Published online Jan 21, 2023. doi: 10.3748/wjg.v29.i3.549
Peer-review started: September 24, 2022
First decision: October 30, 2022
Revised: November 14, 2022
Accepted: December 23, 2022
Article in press: December 23, 2022
Published online: January 21, 2023
Processing time: 110 Days and 1.6 Hours
In 2020, an international expert panel proposed a new definition of fatty liver: Metabolic dysfunction-associated fatty liver disease (MAFLD). The MAFLD added the criteria for defining metabolic dysfunctions, which are high-risk factors for liver-related and cardiovascular events. Contrary to the non-alcoholic fatty liver disease (NAFLD) definition, it allows the coexistence of MAFLD and sig
To review the existing data that evaluate the clinical profile and long-term outcome difference between the patients identified as MAFLD and NAFLD.
Databases MEDLINE via PubMed and EMBASE were searched and relevant publications up to June 28, 2022 were assessed. Studies were included if they involved human participants diagnosed with MAFLD.
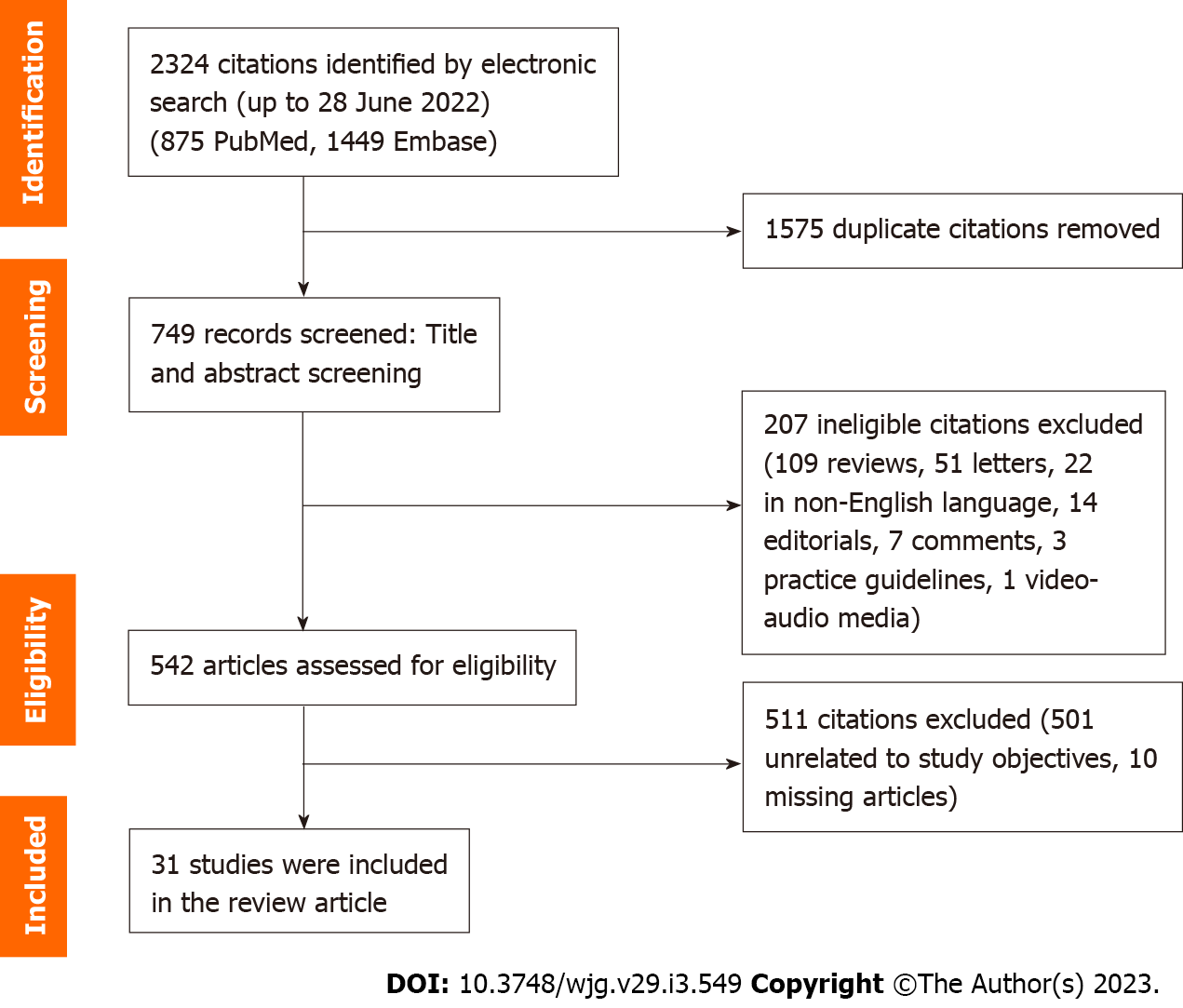
A total of 2324 records were reviewed, of which 1575 duplicate citations were removed. Of the 2324 records screened, 207 articles were excluded, and 542 articles were assessed for their eligibility, for which 511 were excluded. The re
MAFLD is a new definition of fatty liver disease that is gaining increasing acceptance. It is based on empirical clinical practice on positive inclusion of metabolic risk factors and recent evidence suggests that it helps to identify patients with higher risk for liver-related as well as cardiovascular events.
Core Tip: Metabolic dysfunction-associated fatty liver disease (MAFLD) is a new definition of fatty liver disease that is based on positive inclusion of metabolic risk factors. Studies have shown that patients included using the MAFLD criteria were associated with higher risks of hepatic fibrosis and all cause mortality when compared to non-alcoholic fatty liver disease.
