, health check-up data are also included for individuals who underwent specific health check-ups. We compared the development of diseases (defined as the first diagnosis) known to be related to H. pylori infection and eradication before and after eradication in patients who underwent primary H. pylori eradication therapy. We also assessed changes in body mass index (BMI) and MS development before and after eradication.
This claims-based study used NDB data[30] (April 2009-March 2020). The dataset contained medical claims data, diagnosis procedure combination (DPC) claims data (claims related to bundled payments during DPC hospitalization), and pharmacy claims data from patients who received primary H. pylori eradication therapy or those who had at least one diagnosis of GERD. Specific health check-up data available for patients who underwent check-ups included anthropometric data, laboratory values, and answers to questionnaires. The observation period was defined as the entire period of data availability for each patient.
Patients with a prescription for primary H. pylori eradication therapy covered by national health insurance were identified and designated in our study as those who received primary H. pylori eradication therapy. The three medications approved for this therapy are amoxicillin and clarithromycin with either a proton pump inhibitor (PPI) or potassium-competitive acid blocker (P-CAB); these can be prescribed as a combination of single drugs in the same month or as a combination pack (Supp
We compared the incidences of GERD, BE, other upper gastrointestinal diseases [including some diseases of the digestive system other than those categorized as International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) code K92], and IBD, and the prevalence of allergic diseases before and after primary H. pylori eradication therapy (see Supplementary Table 2 for definition of the diseases). Furthermore, IBD was classified into Crohn’s disease (CD) and ulcerative colitis (UC) according to the ICD-10 classification. Other upper gastrointestinal diseases were classified into 10 types [esophagitis, other diseases of the esophagus, gastric ulcer (GU), duodenal ulcer (DU), peptic ulcer, site unspecified, anastomotic ulcer, gastritis and duodenitis, functional dyspepsia (FD), other diseases of the stomach and duodenum, and other diseases of the digestive system] according to the ICD-10 classification, and allergic diseases were classified into five types (allergy, pollinosis, asthma, hypersensitivity, and atopy) by disease name (Supplementary Table 2). It should be noted that since allergic diseases were initially defined according to the ICD-10 classification and atopic asthma was classified into the asthma group, the atopy group included only atopic cough. The month of primary eradication therapy was defined as the earliest month in which primary eradication medications were prescribed for each patient. The term ‘year of eradication’ refers to the year corresponding to this month. The incidence of a disease was calculated on the basis of the earliest first diagnosis of each disease, which was identified using the record of the first diagnosis date by the medical institution on medical claims or DPC claims in the database.
The incidence and prevalence of each disease was calculated for each elapsed year from the eradication, which was calculated as the integer part of the difference of the time and the time of the eradication. If the difference was negative, the elapsed year was the integer part minus one, as in SAS (version 9.4, SAS Institute, Cary, NC), the integers of the negative numbers were rounded up.
We also compared the incidence and prevalence of each disease between the 3-year period before eradication (from the 4th to 2nd year prior to the year of eradication) and the 3-year period after eradication (from the 1st to 3rd year after the year of eradication) based on the age category and calendar year and month, represented by calendar month and year from July 2014 to June 2018.
To assess GERD status in patients with and without H. pylori eradication, we compared treatments in patients who received eradication therapy before GERD development with those who did not undergo eradication therapy during the observation period. Patients with available health check-up data for the year of the first GERD diagnosis were included in this analysis (eradication group), and the data were used to develop a propensity score (PS). Patients in the non-eradication group were identified from the patients with GERD, those who did not undergo eradication therapy, and those who had their health check-up data. The patients identified in the non-eradication group were those whose sex, age at the first GERD diagnosis, and the month of the first GERD diagnosis matched with those of anyone in the eradication group. After adjusting for confounding factors using the PS, the types of drugs prescribed for GERD were compared by year from first GERD diagnosis and by calendar year between the eradication and non-eradication groups. The PS was estimated using a logistic regression model with eradication (dummy variable) as the explained variable, and age, sex, and specific health check-up data (BMI, abdominal circumference, systolic blood pressure, diastolic blood pressure, triglyceride, HDL-C, low-density lipoprotein cholesterol, aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transpeptidase, fasting blood glucose, and hemoglobin A1c) as explanatory variables. The PS was divided into four classes, and patients within each group were assigned the same weight to make the total weight comparable between groups in each PS class. The following drug classes for GERD treatment were analyzed: Histamine H2 receptor antagonist, P-CAB, PPI, and others (Supple
Changes in BMI and proportion of patients with MS were examined before and after eradication in patients for whom these data of specific health check-up were available at least once or yearly for the 3 years before and after the year of eradication. If multiple values were recorded for a patient in the same year, the average value was used. Patients with MS were defined as those with an abdominal circumference of ≥ 85 cm in men or ≥ 90 cm in women, and who met at least two of the following criteria: (1) Triglyceride ≥ 150 mg/dL or HDL-C < 40 mg/dL; (2) Systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg; and (3) Fasting plasma glucose ≥ 110 mg/dL[31]. We used the statistical software packages SAS for the analyses. In the analyses using the specific health check-up data, missing values were imputed based on all other factors using the MI statement in PROC MI of the SAS software.
This study was approved by the Ethics Committee of Oita University, Faculty of Medicine (No. 1692). All procedures followed were in accordance with the World Medical Association’s Declaration of Helsinki (1964, and its later amendments). Informed consent was not obtained because this study used anonymized claims data.
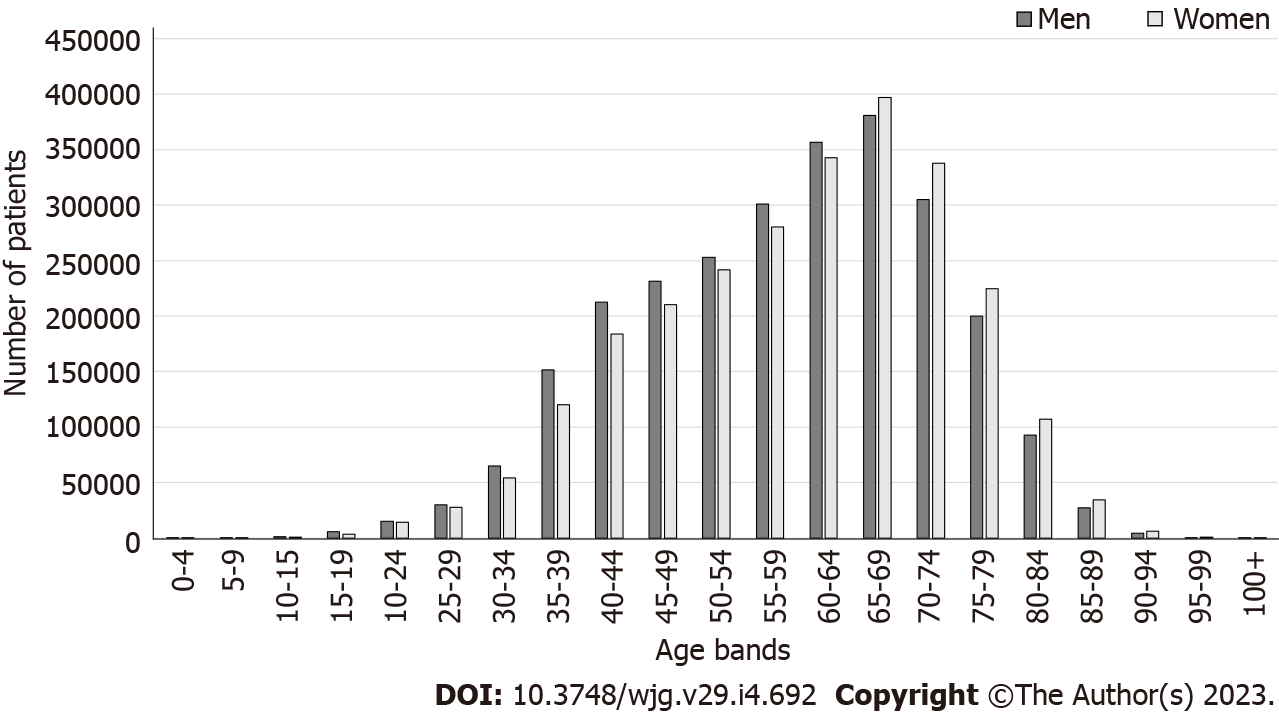
The dataset included 5219731 patients who received primary H. pylori eradication therapy. Maximum number of patients were in the 65-69 years age bracket at the time of eradication. There were more men aged < 65 years, but more women aged ≥ 65 years (Figure 1).