- Citation: Tang SY, Tan JS, Pang XZ, Lee GH. Metabolic dysfunction associated fatty liver disease: The new nomenclature and its impact. World J Gastroenterol 2023; 29(3): 549-560
- URL: https://www.wjgnet.com/1007-9327/full/v29/i3/549.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i3.549
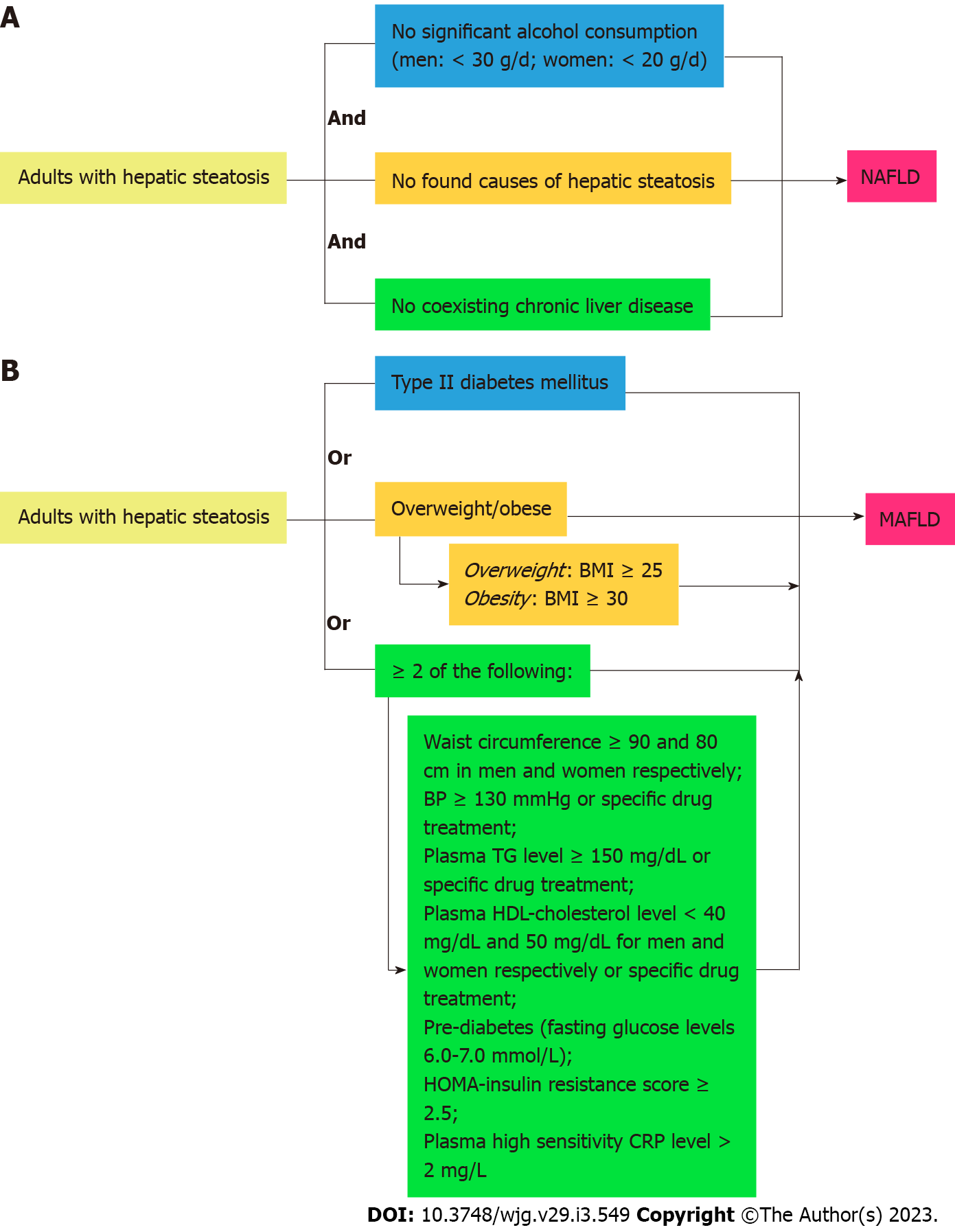
The global prevalence of fatty liver has been rising in recent times, along with metabolic syndrome which are both independently significant contributors to mortality and morbidity worldwide. Since 2020, experts have suggested the change of terminology from non-alcoholic fatty liver disease (NAFLD) to metabolic dysfunction-associated fatty liver disease (MAFLD)[1]. The shift connotes a transition from subtyping patients with hepatic steatosis and no discernible cause of fatty liver, to inclusion criteria characterized by metabolic dysfunction and associated risk factors. NAFLD is an independent disease entity that does not take into account alcohol intake and other causes of pre-existing liver diseases (Figure 1A: Flowchart for the diagnostic criteria of NAFLD).
Metabolic dysfunction in our paper will follow the 1999 World Health Organization definition of metabolic syndrome, which consists of insulin resistance, high fasting glucose, and at least 2 of the following: High-density lipoprotein (HDL)-cholesterol, triglycerides (TG), blood pressure and the presence of obesity. The new proposed MAFLD diagnostic criteria are as follows in Figure 1B (flowchart for the diagnostic criteria for MAFLD): Since the conception of new diagnostic criteria for MAFLD, there have been numerous debates regarding whether this new term should be adopted. There is still a lack of awareness regarding the new terminology and diagnostic criteria amongst many healthcare professionals across the world. This study aims to summarize existing data that evaluate the long-term outcome differences of the change from NAFLD to MAFLD. The study also evaluated the classification of hepatic steatosis by the new MAFLD diagnostic criteria, histopathological classification, as well as risk factors and pathophysiological mechanisms of the new proposed disease entity.
We included studies ranging from case reports to randomized control trials that have been published till June 28, 2022. We excluded abstracts in this review and have restricted to only studies in English. We excluded studies with insufficient information concerning our outcomes of interest and areas of comparison, e.g., survival, incidence of liver steatosis and severity of fibrosis. A PRISMA checklist was also used to guide the development of the systematic review.
A comprehensive systematic search of databases and conference proceedings was conducted to identify all relevant studies up to June 28, 2022. The following electronic databases were searched: MEDLINE via PubMed, and EMBASE, with reference to PRISMA guidelines. We used both text words and medical subject heading terms. The literature search strategy was adapted to suit each database. Our search terms included: “Metabolic-Associated Fatty Liver disease” OR “Metabolic dysfunction-associated fatty liver disease” OR “MAFLD vs Non-Alcoholic Fatty Liver disease” or “MAFLD vs Non-alcoholic Steatohepatitis” OR “Metabolic Associated Steatohepatitis”. The methods for data collection and analysis were based on the Cochrane Handbook of Systematic Reviews for Interventions. Where clarification of information in published data was required, corresponding authors were contacted through electronic mail for clarification.
Two authors (Tan JS and Pang XZ) independently selected potentially eligible studies using the data management software Rayyan QCRI. The initial screening was based on title and abstract, while final inclusion was based on full texts where available. After reading the titles and abstracts of the identified articles, full-text articles of all citations deemed to meet the inclusion criteria were sought. Duplicates were excluded. Each article was independently inspected to verify that they meet the pre-specified inclusion criteria. The study selection process is summarized in Figure 2 (summary of study selection process). Studies that were included in this systematic review are included in Tables 1 and 2 and Supplementary Tables 1-4. The authors included observational studies reporting the implications of MAFLD vs NAFLD.
| Main outcome | Number of studies | Sample | Conclusion | |
| Hepatic steatosis and fibrosis identification in MAFLD terminology change | ||||
| Steatosis and fibrosis | 10 | 38686 subjects | MAFLD definition is able to capture more subjects with fatty liver disease | |
| MAFLD group showed either no difference or higher in fibrosis or liver stiffness compared to NAFLD group | ||||
| Long-term outcome differences in MAFLD terminology change | ||||
| All cause mortality risks and cause specific mortality | 4 | 183380 subjects | MAFLD is associated with an increased risk of mortality compared to NAFLD | |
| MAFLD mortality is largely contributed by the presence of metabolic disorders | ||||
| All cause mortality risks | 1 | 12878 subjects | MAFLD and NAFLD share similar all-cause mortality risk | |
| MAFLD mortality is hence likely caused by ALD, while NAFLD mortality seems to be caused by metabolic abnormalities | ||||
| MAFLD and correlation to non-liver diseases | ||||
| CVD, ASCVD, cardiovascular events | 3 | 2458240 subjects | The risk of CVD is higher in MAFLD compared to NAFLD | |
| MAFLD is superior over NAFLD in predicting ASCVD risk, contributed by the presence of metabolic risk factors | ||||
| Clinical and histopathological features of MAFLD | ||||
| Risk factors, steatosis, advanced fibrosis | 9 | 237679 subjects | T2DM and obesity are significant drivers of MAFLD pathogenesis | |
| MAFLD patients had higher BMI, LDL-C and prevalence of T2DM as compared to NAFLD patients | ||||
| Older age, females and menopausal status are risks factors for developing MAFLD | ||||
| Ref. | Type of study | Sample | Main outcomes | Results | Conclusion |
| Taheri et al[29] | Case-control study | 968 subjects from Iran | DIS, LIS | Risks of MAFLD (OR): High LIS and DIS > high LIS > high DIS (2.56 vs 1.96 vs 1.84; P < 0.001) | Pro-inflammatory dietary and lifestyle exposures are associated with higher risk of MAFLD regardless of gender. Inflammation may be a primary pathogenic mechanism behind dietary risks of MAFLD development |
| Mu et al[30] | Case-control study | 564 subjects from Xinjiang Uygur Autonomous Region, China | SNP | Risks of MAFLD (OR): PNPLA3 rs738409 CC genotype > MBOAT7 rs64173 TT genotype > STAT3 rs74416 AA genotype (1.402 vs 1.299 vs 0.738; P < 0.005) | The CC genotype of PNPLA3 rs738409 and TT genotype of MBOAT7 rs64173 genes are associated with higher risks of MAFLD. The AA genotype of STAT3 rs744166 gene is associated with lower risks of MAFLD. The genes TM6SF2 rs58542926 and GATAD2A rs4808199 show no significant correlation with MAFLD |
| Panera et al[31] | Cohort study-retrospective | 1111 subjects from Milan, Italy | Hepatic fibrosis | Associations of KLB rs17618244 variant (OR): Hepatic fibrosis (1.23; P = 0.04) | The KLB rs17618244 variant was associated with hepatic fibrosis (P = 0.04) but showed no statistical significance in the correlation with steatosis, inflammation and ballooning (P = 0.37, 0.12, 0.16 respectively) |
| Oses et al[32] | Cross-sectional study | 115 children (8-12 years old) | Fasting blood biochemical parameters, SNP | TG, insulin, HOMA-IR, ALT, AST, GGT, ferritin: MAFLD > non-MAFLD (P < 0.05). Percentage of risk of allele carriers: PNPLA3 rs4823173 > PPARG rs1801282 > PPARG rs13081389, HFE rs1800562 (46% vs 33% vs 21%; P < 0.05) | The genetic risk score based on 4 SNPs associated with MAFLD showed limited discriminatory capacity (67% sensitivity and 65% specificity) and did not improve the accuracy of the prediction protocol for MAFLD developed in the study |
A total of 2324 records were reviewed, of which 1575 duplicate citations were removed. Of the 2324 records screened, 207 articles were excluded, and 542 articles were assessed for their eligibility, for which 511 were excluded. The remaining 31 articles that were selected explored various themes, such as the long-term outcome differences of using the MAFLD criteria as compared to the NAFLD criteria, the fibrosis burden in MAFLD as compared to NAFLD, the correlation of MAFLD with other diseases, the histopathological characteristics of MAFLD, as well as risk factors and pathophysiological mechanisms of the new proposed disease entity. Articles that did not compare MAFLD and NAFLD criteria were excluded.
In capturing subjects with hepatic steatosis, the majority of the studies reviewed display a preference for the new MAFLD diagnostic criteria compared with the previous NAFLD, with the new definition being able to identify individuals with dual liver disease etiologies on top of all previously diagnosed NAFLD subjects[2-5]. Results from the Plinio Study also demonstrated that applying the MAFLD criteria reduces the unexplained form of lean NAFLD by identifying the presence of metabolic risk factors in these patients[6]. The Rotterdam Study was also able to identify more individuals with fatty liver disease by applying the MAFLD criteria, where the prevalence of modified MAFLD was higher than NAFLD (34.4% and 29.5%) in their population[7]. MAFLD criteria are also useful in determining the disease severity of patients with diagnosed hepatic steatosis; people with hepatic steatosis who do not fulfil MAFLD criteria are less likely to have significant liver disease as compared to those who are diagnosed with MAFLD (Table 1 and Supplementary Table 1).
In detecting subjects with liver fibrosis, MAFLD criteria also proved superior or concordant with NAFLD in many studies included in this paper[3-5,8],. Results show that the prevalence of significant fibrosis and liver stiffness is considerable in the MAFLD-only group, with marginal differences between the NAFLD-only group and metabolically healthy subjects. One study reported that liver stiffness was higher in MAFLD participants compared to NAFLD participants (7.7 vs 6.8 kPa, P = 0.0010)[5]. Compared to NAFLD participants, MAFLD participants also had higher serum liver enzymes (aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase), fatty liver index, and fibrosis scores including aminotransferase/platelet ratio index (APRI) and NAFLD fibrosis scores. In MAFLD participants with excessive alcohol intake (≥ 30 g/d for males and ≥ 20 g/d for females), it was found that they have a significantly higher APRI score compared to those without excessive alcohol intake[2] (Table 1 and Supplementary Table 1).