The incidences of upper gastrointestinal diseases and IBD were highest in the year of eradication and the preceding year, respectively (Table 1). After eradication, the incidences of GERD and IBD decreased yearly, reaching similar levels to those before eradication. The incidences of other upper gastrointestinal diseases markedly decreased after eradication. In contrast, the incidence of BE increased after eradication. The prevalence of allergic diseases increased in the years before eradication and continued to increase after eradication; the prevalence at 5 years after eradication was almost double the prevalence approximately 10 years before eradication (Table 1).
| Year | Denominator (patient-year) | Average age | Upper gastrointestinal diseases | IBD | Allergy | ||
| GERD | Barrett’s esophagus | Other upper gastrointestinal diseases | |||||
| -10 | 1146497 | 48.9 | 2.31% (2.28%, 2.33%) | 0.004% (0.002%, 0.005%) | 11.45% (11.39%, 11.51%) | 0.029% (0.026%, 0.032%) | 17.5% (17.4%, 17.5%) |
| -9 | 1943505 | 50.1 | 2.55% (2.52%, 2.57%) | 0.006% (0.005%, 0.008%) | 11.10% (11.05%, 11.14%) | 0.028% (0.025%, 0.030%) | 20.0% (19.9%, 20.0%) |
| -8 | 2761765 | 51.3 | 2.75% (2.73%, 2.77%) | 0.007% (0.006%, 0.008%) | 10.57% (10.54%, 10.61%) | 0.026% (0.024%, 0.028%) | 21.3% (21.2%, 21.3%) |
| -7 | 3364755 | 52.5 | 3.02% (3.00%, 3.04%) | 0.010% (0.009%, 0.011%) | 9.89% (9.86%, 9.92%) | 0.027% (0.025%, 0.029%) | 23.1% (23.1%, 23.2%) |
| -6 | 4001760 | 53.7 | 3.18% (3.16%, 3.19%) | 0.011% (0.010%, 0.012%) | 9.27% (9.24%, 9.30%) | 0.027% (0.025%, 0.028%) | 24.4% (24.3%, 24.4%) |
| -5 | 4520587 | 54.9 | 3.38% (3.37%, 3.40%) | 0.015% (0.014%, 0.016%) | 8.67% (8.64%, 8.70%) | 0.028% (0.026%, 0.029%) | 25.7% (25.7%, 25.7%) |
| -4 | 4746859 | 55.9 | 3.48% (3.47%, 3.50%) | 0.018% (0.017%, 0.019%) | 7.54% (7.51%, 7.56%) | 0.027% (0.025%, 0.028%) | 27.4% (27.3%, 27.4%) |
| -3 | 4880888 | 56.9 | 3.58% (3.56%, 3.59%) | 0.023% (0.022%, 0.024%) | 6.50% (6.48%, 6.53%) | 0.027% (0.026%, 0.028%) | 28.8% (28.7%, 28.8%) |
| -2 | 5025747 | 57.9 | 3.80% (3.78%, 3.81%) | 0.030% (0.028%, 0.031%) | 6.04% (6.02%, 6.06%) | 0.029% (0.027%, 0.030%) | 30.3% (30.3%, 30.4%) |