However, all the studies reviewed could only provide an estimate of fibrosis and steatosis as the gold-standard technique for diagnosis (liver biopsy) was not done in these large population-based studies. The definition of fibrosis also differed among the studies with one study[7] using liver stiffness ≥ 8.0 kPa as the definition of fibrosis while another[9] defined fibrosis by liver stiffness measure ≥ 9.7 kPa and controlled attenuation parameter ≥ 274 dB/m (Table 1 and Supplementary Table 1).
Park et al[10] categorized MAFLD subjects into metabolic health - MAFLD group (≤ 1 risk factor and no diabetes) and metabolic unhealthy MAFLD group (having diabetes and/or ≥ 2 metabolic risk abnormalities) and found that the MH - MAFLD group showed no difference in the prevalence of significant or advanced hepatic fibrosis or carotid artery plaque formation compared with the healthy control group. Between the groups, there were marked differences in comorbidities and hepatic fibrosis burden, suggesting that the MAFLD definition involves an inhomogeneous population at risk of hepatic fibrosis and hence the need for a more elaborate definition (Table 1 and Supplementary Table 1).
There is also a gap in the literature surrounding the application of MAFLD criteria in the pediatric population. Although Ciardullo et al[11] managed to find the MAFLD criteria being fulfilled in most of their population (United States adolescents with evidence of hepatic steatosis), it did not affect the prevalence of significant fibrosis and liver stiffness between MAFLD patients and non-MAFLD steatotic patients. This might be due to the inherent chronicity in the progression of hepatic steatosis to liver fibrosis; more time should be granted to investigate the correlation between the new diagnostic criteria and long-term outcomes prospectively in the pediatric population (Table 1 and Supplementary Table 1).
Prospectively, many of the included studies show that individuals with MAFLD demonstrate higher all-cause, cardiovascular-related and cancer-related mortality as compared to individuals with NAFLD, or individuals with neither MAFLD or NAFLD[12-15]. A United States study that analyzed 7761 participants with a median follow-up of 23 years, noted that MAFLD patients who do not meet NAFLD criteria have a 1.7-fold higher risk of all-cause mortality, an association not demonstrated in patients with NAFLD or simple hepatic steatosis[15]. Even among MAFLD patients, individuals who meet all 3 criteria of its definition seem to exhibit higher all-cause mortality than those only fulfilling 1 or 2 of the criteria. Individuals who fulfilled all 3 MAFLD criteria had the highest hazard ratio [hazard ratio (HR)] for all-cause mortality risk (HR = 2.05), followed by individuals with metabolic dysfunction and type 2 diabetes mellitus (T2DM) (HR = 1.83), and lastly individuals with only metabolic dysfunction (HR = 1.30)[12] (Table 1 and Supplementary Table 2).
All-cause mortality in MAFLD patients is postulated to be driven by its individual metabolic constituents. Of which, T2DM has the strongest association, followed by metabolic dysfunction and elevated body mass index (BMI)[12-14]. In a United States population study[12], participants with MAFLD were sub-grouped into 1 of the 3 MAFLD criteria and were subsequently analyzed. Interestingly, the overweight (BMI ≥ 25.0 kg/m2) subgroup was not associated with cancer-related mortality while the metabolic dysregulation subgroup (lean individuals with ≥ 2 metabolic risk factors among non-diabetic participants) was only associated with all-cause mortality, suggesting that T2DM is the most multifaceted cause of mortality in MAFLD patients. A similar study conducted in Kailuan, China showed similar results in that T2DM and metabolic dysfunction have the highest mortality risks (HR = 2.16, 1.79 respectively) among the MAFLD subtypes[14]. A suggested explanation is that on top of proinflammatory, pro-atherogenic and diabetogenic mediators released by livers of patients with NAFLD, the constant exposure to hyperglycaemia and raised concentrations of circulating insulin stimulated cancer progression[12] (Table 1 and Supplementary Table 2).
Age and gender seem to play a role in the mortality risks of MAFLD patients too. Among Kailuan Chinese adults, mortality risks have also been found to be higher in younger adults with MAFLD, with risks declining with age regardless of gender[14]. This association seems to suggest that early-onset metabolic comorbidities are more deleterious in MAFLD patients than when presented at later ages. It is also worth noting that the same study found that obesity has a negative association with mortality risks in older age groups (males above 40 years of age and females above 50 years of age). The non-concordant results could be explained by the obesity paradox, whereby excess adipose tissue could serve as an energy reserve, which could grant a survival advantage in older patients. This might be particularly significant in cancer-related mortality in older MAFLD patients, who are more likely to suffer from malnutrition or poor appetite. A study using LASSO regularisation for variable adjustment found that MAFLD association with cardiovascular-related and cancer-related mortality lost significance once age, gender and ethnicity were accounted for[13], signifying that age and gender are secondarily important in mortality pathways in MAFLD patients (Table 1 and Supplementary Table 2).
A study of contention points out that the MAFLD definition has failed to capture the impact of metabolic dysfunction on long-term mortality outcomes, attributing the cause of increased all-cause mortality in the MAFLD group to the inclusion of alcoholic liver disease[16] rather than predisposing metabolic derangements. The study demonstrated good concordance between MAFLD and NAFLD groups with similar clinical characteristics except in components of each definition (e.g., alcohol use for MAFLD) and concluded that there was no difference in cumulative all-cause and cause-specific mortality. In another study, individuals with MAFLD, advanced fibrosis was also associated with a higher risk of all-cause mortality [HR = 1.95; 95% confidence interval (CI): 1.46-2.60; P < 0.001], while individuals with NAFLD and advanced fibrosis were not significantly associated with all – cause mortality (HR = 1.33; 95%CI: 0.91-1.94; P = 0.144)[15]. These findings suggest that MAFLD’s strong association with all-cause mortality is independent of known metabolic risk factors, though a point to consider is that mortality risk factors were only retrospectively available for NHANES III data set[15] and not for NHANES 2017-2018 data set reported in the study, which led to fibrosis being used as a surrogate marker for mortality. Contrarily, a study conducted using the Third National Health and Nutrition Examination Survey showed that MAFLD participants had a higher mortality risk regardless of excessive alcohol consumption status over a median follow up of 23.2 years[12] (Table 1 and Supplementary Table 2).
NAFLD is tied very closely to cardiovascular diseases (CVD), with CVD being the most important cause of death in NAFLD patients. Hepatic steatosis is independently associated with coronary plaques and both hepatic steatosis and fibrosis are significantly associated with diastolic heart dysfunction. Multiple reports have shown that MAFLD is largely superior to NAFLD in the identification of high-risk patients for atherosclerotic cardiovascular diseases[17-19]. In a retrospective cohort study of 2,452,949 Japanese patients, of which the prevalence of MAFLD was estimated to be 9.7% (n = 237242), the overall prevalence of hypertriglyceridemia, DM and both were 13.6%, 4.3% and 1.1% in non MAFLD patients, compared to 64.1%, 20.6% and 12.9% respectively, in the MAFLD group[17]. The same study also demonstrated that risks of coronary artery disease and CVD were higher in the MAFLD group than in the non-MAFLD group, but the CVD risks were almost the same in NAFLD and non-NAFLD group (HR = 1.02) after adjustments for metabolic syndrome factors, low-density lipoprotein cholesterol (LDL-C), statin use, age, gender, and smoking (Table 1 and Supplementary Table 3).
A single-center cohort study in Japan demonstrated that MAFLD, but not NAFLD, was an independent risk factor for the worsening of atherosclerotic disease[18]. It also identified that the presence of metabolic dysfunction might be the main risk factor for developing cardiovascular disease in MAFLD, instead of alcohol consumption. This suggests that the MAFLD criteria were superior to NAFLD in identifying patients at risk of CVD (Table 1 and Supplementary Table 3).
Patients diagnosed with the MAFLD criteria, but not fulfilling the NAFLD definition, had higher baseline metabolic derangements, except low HDL, compared to patients diagnosed with NAFLD but not fulfilling MAFLD criteria[19]. The same group of patients was also found to have a higher risk of developing general obesity, DM, and cardiovascular events at the end of a 7-year follow (Table 1 and Supplementary Table 3).
With the new MAFLD definition gaining traction, many studies have explored methods to characterize the typical patient profile. MAFLD patients tend to be older, have higher BMI, and have more metabolic comorbidities as compared to healthy controls[20]. Unsurprisingly, the presence of metabolic traits meant a higher likelihood of inclusion into the MAFLD population. Compared to NAFLD, the MAFLD population has higher metabolic traits, including high TG, overweight or obesity, glucose intolerance and higher liver enzymes[21]. This result was similar to a study conducted in Fujian, China, where it was found that the MAFLD had higher BMI, LDL-C and T2DM prevalence as compared to NAFLD patients or healthy controls[22] (Table 1 and Supplementary Table 4).
It seems that the number of co-existing metabolic characteristics play an important role in defining the clinical characteristics of MAFLD patients. Patients with two or more metabolic conditions at diagnosis, had a higher grade of hepatic and renal injury compared to those with only one metabolic condition. As the number of concomitant metabolic comorbidities increased, MAFLD patients tended to be older, females, had renal impairment clinically and were more likely to have advanced fibrosis[23] (Table 1 and Supplementary Table 4).
The peak prevalence of MAFLD in the female population is older as compared to the male population[24,25]. This could be due to menopausal factors, where estrogen is postulated to have a protective effect on metabolic disorders. Post-menopausal, lower estrogen levels can lead to fat redistribution and hence result in metabolic disorders such as glucose intolerance, dyslipidemia and MAFLD[24]. It was also found that the odds ratio (OR) of MAFLD was 1.74 times higher for females over 50 years old, than those under 50 years old[26]. On the other hand, older men had a lower prevalence of MAFLD than middle aged men with the prevalence rising rapidly between the age of 18-39, and more slowly after the age of 40 years with a peak prevalence at 42% in the 50-54 age before declining[25] (Table 1 and Supple
Among the metabolic subtypes, DM superseded metabolic dysfunction and obesity in prevalence, as well as risks and severity of advanced fibrosis. Among Shanghai Chinese adults, the prevalence of MAFLD and advanced fibrosis was greatest in patients with T2DM, followed by obese and then overweight individuals[20]. In terms of severity, an NHANES III study population found higher fibrosis-4 index (FIB4) scores among MAFLD patients with DM, as compared to metabolic dysfunction and obesity[23]. Similarly, a Taiwanese study found that DM was second to hepatitis B Virus (HBV) infection in its risk of advanced fibrosis in its local MAFLD population, before hypertension or dyslipidemia[27]. More cases of hepatic steatosis and advanced liver fibrosis were found in MAFLD individuals as compared to NAFLD or healthy control groups[21], which might corroborate previous discussions on MAFLD efficacy in identifying liver disease and adverse liver outcomes (Table 1 and Supplementary Table 4).
Different conclusions were made in studies from Fujian, China and Korea. While the former drew similar conclusions in that MAFLD had a higher prevalence of moderate-severe hepatic steatosis than steatotic patients with no metabolic risks, the correlation could not be said the same for the prevalence of advanced fibrosis. However, it is worth considering that many of its participants are selected from a single center with a high proportion of HBV infection and low BMI, which might not adequately capture the relationship between metabolic dysfunction on advanced fibrosis in isolation[22]. In the Korean study, it showed that while metabolic dysfunction did have a positive correlation with risks of liver fibrosis, obesity seemed to be a more contributory factor than DM[28]. An important point worth bringing up is that the mentioned studies used different definitions of advanced fibrosis. While most of the studies collected biopsy-proven liver fibrosis, the definition of advanced differed slightly; the Korean study used defined advanced fibrosis as LSM value ≥ 7.0 kPa[28], the Fujian study as having a score of ≥ 3 on the Scheuer scale[22], the Taiwanese study as stage 3-4 on the NASH CRN fibrosis staging system[27]. To complicate things, some studies used FIB4 scoring as a marker of fibrosis[20,23], which is a measurement done clinically rather than histologically (Table 1 and Supplementary Table 4).
There are few studies comparing the histological profile in NAFLD and MAFLD due to the invasiveness of liver biopsy. One study of 1217 cases did not identify any significant differences in inflammation, advanced fibrosis, and grade of steatosis between MAFLD and NAFLD patients on histology[22]. The same study identified a third group of patients without obesity, T2DM or metabolic dysregulation but with liver steatosis on liver biopsy (non-metabolic related steatosis). Non-metabolic related steatosis patients demonstrated the similar extent of inflammation and degree of fibrosis as MAFLD and NAFLD patients despite being healthier from the metabolic syndrome point of view, hence suggesting that the MAFLD criteria may still miss out on some steatotic patients with significant liver injury (Table 1 and Supplementary Table 4).