| -1 | 4732998 | 58.8 | 11.01% (10.99%, 11.04%) | 0.164% (0.161%, 0.168%) | 19.31% (19.27%, 19.35%) | 0.046% (0.044%, 0.048%) | 33.7% (30.3%, 30.4%) |
| 0 | 4892585 | 59.8 | 11.33% (11.30%, 11.36%) | 0.223% (0.219%, 0.227%) | 16.48% (16.45%, 16.51%) | 0.053% (0.051%, 0.055%) | 34.7% (34.7%, 34.8%) |
| 1 | 4137930 | 61.0 | 3.84% (3.82%, 3.86%) | 0.083% (0.080%, 0.086%) | 0.15% (0.15%, 0.16%) | 0.034% (0.032%, 0.036%) | 35.0% (34.9%, 35.0%) |
| 2 | 3344893 | 62.2 | 3.30% (3.28%, 3.32%) | 0.080% (0.077%, 0.083%) | 0.12% (0.12%, 0.13%) | 0.030% (0.028%, 0.032%) | 35.7% (35.7%, 35.8%) |
| 3 | 2520763 | 63.4 | 2.98% (2.96%, 3.00%) | 0.076% (0.072%, 0.079%) | 0.10% (0.09%, 0.10%) | 0.030% (0.028%, 0.032%) | 36.7% (36.7%, 36.8%) |
| 4 | 1900509 | 64.5 | 2.76% (2.74%, 2.78%) | 0.076% (0.072%, 0.080%) | 0.09% (0.08%, 0.09%) | 0.028% (0.026%, 0.031%) | 36.5% (36.4%, 36.6%) |
| 5 | 1275881 | 65.4 | 2.51% (2.49%, 2.54%) | 0.073% (0.069%, 0.078%) | 0.07% (0.07%, 0.07%) | 0.028% (0.025%, 0.031%) | 38.6% (38.5%, 38.7%) |
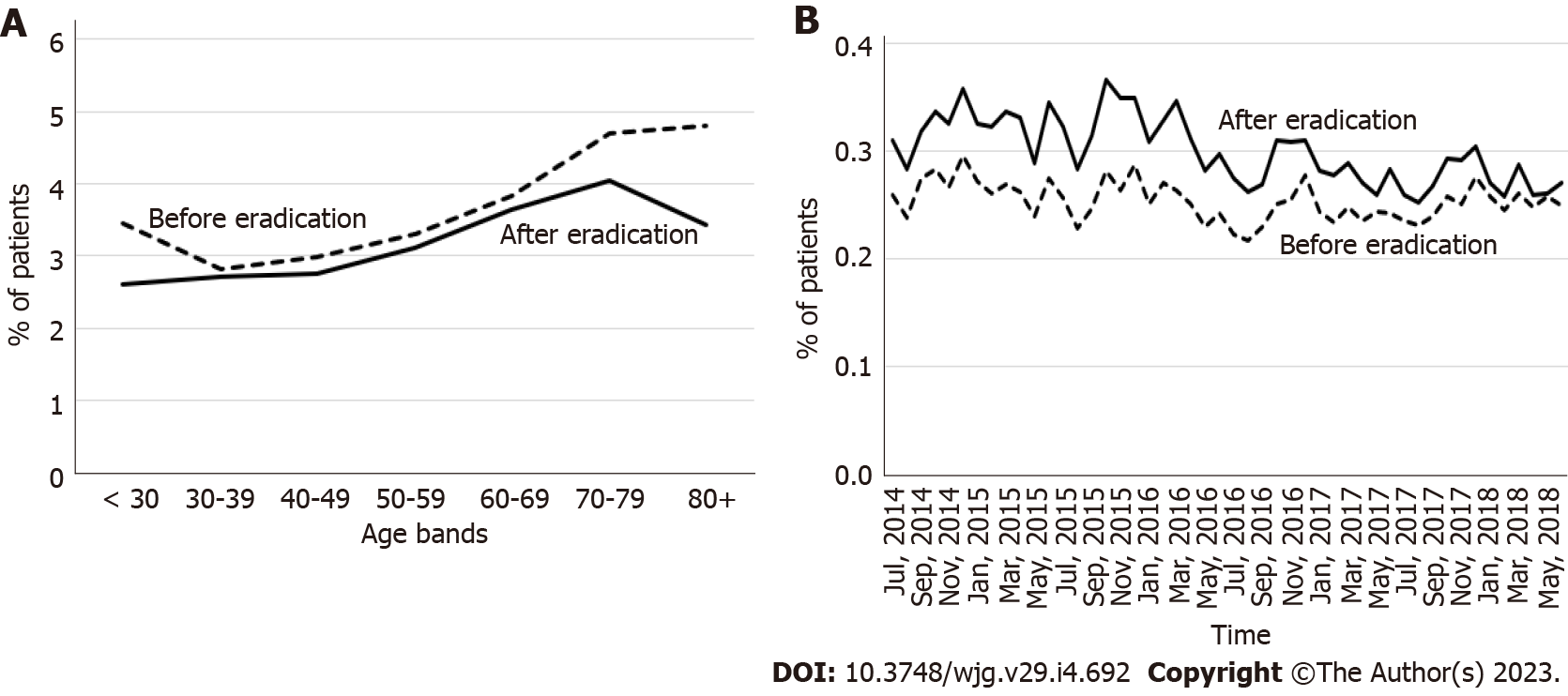
The incidence of GERD in the 3-year period before eradication was similar to or slightly higher than that in the period after eradication for all age groups, and it tended to increase with age regardless of eradication status (Figure 2A). When data were analyzed according to the calendar year and month, the incidence was higher after eradication, although this difference disappeared in later years (Figure 2B).
When comparing GERD treatment status between the eradication group (76367 patients) and non-eradication group (1008539 patients), there was no remarkable difference in the trend of GERD medication by year from first GERD diagnosis or calendar year, although the percentage of patients receiving each type of GERD drug was slightly higher in the eradication group (Supple
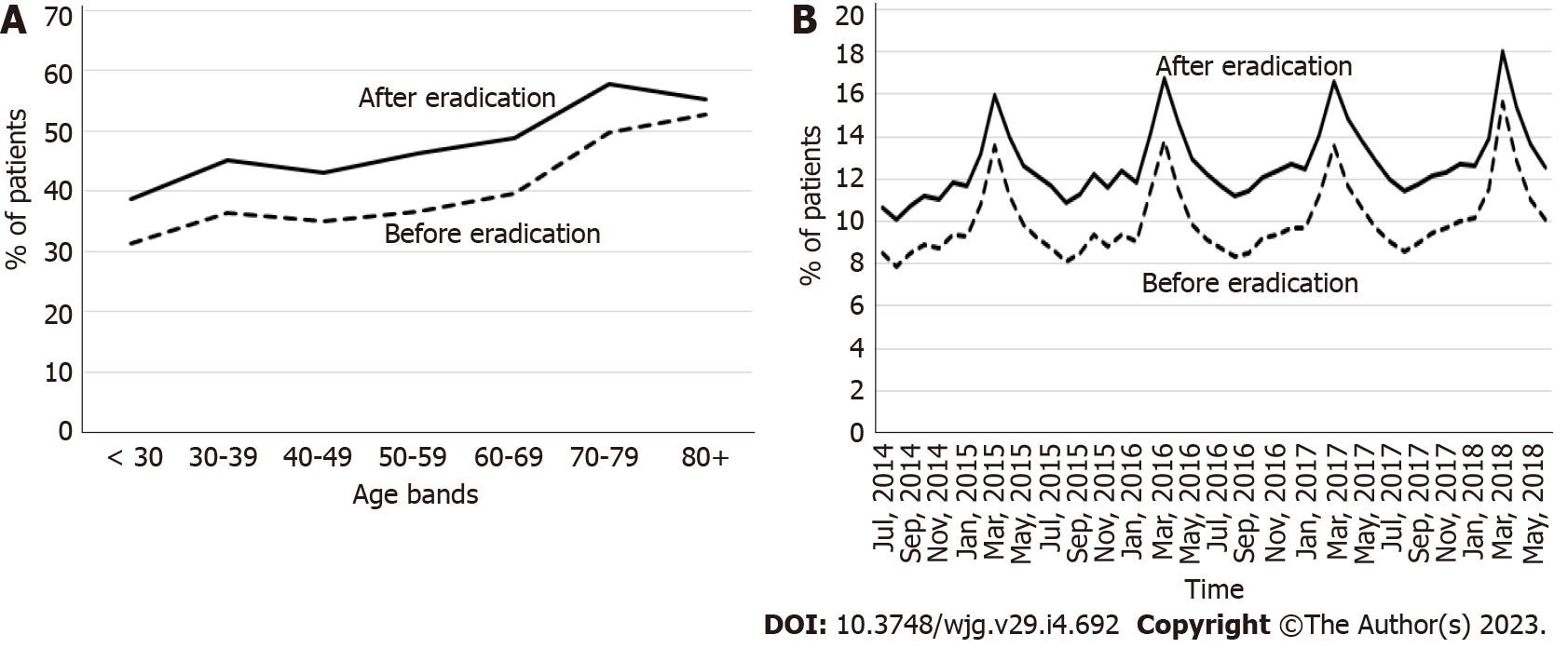
The incidence of BE was higher in the 3-year period after eradication than in the period before eradication for all age categories (Figure 3A), and throughout the observation period (Figure 3B). The incidence of BE increased with age regardless of H. pylori eradication status in patients < 70 years of age and decreased thereafter beyond the age of 70 years (Figure 3A).
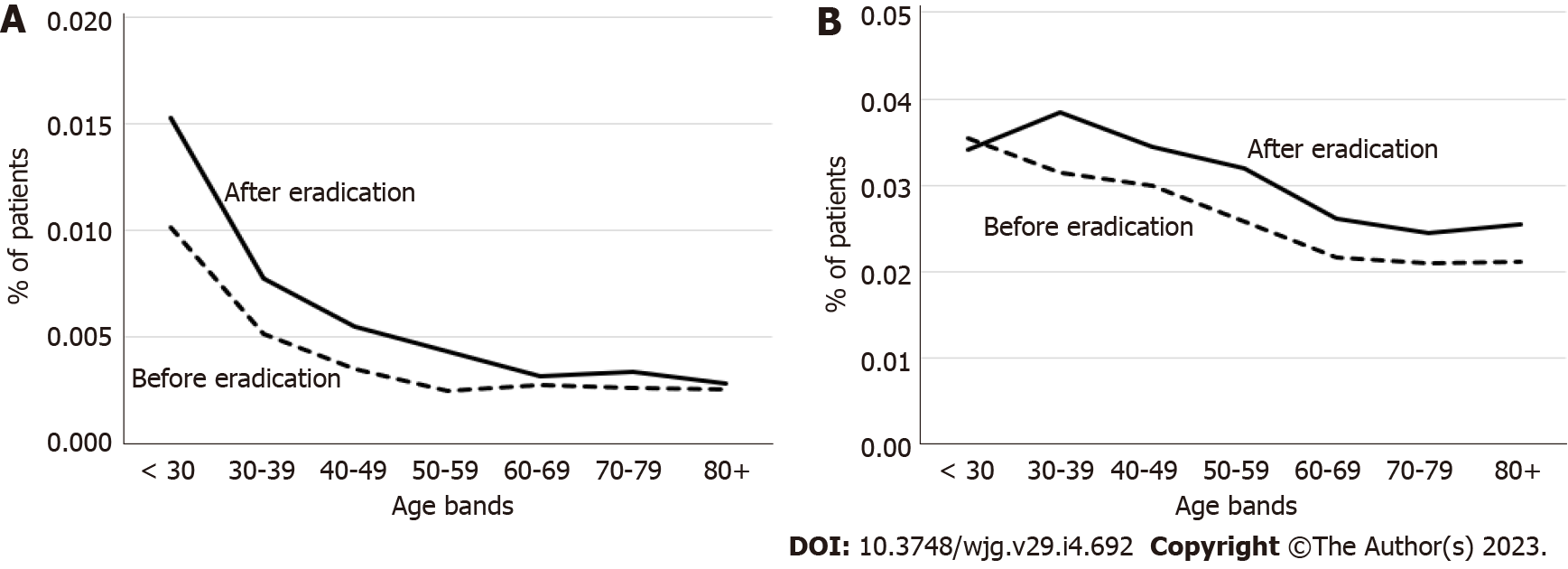
The incidence of other upper gastrointestinal diseases was lower in the 3-year period after eradication than in the period before eradication for all age categories (Supplementary Figure 3A), and throughout the study period (Supplementary Figure 3B).
Gastritis and duodenitis showed the highest incidences before eradication, and their incidences decreased after eradication (Supplementary Figure 4). The incidences of GU and DU were also higher before eradication than after eradication (Supplementary Figure 4). The incidences of other diseases, including FD, esophagitis, and other diseases of the esophagus, increased with time and were higher after eradication than before (Supplementary Figure 4). Of note, the overall incidence of these diseases was lower than that of the other upper gastrointestinal diseases.
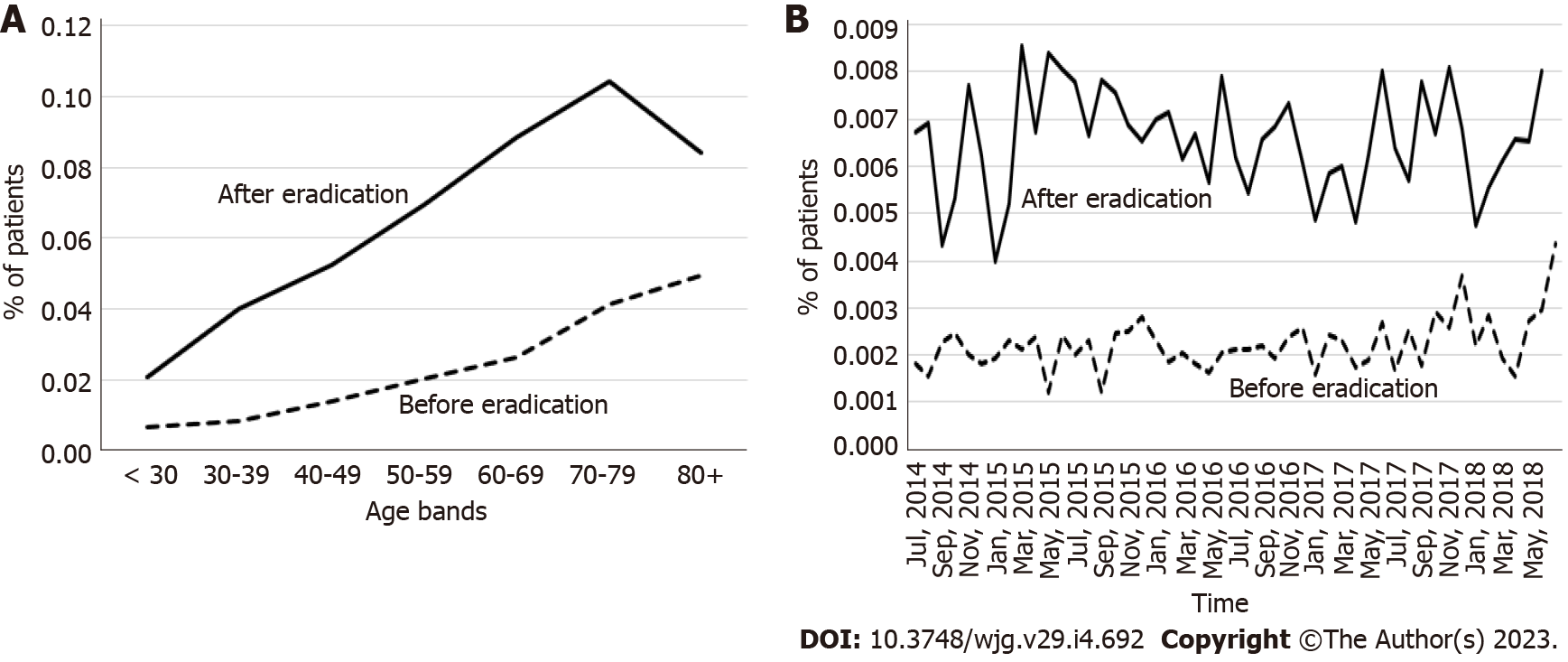
The incidence of CD was higher in the 3-year period after eradication than in the period before eradication for younger age categories. This difference decreased with increasing age, particularly for patients aged ≥ 60 years (Figure 4A). The incidence of UC was higher in the 3-year period after eradication than in the period before eradication for patients aged ≥ 30 years (Figure 4B). For both diseases, the incidence tended to decrease with increasing age up to 50-59 years; the incidence of CD was very low for this and older age groups (Figures 4A and B).