To date, the exact pathophysiology of MAFLD is not exactly well-understood. Many studies have, however, explored its correlations with genetic variants and modifiable lifestyle practices. Among Iranian adults, higher inflammatory scores secondary to dietary and lifestyle exposures such as smoking and sedentary lifestyles are associated with higher risks of MAFLD. The study suggests that inflammatory mechanisms are intrinsic in the pathophysiologic pathways in MAFLD development and progression[29]. Genetic variants have also been proven to show a link with MAFLD. Among the wide array of variants associated with higher risks of MAFLD include PNPLA3 rs738409 and MBOAT7 rs64173, while variants such as STAT3 rs74416 had been shown to have a protective effect instead. TM6SF2 rs58542926 did not show a significant correlation with MAFLD in the same study[30]. It is worth noting that the three single nucleotide polymorphisms are associated with NAFLD, which implies some degree of shared genetic predisposition to liver disease development. A variant KLB rs17618244 has emerged recently among Italian patients, and results show a predilection for hepatic fibrosis but no correlation to liver steatosis and inflammation[31]. However, the clinical practicality of genetic variant is not yet well-founded; in a pediatric MAFLD population, the genetic risk scores associated with PNPLA3 and PPARG single nucleotide polymorphisms showed little discriminatory value in predicting MAFLD patients[32]. Currently, many studies around MAFLD pathophysiology are limited by small subject groups, and more research should aim toward gaining a deeper and clinically relevant understanding of disease biomechanisms. In comparison, MAFLD shares similar genes as NAFLD, such as PNPLA3, MBOAT7 and TM6SF2[33], although most variants differ between the 2. A meta-analysis found that the PNPLA3 rs738409, also found in MAFLD, showed a positive association with NAFLD, with its G allele being frequently observed in NAFLD individuals (GG vs CC OR = 4.01 and GC vs CC OR = 1.88)[34] (Table 2).
The proposed change of the term from ‘NAFLD’ to ‘MAFLD’ aims to better reflect and focus on the underlying metabolism-related etiology of the disease and not just on the exclusion of alcohol intake or other liver diseases. Our review noted that the MAFLD diagnostic criteria were able to identify more individuals with fatty liver. In terms of advanced fibrosis, the MAFLD criteria were superior or concordant with NAFLD in many studies. All-cause mortality, cardiovascular disease-related and cancer-related mortality were shown to be higher in MAFLD patients. MAFLD patients also had higher baseline metabolic derangement, and risks of developing obesity, diabetes, and cardiovascular events.
Within the subtypes of MAFLD, patients with more metabolic conditions at the time of diagnosis had worse hepatic and liver injury compared to those with a single metabolic condition. This highlights the importance of individualized treatment in MAFLD patients. Non-modifiable risk factors identified for MAFLD include older age, female, post menopause, lower education level, and urban residence and modifiable risk factors include physical activity and BMI. While there are preliminary studies to suggest genetic variants associated with MAFLD, more investigations should be done to explore the mechanism behind them.
From the start, the level of acceptance for the proposal of MAFLD had been varied. So far, the Middle East and North Africa consensus panel and the Latin American Association for the Study of the Liver had endorsed the renaming of NAFLD to MAFLD[35,36]. The Latin American association had also indicated that a change in terminology could increase patients’ willingness to openly discuss their disease, as the term “alcohol” leads to stigmatization. The Asian Pacific Association for the Study of the Liver had published clinical practice guidelines for the diagnosis and management of MAFLD[37], noting that dual etiology liver diseases, particularly a combination of MAFLD with viral hepatitis or alcohol, are common in this region. The change in terminology is still being debated in North America and Europe, even though the original expert consensus proposing MAFLD criteria was published in the Journal of Hepatology. Recently, it has been proposed that changing the terminology requires a new understanding of the molecular basis of the disease entity and new insights into risk stratification or other important aspects of this liver disease[38]. Central to the debate about the new nomenclature is whether NAFLD is an appropriate name as the term ‘non-alcoholic’ overemphasizes the absence of alcohol use and underemphasizes the importance of the metabolic risk factors which are the main drivers of disease progression. Further, several investigators have suggested that MAFLD but not NAFLD is associated with increased fibrosis and mortality. The opponents to “MAFLD” raised the concern that there is a lack of a general consensus on the definition of ‘metabolic health’. Younossi et al[38] reported excess alcohol use was documented in approximately 15% of patients with MAFLD in an NHANES cohort, and contribute to liver-specific mortality for MAFLD (HR = 4.50; 95%CI: 1.89-10.75) but not NAFLD. In the same study, insulin resistance predicted liver-specific mortality in NAFLD (HR = 3.57; 95%CI: 1.35-9.42) but not MAFLD (HR = 0.84; 95%CI: 0.36-1.95). However, as seen, most of the publication to date do report higher fibrosis score.
The major limitation of our study Is, to date, most published studies on MAFLD are retrospective or cross-sectional, with very few prospective studies (which are really “retrospective-prospective”, designed before the MAFLD was defined). This is not surprising since the consensus statement was only published in 2020. Second, many large database studies contain data obtained more than 10 years ago. The subjects were unlikely to have been screened comprehensively using the metabolic risk tests as listed in Figure 1B, or received the pharmacotherapies available today. Also, as MAFLD overlaps with NAFLD patients, the use of student t-tests and most parametric tests for comparison between the two groups is inappropriate as they are not independent groups. Publishing bias may exist as published studies are mostly positive studies and negative studies may not be reported. Lastly, most of the studies that have been included are over-represented by the Western population, and the generalizability of the results to the rest of the world can be questioned.
In conclusion, MAFLD is a new definition of fatty liver disease that is gaining wide acceptance, especially in Asia, Latin America, and Africa. There are still questions in hot debates. The concept is based on empirical clinical practice on positive inclusion of metabolic risk factors and recent evidence suggests that it helps to identify patients with higher risk for liver-related as well as cardiovascular events. MAFLD also consists of three subtypes, each with a unique metabolic dysfunction, which may be useful for the development of new pharmacotherapy. The nomenclature and metabolic risk factor criteria will likely evolve with time. However, the principle of having “positive criteria” for metabolic dysfunction as an etiology for fatty liver disease, independent of alcohol intake, will probably prevail. More high-quality scientific evidence is still required before the widespread acceptance of this new definition.