The prevalence of allergic diseases was higher in the 3-year period after eradication than in the period before eradication for all age categories (Figure 5A), and throughout the entire study period (Figure 5B). The prevalence of allergic diseases tended to be higher in older patients (Figure 5A); moreover, periodic seasonal changes were observed, with a higher incidence in early spring (Figure 5B), both before and after eradication. The prevalence of all types of allergies increased after eradication (Supp
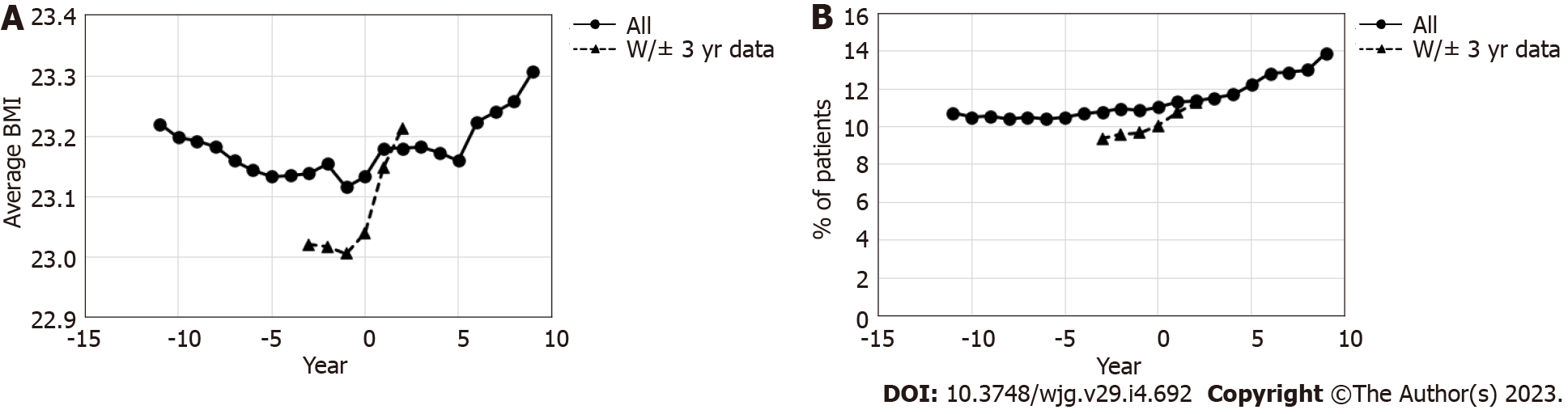
At least one set of data regarding BMI was available for 1701111 patients during the observation period, and 100954 patients had BMI data available for every year of the 3 years before and after the year of eradication. Among patients with at least one data point, the average BMI slightly decreased toward the year of eradication, following which it slightly increased (Figure 6A). This increase was even more remarkable among those with complete BMI data in the 3 years before and after eradication.
In all patients with health check-up data available at least once (1701111 patients), the percentage of patients with MS slightly increased following eradication (Figure 6B). An even larger increase after eradication was observed in patients with all data from 3 years before and after eradication.
This study comprehensively analyzed the effects of H. pylori eradication, including both favorable and unfavorable aspects, in Japan. The database used in this study is a nationwide claims database comprising data from almost the entire Japanese population, including more than 5 million people who received primary H. pylori eradication therapy. While favorable effects on GU, DU, and gastritis were confirmed, the treatment could result in the development of several concerning diseases. In particular, the possibility of an increase in allergic diseases is a new finding that has not been reported in the past.
When the effect of aging and reporting period was considered, there was no significant increase in the incidence of GERD after eradication. Previous studies reported conflicting results regarding the relationship between H. pylori infection and GERD development. Among Japanese H. pylori-positive patients, the odds ratio (OR) for GERD development is reported to be 0.35-0.50[10,32,33]. The lower rates of GERD development in patients with H. pylori is reportedly due to the suppression of gastric acid production caused by H. pylori infection. However, a systematic review based on randomized controlled trials (RCTs) of patients who underwent H. pylori eradication indicated no significant increase in GERD occurrence at 6, 12, and 24 mo following eradication[12]. No significant effects of H. pylori eradication on GERD development were suggested by another systematic review analyzing RCTs[34]. In addition, another RCT showed that H. pylori eradication did not affect the recurrence rate of GERD after eradication[35]. In contrast, an increased risk following eradication therapy was suggested by a meta-analysis of cohort studies and RCTs[36]. These contradictory results might be due to differences in baseline diseases and study design. In fact, another systematic review suggested that eradication is not associated with GERD development in dyspeptic patients, whereas an association in patients with peptic ulcers is suggested by results of cohort studies but not of RCTs[13]. Collectively, the findings of previous reports together with our results suggest the lack of an association between H. pylori eradication and GERD development. Moreover, the type of GERD medication did not differ between patients with and without eradication. This suggests that a change in GERD treatment including a switch to more powerful P-CAB or an increase in dosage of PPI is not required following eradication.
After eradication, a higher incidence of BE was observed in all age groups and over time. A previous case-control study reported a lower risk of BE in patients with H. pylori infection, with an overall OR of 0.55 [95% confidence interval (CI): 0.35-0.84] after adjusting for age and white race, as well as an OR of 0.28 (0.15-0.50) in those with corpus atrophy or antisecretory drug use[37]. The diagnostic criteria of BE is different in Japan and Western countries, so direct comparisons are difficult[38]. However, a possible explanation for the higher incidence of BE may have to do with the palisade vessel at the distal esophagus, a landmark of the gastroesophageal junction used in Japan, being more clearly identifiable by endoscopy after eradication, as H. pylori may colonize the esophageal mucosa, causing inflammation[39,40]. After eradication of H. pylori, small areas of columnar metaplasia may become visible due to resolution of mucosal inflammation. Utilization of advanced image-enhanced endoscopy, such as linked color imaging may also contribute to a higher diagnostic performance for BE[41]. Notably, BE lesions in Japanese patients were very short (< 1 cm) in most cases, and patients with ≥ 3-cm lesions were rare (approximately ≤ 1%)[42]. A previous Japanese case report described a case of esophageal adenocarcinoma after H. pylori eradication[43]. However, a Swedish cohort study indicated no evidence of an increased risk of BE or esophageal adenocarcinoma after H. pylori eradication[44]. Further research, including an evaluation of the characteristics of BE and its association with cancer, is warranted to evaluate the true effects of eradication on BE in Japanese patients.
Both age-dependent and monthly analyses suggest lower incidences of other upper gastrointestinal diseases following eradication therapy. Among these diseases, GU, DU, and gastritis and duodenitis (analyzed together) were the most common before eradication. The effect of H. pylori eradication on the risk of GU, DU, and gastritis has been previously established[1,4]; therefore, the reduced incidence of these diseases after eradication was not surprising. Further, we found an increase in FD, as recorded in the claims data, after eradication (Supplementary Figure 3), contrary to previous meta-analysis on RCTs reporting improvement or no change after eradication[45-47]. This discrepancy may be explained by our insurance claim system. For patients with dyspepsia symptoms and H. pylori infection, their diagnosis would be H. pylori-positive gastritis, which allows them to receive eradication therapy under insurance coverage. However, if they still had dyspeptic symptoms after successful eradication, their diagnosis would be converted to FD, thereby increasing the number of patients with this label.
A protective effect of H. pylori infection against IBD has been suggested by an observational cohort study[48] and meta-analyses[19-21]. This relationship is explained by the effects of H. pylori infection on the immune system[18]. H. pylori infection increases gastric mucosal expression of a regulatory T cell marker, forkhead box P3, which decreases inflammatory Th1/Th17 responses and leads to suppression of inflammatory diseases including IBD[18,21]. Recently, metagenomic studies demonstrated an uneven recovery of the human gut microbiome after treatment with antibiotics[49,50], and the use of antibiotics was associated with an increased risk of developing both new-onset CD and UC. This risk was highest in the 1st year after antibiotic intake[51]. However, a higher incidence after eradication was also observed in all ages when comparing the 3-year period before and after eradication, and the incidence of CD and UC was higher in younger patients. In previous reports, eradication was not associated with an increased risk of IBD[52], or numerically, but not significantly increased[53]. Meanwhile, a significant increase in the risk of autoimmune diseases including IBD after eradication has also been reported[54]. A case-control study using Swedish national registry data showed that a history of antibiotic use is associated with IBD development, and that dispensation of multiple antibiotics increases the risk[55]. Our results are therefore consistent with the Swedish national registry data and should be investigated in other populations.
An inverse correlation of H. pylori infection with allergy has been reported in Japan[17] and other countries, and several mechanisms have been proposed[14-16]. H. pylori neutrophil-activating protein (HP-NAP), a virulence factor of H. pylori, reportedly stimulates neutrophils, increasing their production of oxygen radicals and adhesion to endothelial cells[56]. HP-NAP was shown to inhibit eosinophil infiltration and serum immunoglobulin E production in the lung, resulting in inhibition of interleukin (IL)-4 and IL-5 production in a mouse model[57]. These immunological changes in response to H. pylori eradication may be associated with the development of allergic diseases, as supported by our own findings. While the estimated number needed to treat for 3 years before and after eradication was calculated to be 58.8 for GU, 714.3 for DU, and 19.2 for gastritis and duodenitis, the estimated number needed to harm (NNH) for this period was found to be 12.3 for allergic diseases (unpublished data); this highlights the necessity of monitoring the effects of eradication therapy on allergy. Nevertheless, the effects of eradication therapy on chronic urticaria remain controversial; eradication therapy is recommended for those with chronic urticaria in Japanese guidelines[58]. Further large-scale prospective studies are warranted to understand the association between eradication and allergic diseases.
There are many reports on the relationship between H. pylori eradication and BMI and MS (Supplementary Table 4). Previous reports indicate that eradication generally increases BMI. An RCT conducted in South West England including H. pylori-infected patients with an average BMI of approximately 27 kg/m2 reported an increase in BMI after eradication[59]. The increase in BMI after eradication is reportedly caused by the improvement of dyspepsia symptoms[59] and effects on the hormonal systems via ghrelin and leptin, which regulate food intake and appetite[24,59].
On the other hand, controversy remains about MS. Previous reports, including a Japanese study before 2010, have reported that dyslipidemia is exacerbated by eradication. However, recent reports have suggested that the factors that make up MS, especially dyslipidemia and diabetes, tend to improve after eradication (Supplementary Table 4). Recent Korean studies examining patients with an average BMI of approximately 24 kg/m2 suggested an association between H. pylori infection and the risk of dyslipidemia[24,27], as well as a decrease in this risk after successful eradication[27]. The difference between the previous reports and our results may be due to the difference in the background of the population and the length of the observation period. Dyslipidemia is greatly affected not only by H. pylori status but also by age, sex, socioeconomic status, BMI, smoking status, diet, alcohol consumption, and exercise. Recently, changes in the gut microbiota after eradication and its effects on metabolism have been discussed[26,60], and further elucidation of this pathophysiology is awaited.
To our knowledge, this is the first study to investigate the effects of H. pylori eradication on a comprehensive disease spectrum in one of the largest target populations with a long observation period. However, this study has several limitations. First, we defined patients who received H. pylori eradication therapy based on prescription data. As such, there are no data about actual drug intake and the results of eradication success. However, we can assume that eradication rate is approximately 80% with PPI-based triple therapy, and 90% with the use of P-CAB-based triple therapy[61]. For those who could not achieve eradication, nearly 100% success can be achieved with a secondary regimen with metronidazole. Second, disease incidences were based on records of the diagnoses; thus, it was not possible to confirm the true onset of the development of the disease. In addition, some patients may have been diagnosed at the time of the medical visit or examination for eradication treatment, as suggested by the highest incidence of gastrointestinal diseases around the year of eradication. Consequently, the incidence might be overestimated around the year of eradication or later. Furthermore, it is possible that some diagnoses recorded in the claims based on drug prescriptions or diagnostic tests could be tentative or even false, although regular audit of the claims is done. In order to reduce such short-term influences, we also analyzed changes of disease incidence in a longer time-span. Third, although the NDB database is comprehensive, we only included a limited subset of patients when assessing the GERD status in patients with and without eradication, (only patients having health check-up data in the year of first GERD diagnosis were included) or when evaluating its effects on BMI and MS (only patients having health check-up data within 3 years before and after eradication were included). This affects the generalizability of these analyses. Finally, these findings may not be applicable to other countries, particularly those with different dietary habits and physical constitutions.
Our data obtained from a nationwide Japanese claims and health check-up database suggest an increased development of BE, IBD, allergic diseases, BMI, and MS, but not of GERD after H. pylori eradication. Although we observed a drastic decrease in the incidence of GU, DU, and gastritis, a large increase in allergic diseases may cancel out these beneficial effects, considering NNH. We believe that a comprehensive long-term assessment of the treatment effects, considering both favorable and unfavorable outcomes, is necessary for evaluating the true value of H. pylori eradication.
Helicobacter pylori (H. pylori) eradication therapy can prevent some diseases, including peptic ulcer disease and gastric cancer. However, potentially unfavorable effects of eradication therapy have also been reported for some diseases, such as gastroesophageal reflux disease (GERD), Barrett’s esophagus (BE), inflammatory bowel disease (IBD), allergic diseases, and metabolic diseases. Consequently, both positive and negative impacts should be considered when assessing the effects of H. pylori eradication therapy.
This study compared the incidence of some diseases before and after H. pylori eradication therapy in order to assess the positive as well as negative effects of the therapy. The comprehensive evaluation of these effects in Japan, where large-scale eradication therapy was commenced earlier than it was in other countries because of coverage by universal healthcare, might provide useful information for clinicians worldwide.
The objective of this study was to compare the incidence of some diseases, which appear to be associated with H. pylori eradication therapy, before and after the eradication in order to obtain a comprehensive overview of the treatment effects.
This study used a Japanese nationwide health claims database, the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB; April 2009-March 2020), developed by the Japanese Ministry of Health, Labour and Welfare. The database contains almost all (≥ 95%) health insurance claims data issued in Japan as well as health check-up data for individuals who underwent specific health check-ups. Patients with a prescription for primary H. pylori eradication therapy covered by national health insurance were analyzed as those who received primary H. pylori eradication therapy. The incidences of GERD, BE, other upper gastrointestinal diseases, and IBD; the prevalence of allergic diseases and metabolic syndrome (MS); and changes in body mass index (BMI) were examined before and after primary H. pylori eradication therapy.
In total, 5219731 patients who received primary eradication therapy were identified in the database. There was no significant increase in the incidence of GERD after eradication when considering the effects of aging and the reporting period. The incidence of BE was higher in the 3-year period after eradication than in the period before eradication for all age categories. The incidence of IBD and prevalence of allergic disease were also higher after eradication. In contrast, the incidences of gastric and DUs and gastritis were decreased after eradication. Among patients with at least one entry of health check-up data (1701111 patients), the percentage of patients with MS showed a slight increase following eradication (11.0% in the year of eradication and 12.2% after 5 years). Because this study only used information recorded in the claims database, the disease incidences were based on records of the diagnoses, and it was not possible to confirm the true onset of the development of the disease. The accuracy of the records of diagnoses also affected the results of this study.
To our knowledge, this is the first study to examine the effects of H. pylori eradication therapy using a large-scale database in Japan. The results suggest that there is an increase in BMI and the development of BE, IBD, allergic diseases, and MS, but not in the development of GERD, after H. pylori eradication therapy. Although the treatment can drastically decrease the incidences of gastric and duodenal ulcers and gastritis, a considerable increase in allergic diseases may cancel out these beneficial effects.
A comprehensive, long-term assessment of the treatment effects, with consideration of both favorable and unfavorable effects, is necessary for evaluating the true value of H. pylori eradication therapy.