Metabolic dysfunction-associated fatty liver disease (MAFLD) was proposed in 2020 as the new definition of fatty liver. Compared to nonalcoholic fatty liver disease (NAFLD), MAFLD consists of inclusion criteria characterized by metabolic dysfunction and associated risk factors. There is still a lack of awareness regarding this new MAFLD terminology and its impact on clinical practice.
There have been numerous debates regarding whether the new term MAFLD should be adopted. The definition of MAFLD reflects a shift in the focus from sub typing patients with hepatic steatosis and no discernible cause of fatty liver to the underlying metabolism - related etiology of the disease.
This study summaries existing data that evaluate the long-term outcome differences of the terminology change from NAFLD to MAFLD, classification of hepatic steatosis, histopathological classification, risk factors and pathophysiological mechanisms of the new proposed terminology.
A systemic search of database MEDLINE via PubMed and EMBASE were conducted to identify relevant studies up to June 28, 2022.
Of the 2324 records screened, 1575 duplicates were removed, following which 207 articles were excluded and a remaining 542 articles were assessed for eligibility. 511 articles were excluded and a remaining 31 articles were selected for review. Studies show that MAFLD patients were able to identify more patients with fatty liver compared to NAFLD. MAFLD criteria was also superior or concordant in terms of advanced fibrosis. MAFLD is also associated with higher all-cause mortality, cardiovascular disease - related and cancer - related mortality compared to NAFLD patients.
MAFLD is gaining acceptance as a new definition of fatty liver disease. The nomenclature and definition of MAFLD highlights the metabolic risk factor which are main drivers of disease progression.
MAFLD consists of 3 subtypes, each with a unique metabolic dysfunction profile that may be useful for development of new pharmacotherapy. However, further understanding is required to determine the molecular basis of MAFLD as a disease entity and new insights into risk stratification.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fan JG, China; Tai DI, Taiwan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2841] [Article Influence: 568.2] [Reference Citation Analysis (1)] |
| 2. | Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, Wu Y, Wang X, Zhu Y. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40:2082-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 400] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 3. | Park H, Yoon EL, Kim M, Kim JH, Cho S, Jun DW, Nah EH. Fibrosis Burden of Missed and Added Populations According to the New Definition of Metabolic Dysfunction-Associated Fatty Liver. J Clin Med. 2021;10:4625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Kemp W, Clayton-Chubb D, Majeed A, Glenister KM, Magliano DJ, Lubel J, Bourke L, Simmons D, Roberts SK. Impact of renaming NAFLD to MAFLD in an Australian regional cohort: Results from a prospective population-based study. J Gastroenterol Hepatol. 2022;37:395-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Yamamura S, Eslam M, Kawaguchi T, Tsutsumi T, Nakano D, Yoshinaga S, Takahashi H, Anzai K, George J, Torimura T. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int. 2020;40:3018-3030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 313] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 6. | Baratta F, Ferro D, Pastori D, Colantoni A, Cocomello N, Coronati M, Angelico F, Del Ben M. Open Issues in the Transition from NAFLD to MAFLD: The Experience of the Plinio Study. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | van Kleef LA, Ayada I, Alferink LJM, Pan Q, de Knegt RJ. Metabolic dysfunction-associated fatty liver disease improves detection of high liver stiffness: The Rotterdam Study. Hepatology. 2022;75:419-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 8. | Wong VW, Wong GL, Woo J, Abrigo JM, Chan CK, Shu SS, Leung JK, Chim AM, Kong AP, Lui GC, Chan HL, Chu WC. Impact of the New Definition of Metabolic Associated Fatty Liver Disease on the Epidemiology of the Disease. Clin Gastroenterol Hepatol. 2021;19:2161-2171.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 9. | Ciardullo S, Perseghin G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int. 2021;41:1290-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 10. | Park H, Yoon EL, Kim M, Cho S, Nah EH, Jun DW. Nomenclature Dilemma of Metabolic Associated Fatty Liver Disease (MAFLD): Considerable Proportions of MAFLD Are Metabolic Healthy. Clin Gastroenterol Hepatol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Ciardullo S, Carbone M, Invernizzi P, Perseghin G. Impact of the new definition of metabolic dysfunction-associated fatty liver disease on detection of significant liver fibrosis in US adolescents. Hepatol Commun. 2022;6:2070-2078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Zhang YC, Lyu ZY, Ma B, Li LM, Wang W, Sheng C, Dai HJ, Huang YB, Song FF, Song FJ, Chen KX. A new risk stratification strategy for fatty liver disease by incorporating MAFLD and fibrosis score in a large US population. Hepatol Int. 2022;16:835-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Huang Q, Zou X, Wen X, Zhou X, Ji L. NAFLD or MAFLD: Which Has Closer Association With All-Cause and Cause-Specific Mortality? Front Med (Lausanne). 2021;8:693507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 14. | Wang X, Wu S, Yuan X, Chen S, Fu Q, Sun Y, Lan Y, Hu S, Wang Y, Lu Y, Qu S, Wang L. Metabolic Dysfunction-associated Fatty Liver Disease and Mortality Among Chinese Adults: a Prospective Cohort Study. J Clin Endocrinol Metab. 2022;107:e745-e755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 15. | Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. 2021;75:1284-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 305] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 16. | Younossi ZM, Paik JM, Al Shabeeb R, Golabi P, Younossi I, Henry L. Are there outcome differences between NAFLD and metabolic-associated fatty liver disease? Hepatology. 2022;76:1423-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 132] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 17. | Yoneda M, Yamamoto T, Honda Y, Imajo K, Ogawa Y, Kessoku T, Kobayashi T, Nogami A, Higurashi T, Kato S, Hosono K, Saito S, Nakajima A. Risk of cardiovascular disease in patients with fatty liver disease as defined from the metabolic dysfunction associated fatty liver disease or nonalcoholic fatty liver disease point of view: a retrospective nationwide claims database study in Japan. J Gastroenterol. 