The authors thank Mr. Kosuke Iwasaki at the University of Tokyo for data analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Jayaweera J, Sri Lanka; Reshetnyak VI, Russia; Wen XL, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Asaka M, Kato M, Sugiyama T, Satoh K, Kuwayama H, Fukuda Y, Fujioka T, Takemoto T, Kimura K, Shimoyama T, Shimizu K, Kobayashi S; Japan Helicobacter pylori Eradication Study Group. Follow-up survey of a large-scale multicenter, double-blind study of triple therapy with lansoprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. J Gastroenterol. 2003;38:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Yoon SB, Park JM, Lim CH, Cho YK, Choi MG. Effect of Helicobacter pylori eradication on metachronous gastric cancer after endoscopic resection of gastric tumors: a meta-analysis. Helicobacter. 2014;19:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 3. | Nakamura S, Sugiyama T, Matsumoto T, Iijima K, Ono S, Tajika M, Tari A, Kitadai Y, Matsumoto H, Nagaya T, Kamoshida T, Watanabe N, Chiba T, Origasa H, Asaka M; JAPAN GAST Study Group. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut. 2012;61:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 4. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1322] [Cited by in RCA: 1184] [Article Influence: 118.4] [Reference Citation Analysis (0)] |
| 5. | Franchini M, Cruciani M, Mengoli C, Pizzolo G, Veneri D. Effect of Helicobacter pylori eradication on platelet count in idiopathic thrombocytopenic purpura: a systematic review and meta-analysis. J Antimicrob Chemother. 2007;60:237-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 6. | Hudak L, Jaraisy A, Haj S, Muhsen K. An updated systematic review and meta-analysis on the association between Helicobacter pylori infection and iron deficiency anemia. Helicobacter. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 7. | Graham DY, Yamaoka Y. H. pylori and cagA: relationships with gastric cancer, duodenal ulcer, and reflux esophagitis and its complications. Helicobacter. 1998;3:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Richter JE, Falk GW, Vaezi MF. Helicobacter pylori and gastroesophageal reflux disease: the bug may not be all bad. Am J Gastroenterol. 1998;93:1800-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Raghunath A, Hungin AP, Wooff D, Childs S. Prevalence of Helicobacter pylori in patients with gastro-oesophageal reflux disease: systematic review. BMJ. 2003;326:737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 233] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Chiba H, Gunji T, Sato H, Iijima K, Fujibayashi K, Okumura M, Sasabe N, Matsuhashi N, Nakajima A. A cross-sectional study on the risk factors for erosive esophagitis in young adults. Intern Med. 2012;51:1293-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Nam SY, Choi IJ, Ryu KH, Kim BC, Kim CG, Nam BH. Effect of Helicobacter pylori infection and its eradication on reflux esophagitis and reflux symptoms. Am J Gastroenterol. 2010;105:2153-2162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Qian B, Ma S, Shang L, Qian J, Zhang G. Effects of Helicobacter pylori eradication on gastroesophageal reflux disease. Helicobacter. 2011;16:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Yaghoobi M, Farrokhyar F, Yuan Y, Hunt RH. Is there an increased risk of GERD after Helicobacter pylori eradication? Am J Gastroenterol. 2010;105:1007-13; quiz 1006, 1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | McCune A, Lane A, Murray L, Harvey I, Nair P, Donovan J, Harvey R. Reduced risk of atopic disorders in adults with Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2003;15:637-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167:821-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 282] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 16. | Blaser MJ, Chen Y, Reibman J. Does Helicobacter pylori protect against asthma and allergy? Gut. 2008;57:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Shiotani A, Miyanishi T, Kamada T, Haruma K. Helicobacter pylori infection and allergic diseases: epidemiological study in Japanese university students. J Gastroenterol Hepatol. 2008;23:e29-e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Arnold IC, Hitzler I, Müller A. The immunomodulatory properties of Helicobacter pylori confer protection against allergic and chronic inflammatory disorders. Front Cell Infect Microbiol. 2012;2:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Wu XW, Ji HZ, Yang MF, Wu L, Wang FY. Helicobacter pylori infection and inflammatory bowel disease in Asians: a meta-analysis. World J Gastroenterol. 2015;21:4750-4756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 20. | Rokkas T, Gisbert JP, Niv Y, O'Morain C. The association between Helicobacter pylori infection and inflammatory bowel disease based on meta-analysis. United European Gastroenterol J. 2015;3:539-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Luther J, Dave M, Higgins PD, Kao JY. Association between Helicobacter pylori infection and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Inflamm Bowel Dis. 2010;16:1077-1084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 22. | Roka K, Roubani A, Stefanaki K, Panayotou I, Roma E, Chouliaras G. The prevalence of Helicobacter pylori gastritis in newly diagnosed children with inflammatory bowel disease. Helicobacter. 2014;19:400-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Satoh H, Saijo Y, Yoshioka E, Tsutsui H. Helicobacter Pylori infection is a significant risk for modified lipid profile in Japanese male subjects. J Atheroscler Thromb. 2010;17:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Kim TJ, Lee H, Kang M, Kim JE, Choi YH, Min YW, Min BH, Lee JH, Son HJ, Rhee PL, Baek SY, Ahn SH, Kim JJ. Helicobacter pylori is associated with dyslipidemia but not with other risk factors of cardiovascular disease. Sci Rep. 2016;6:38015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, Urabe A. Helicobacter pylori infection is significantly associated with metabolic syndrome in the Japanese population. Am J Gastroenterol. 2008;103:3005-3010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Liou JM, Chen CC, Chang CM, Fang YJ, Bair MJ, Chen PY, Chang CY, Hsu YC, Chen MJ, Lee JY, Yang TH, Luo JC, Chen CY, Hsu WF, Chen YN, Wu JY, Lin JT, Lu TP, Chuang EY, El-Omar EM, Wu MS; Taiwan Gastrointestinal Disease and Helicobacter Consortium. Long-term changes of gut microbiota, antibiotic resistance, and metabolic parameters after Helicobacter pylori eradication: a multicentre, open-label, randomised trial. Lancet Infect Dis. 2019;19:1109-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (1)] |
| 27. | Park Y, Kim TJ, Lee H, Yoo H, Sohn I, Min YW, Min BH, Lee JH, Rhee PL, Kim JJ. Eradication of Helicobacter pylori infection decreases risk for dyslipidemia: A cohort study. Helicobacter. 2021;26:e12783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Furuta T, Shirai N, Xiao F, Takashima M, Hanai H. Effect of Helicobacter pylori infection and its eradication on nutrition. Aliment Pharmacol Ther. 2002;16:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Kamada T, Hata J, Kusunoki H, Ito M, Tanaka S, Kawamura Y, Chayama K, Haruma K. Eradication of Helicobacter pylori increases the incidence of hyperlipidaemia and obesity in peptic ulcer patients. Dig Liver Dis. 2005;37:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Tsuneishi M, Yamamoto T, Yamaguchi T, Kodama T, Sato T. Association between number of teeth and Alzheimer's disease using the National Database of Health Insurance Claims and Specific Health Checkups of Japan. PLoS One. 2021;16:e0251056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Takahashi K, Bokura H, Kobayashi S, Iijima K, Nagai A, Yamaguchi S. Metabolic syndrome increases the risk of ischemic stroke in women. Intern Med. 2007;46:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Kawai T, Yamamoto K, Fukuzawa M, Yamagishi T, Yagi K, Kataoka M, Kawakami K, Itoi T, Sakai Y, Moriyasu F, Takagi Y, Aoki T. Helicobacter pylori infection and reflux esophagitis in young and middle-aged Japanese subjects. J Gastroenterol Hepatol. 