2021;56:1022-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 18. | Tsutsumi T, Eslam M, Kawaguchi T, Yamamura S, Kawaguchi A, Nakano D, Koseki M, Yoshinaga S, Takahashi H, Anzai K, George J, Torimura T. MAFLD better predicts the progression of atherosclerotic cardiovascular risk than NAFLD: Generalized estimating equation approach. Hepatol Res. 2021;51:1115-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 132] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 19. | Niriella MA, Ediriweera DS, Kasturiratne A, De Silva ST, Dassanayaka AS, De Silva AP, Kato N, Pathmeswaran A, Wickramasinghe AR, de Silva HJ. Outcomes of NAFLD and MAFLD: Results from a community-based, prospective cohort study. PLoS One. 2021;16:e0245762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 20. | Zeng J, Qin L, Jin Q, Yang RX, Ning G, Su Q, Yang Z, Fan JG. Prevalence and characteristics of MAFLD in Chinese adults aged 40 years or older: A community-based study. Hepatobiliary Pancreat Dis Int. 2022;21:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 21. | Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential Clinical Characteristics and Mortality Outcomes in Persons With NAFLD and/or MAFLD. Clin Gastroenterol Hepatol. 2021;19:2172-2181.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 159] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 22. | Huang J, Xue W, Wang M, Wu Y, Singh M, Zhu Y, Kumar R, Lin S. MAFLD Criteria May Overlook a Subtype of Patient with Steatohepatitis and Significant Fibrosis. Diabetes Metab Syndr Obes. 2021;14:3417-3425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Huang J, Ou W, Wang M, Singh M, Liu Y, Liu S, Wu Y, Zhu Y, Kumar R, Lin S. MAFLD Criteria Guide the Subtyping of Patients with Fatty Liver Disease. Risk Manag Healthc Policy. 2021;14:491-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | Yuan Q, Wang H, Gao P, Chen W, Lv M, Bai S, Wu J. Prevalence and Risk Factors of Metabolic-Associated Fatty Liver Disease among 73,566 Individuals in Beijing, China. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 25. | Chen YL, Li H, Li S, Xu Z, Tian S, Wu J, Liang XY, Li X, Liu ZL, Xiao J, Wei JY, Ma CY, Wu KN, Ran L, Kong LQ. Prevalence of and risk factors for metabolic associated fatty liver disease in an urban population in China: a cross-sectional comparative study. BMC Gastroenterol. 2021;21:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 26. | Fan J, Luo S, Ye Y, Ju J, Zhang Z, Liu L, Yang J, Xia M. Prevalence and risk factors of metabolic associated fatty liver disease in the contemporary South China population. Nutr Metab (Lond). 2021;18:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 27. | Huang SC, Su HJ, Kao JH, Tseng TC, Yang HC, Su TH, Chen PJ, Liu CJ. Clinical and Histologic Features of Patients with Biopsy-Proven Metabolic Dysfunction-Associated Fatty Liver Disease. Gut Liver. 2021;15:451-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Huh JH, Kim KJ, Kim SU, Cha BS, Lee BW. Obesity is an important determinant of severity in newly defined metabolic dysfunction-associated fatty liver disease. Hepatobiliary Pancreat Dis Int. 2022;21:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Taheri E, Bostick RM, Hatami B, Pourhoseingholi MA, Asadzadeh Aghdaei H, Moslem A, Mousavi Jarrahi A, Zali MR. Dietary and Lifestyle Inflammation Scores Are Inversely Associated with Metabolic-Associated Fatty Liver Disease among Iranian Adults: A Nested Case-Control Study. J Nutr. 2022;152:559-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Mu T, Peng L, Xie X, He H, Shao Q, Wang X, Zhang Y. Single Nucleotide Polymorphism of Genes Associated with Metabolic Fatty Liver Disease. J Oncol. 2022;2022:9282557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Panera N, Meroni M, Longo M, Crudele A, Valenti L, Bellacchio E, Miele L, D'Oria V, Paolini E, Maggioni M, Fracanzani AL, Alisi A, Dongiovanni P. The KLB rs17618244 gene variant is associated with fibrosing MAFLD by promoting hepatic stellate cell activation. EBioMedicine. 2021;65:103249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Oses M, Cadenas-Sanchez C, Medrano M, Galbete A, Miranda-Ferrua E, Ruiz JR, Sánchez-Valverde F, Ortega FB, Cabeza R, Villanueva A, Idoate F, Labayen I. Development of a prediction protocol for the screening of metabolic associated fatty liver disease in children with overweight or obesity. Pediatr Obes. 2022;17:e12917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. 2018;68:268-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 691] [Article Influence: 98.7] [Reference Citation Analysis (0)] |
| 34. | Dai G, Liu P, Li X, Zhou X, He S. Association between PNPLA3 rs738409 polymorphism and nonalcoholic fatty liver disease (NAFLD) susceptibility and severity: A meta-analysis. Medicine (Baltimore). 2019;98:e14324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 35. | Shiha G, Alswat K, Al Khatry M, Sharara AI, Örmeci N, Waked I, Benazzouz M, Al-Ali F, Hamed AE, Hamoudi W, Attia D, Derbala M, Sharaf-Eldin M, Al-Busafi SA, Zaky S, Bamakhrama K, Ibrahim N, Ajlouni Y, Sabbah M, Salama M, Anushiravani A, Afredj N, Barakat S, Hashim A, Fouad Y, Soliman R. Nomenclature and definition of metabolic-associated fatty liver disease: a consensus from the Middle East and north Africa. Lancet Gastroenterol Hepatol. 2021;6:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 36. | Mendez-Sanchez N, Arrese M, Gadano A, Oliveira CP, Fassio E, Arab JP, Chávez-Tapia NC, Dirchwolf M, Torre A, Ridruejo E, Pinchemel-Cotrim H, Castellanos Fernández MI, Uribe M, Girala M, Diaz-Ferrer J, Restrepo JC, Padilla-Machaca M, Dagher L, Gatica M, Olaechea B, Pessôa MG, Silva M. The Latin American Association for the Study of the Liver (ALEH) position statement on the redefinition of fatty liver disease. Lancet Gastroenterol Hepatol. 2021;6:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 37. | Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, Zheng MH, Shiha G, Yilmaz Y, Gani R, Alam S, Dan YY, Kao JH, Hamid S, Cua IH, Chan WK, Payawal D, Tan SS, Tanwandee T, Adams LA, Kumar M, Omata M, George J. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 555] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 38. | Younossi ZM, Rinella ME, Sanyal AJ, Harrison SA, Brunt EM, Goodman Z, Cohen DE, Loomba R. From NAFLD to MAFLD: Implications of a Premature Change in Terminology. Hepatology. 2021;73:1194-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 290] [Article Influence: 72.5] [Reference Citation Analysis (0)] |