2010;25 Suppl 1:S80-S85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Gunji T, Sato H, Iijima K, Fujibayashi K, Okumura M, Sasabe N, Urabe A, Matsuhashi N. Risk factors for erosive esophagitis: a cross-sectional study of a large number of Japanese males. J Gastroenterol. 2011;46:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Saad AM, Choudhary A, Bechtold ML. Effect of Helicobacter pylori treatment on gastroesophageal reflux disease (GERD): meta-analysis of randomized controlled trials. Scand J Gastroenterol. 2012;47:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Moayyedi P, Bardhan C, Young L, Dixon MF, Brown L, Axon AT. Helicobacter pylori eradication does not exacerbate reflux symptoms in gastroesophageal reflux disease. Gastroenterology. 2001;121:1120-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 135] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Xie T, Cui X, Zheng H, Chen D, He L, Jiang B. Meta-analysis: eradication of Helicobacter pylori infection is associated with the development of endoscopic gastroesophageal reflux disease. Eur J Gastroenterol Hepatol. 2013;25:1195-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Fischbach LA, Graham DY, Kramer JR, Rugge M, Verstovsek G, Parente P, Alsarraj A, Fitzgerald S, Shaib Y, Abraham NS, Kolpachi A, Gupta S, Vela MF, Velez M, Cole R, Anand B, El Serag HB. Association between Helicobacter pylori and Barrett's esophagus: a case-control study. Am J Gastroenterol. 2014;109:357-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Kuwano H, Nishimura Y, Oyama T, Kato H, Kitagawa Y, Kusano M, Shimada H, Takiuchi H, Toh Y, Doki Y, Naomoto Y, Matsubara H, Miyazaki T, Muto M, Yanagisawa A. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus. 2015;12:1-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 345] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 39. | Henihan RD, Stuart RC, Nolan N, Gorey TF, Hennessy TP, O'Morain CA. Barrett's esophagus and the presence of Helicobacter pylori. Am J Gastroenterol. 1998;93:542-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Contreras M, Salazar V, García-Amado MA, Reyes N, Aparcero M, Silva O, Castro D, Romero R, Gueneau P, Michelangeli F. High frequency of Helicobacter pylori in the esophageal mucosa of dyspeptic patients and its possible association with histopathological alterations. Int J Infect Dis. 2012;16:e364-e370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Adachi K, Ishimura N, Kishi K, Notsu T, Mishiro T, Sota K, Ishihara S. Prevalence of Barrett's Epithelium Shown by Endoscopic Observations with Linked Color Imaging in Subjects with Different H. pylori Infection Statuses. Intern Med. 2021;60:667-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Watari J, Hori K, Toyoshima F, Kamiya N, Yamasaki T, Okugawa T, Asano H, Li ZL, Kondo T, Ikehara H, Sakurai J, Tomita T, Oshima T, Fukui H, Miwa H. Association between obesity and Barrett's esophagus in a Japanese population: a hospital-based, cross-sectional study. BMC Gastroenterol. 2013;13:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Abe Y, Koike T, Iijima K, Imatani A, Ishida K, Yuki T, Miyata G, Shimosegawa T. Esophageal Adenocarcinoma Developing after Eradication of Helicobacter pylori. Case Rep Gastroenterol. 2011;5:355-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Doorakkers E, Lagergren J, Santoni G, Engstrand L, Brusselaers N. Helicobacter pylori eradication treatment and the risk of Barrett's esophagus and esophageal adenocarcinoma. Helicobacter. 2020;25:e12688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 45. | Zhao B, Zhao J, Cheng WF, Shi WJ, Liu W, Pan XL, Zhang GX. Efficacy of Helicobacter pylori eradication therapy on functional dyspepsia: a meta-analysis of randomized controlled studies with 12-month follow-up. J Clin Gastroenterol. 2014;48:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 46. | Kang SJ, Park B, Shin CM. Helicobacter pylori Eradication Therapy for Functional Dyspepsia: A Meta-Analysis by Region and H. pylori Prevalence. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Ford AC, Tsipotis E, Yuan Y, Leontiadis GI, Moayyedi P. Efficacy of Helicobacter pylori eradication therapy for functional dyspepsia: updated systematic review and meta-analysis. Gut. 2022;gutjnl-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 48. | Sonnenberg A, Genta RM. Low prevalence of Helicobacter pylori infection among patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 49. | Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1907] [Cited by in RCA: 1739] [Article Influence: 102.3] [Reference Citation Analysis (1)] |
| 50. | Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4554-4561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1850] [Cited by in RCA: 1652] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 51. | Aniwan S, Tremaine WJ, Raffals LE, Kane SV, Loftus EV Jr. Antibiotic Use and New-Onset Inflammatory Bowel Disease in Olmsted County, Minnesota: A Population-Based Case-Control Study. J Crohns Colitis. 2018;12:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | Rosania R, Von Arnim U, Link A, Rajilic-Stojanovic M, Franck C, Canbay A, Malfertheiner P, Venerito M. Helicobacter pylori eradication therapy is not associated with the onset of inflammatory bowel diseases. A case-control study. J Gastrointestin Liver Dis. 2018;27:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Calvet X, Ducons J, Bujanda L, Bory F, Montserrat A, Gisbert JP; Hp Study Group of the Asociación Española de Gastroenterología. Seven versus ten days of rabeprazole triple therapy for Helicobacter pylori eradication: a multicenter randomized trial. Am J Gastroenterol. 2005;100:1696-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Lin KD, Chiu GF, Waljee AK, Owyang SY, El-Zaatari M, Bishu S, Grasberger H, Zhang M, Wu DC, Kao JY. Effects of Anti-Helicobacter pylori Therapy on Incidence of Autoimmune Diseases, Including Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2019;17:1991-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 55. | Nguyen LH, Örtqvist AK, Cao Y, Simon TG, Roelstraete B, Song M, Joshi AD, Staller K, Chan AT, Khalili H, Olén O, Ludvigsson JF. Antibiotic use and the development of inflammatory bowel disease: a national case-control study in Sweden. Lancet Gastroenterol Hepatol. 2020;5:986-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 56. | Amedei A, Cappon A, Codolo G, Cabrelle A, Polenghi A, Benagiano M, Tasca E, Azzurri A, D'Elios MM, Del Prete G, de Bernard M. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J Clin Invest. 2006;116:1092-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 252] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 57. | Codolo G, Mazzi P, Amedei A, Del Prete G, Berton G, D'Elios MM, de Bernard M. The neutrophil-activating protein of Helicobacter pylori down-modulates Th2 inflammation in ovalbumin-induced allergic asthma. Cell Microbiol. 2008;10:2355-2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 58. | Kato M, Ota H, Okuda M, Kikuchi S, Satoh K, Shimoyama T, Suzuki H, Handa O, Furuta T, Mabe K, Murakami K, Sugiyama T, Uemura N, Takahashi S. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 Revised Edition. Helicobacter. 2019;24:e12597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 59. | Lane JA, Murray LJ, Harvey IM, Donovan JL, Nair P, Harvey RF. Randomised clinical trial: Helicobacter pylori eradication is associated with a significantly increased body mass index in a placebo-controlled study. Aliment Pharmacol Ther. 2011;33:922-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 60. | Suzuki S, Gotoda T, Takano C, Horii T, Sugita T, Ogura K, Ichijima R, Kusano C, Ikehara H. Long term impact of vonoprazan-based Helicobacter pylori treatment on gut microbiota and its relation to post-treatment body weight changes. Helicobacter. 2021;26:e12851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Deguchi H, Uda A, Murakami K. Current Status of Helicobacter pylori Diagnosis and Eradication Therapy in Japan Using a Nationwide Database. Digestion. 2020;101:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |