Published online Feb 28, 2022. doi: 10.3748/wjg.v28.i8.853
Peer-review started: August 13, 2021
First decision: October 2, 2021
Revised: October 29, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: February 28, 2022
Processing time: 195 Days and 0.3 Hours
Helicobacter pylori (H. pylori) infection is known to prevent the occurrence of gastroesophageal reflux disease (GERD) by inducing gastric mucosal atrophy. However, little is known about the relationship between atrophic gastritis (AG) and GERD.
To confirm the inverse correlation between AG and the occurrence and severity of GERD.
Individuals receiving health checkups who underwent upper gastrointestinal endoscopy at Seoul National University Healthcare System Gangnam Center were included. The grade of reflux esophagitis was evaluated according to the Los Angeles classification. Endoscopic AG (EAG) was categorized into six grades. Serologic AG (SAG) was defined as pepsinogen I ≤ 70 ng/mL and pepsinogen I/II ratio ≤ 3.0. The association between the extent of EAG and SAG and the occurrence and severity of GERD was evaluated using multivariate logistic regression analysis.
In total, 4684 individuals with GERD were compared with 21901 healthy controls. In multivariate logistic regression analysis, advanced age, male sex, body mass index > 23 kg/m2, presence of metabolic syndrome, current smoking, and alcohol consumption were associated with an increased risk of GERD. Seropositivity for H. pylori immunoglobulin G antibodies was associated with a decreased risk of GERD. There was an inverse correlation between the extent of EAG and occurrence of GERD: Odds ratio (OR), 1.01 [95% confidence interval (CI): 0.90-1.14] in C1, 0.87 (0.78-0.97) in C2, 0.71 (0.62-0.80) in C3, 0.52 (0.44-0.61) in O1, 0.37 (0.29-0.48) in O2, and 0.28 (0.18-0.43) in O3. Additionally, the extent of EAG showed an inverse correlation with the severity of GERD. The presence of SAG was correlated with a reduced risk of GERD (OR = 0.49, 95%CI: 0.28-0.87, P = 0.014).
The extent of EAG and SAG exhibited strong inverse relationships with the occurrence and severity of GERD. AG followed by H. pylori infection may be independently protect against GERD.
Core Tip: This is a retrospective study to evaluate the inverse correlation of atrophic gastritis and the occurrence and severity of gastroesophageal reflux disease (GERD). Old age, male sex, body mass index over 23 kg/m2, metabolic syndrome, current smoking, and alcohol intake increased the risk of GERD. Seropositivity for Helicobacter pylori (H. pylori) immunoglobulin G antibody decreased the risk of GERD. There was an inverse correlation between extent of endoscopic atrophic gastritis (EAG) and occurrence of GERD. Additionally, extent of EAG showed inverse correlation with severity of GERD. Presence of serologic atrophic gastritis was correlated with reduced risk of GERD. Atrophic gastritis followed by H. pylori infection could be an inde-pendent protective factor against GERD.
- Citation: Han YM, Chung SJ, Yoo S, Yang JI, Choi JM, Lee J, Kim JS. Inverse correlation between gastroesophageal reflux disease and atrophic gastritis assessed by endoscopy and serology. World J Gastroenterol 2022; 28(8): 853-867
- URL: https://www.wjgnet.com/1007-9327/full/v28/i8/853.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i8.853
Gastroesophageal reflux disease (GERD) is a disorder with specific symptoms, such as acid regurgitation and heartburn, caused by the recurrent reflux of gastric contents into the esophagus due to transient relaxation or low pressure of the lower esophageal sphincter[1,2]. A condition with troublesome symptoms accompanied by esophageal structural changes, such as mucosal breaks, erosion, or ulcers (i.e., reflux esophagitis) is defined as erosive reflux disease (ERD); however, only about one-third to one-half of patients with GERD exhibit positive findings on endoscopic examinations[1]. Meanwhile, the presentation of typical esophageal symptoms in the absence of endoscopic changes is classified as non-ERD (NERD).
GERD is a common disease with a prevalence ranging from 18.1% to 27.8% in North America, 8.8% to 25.9% in Europe, and 2.5% to 7.8% in East Asia[3,4]. According to a systematic review conducted in 2014, the prevalence of GERD is significantly increased when comparing studies conducted before and after 1995[3]. Several factors such as a prolonged life expectancy, westernized lifestyle, and increasing prevalence of obesity might influence the increase in GERD prevalence[5]. GERD and its complications, such as reflux esophagitis and Barrett’s esophagus, cause a considerable socioeconomic burden in terms of hospital visits, incur significant medical costs for diagnosis and treatment, and induce several problems related to quality of life. A number of epidemiological studies have evaluated the risk factors for GERD. Male sex, caffeine intake, smoking, alcohol consumption, dietary factors (especially the consumption of large meals and a high-fat diet), a low education level, and obesity are known risk factors for GERD[2,6-8].
Interestingly, an inverse correlation between GERD and Helicobacter pylori (H. pylori) infection has been demonstrated in several studies. In a case-control study performed in Japan, most cases of reflux esophagitis occurred in the absence of H. pylori and atrophic gastritis or in milder cases of gastritis with H. pylori infection[9]. H. pylori infection has been shown to reduce the risk and severity of GERD in several epidemiological studies and systematic reviews[10-12]. Furthermore, the grade of reflux esophagitis and GERD symptoms are aggravated after H. pylori eradication therapy[2,13]. These findings suggest that H. pylori infection might be a protective factor against GERD as a result of a decreased acid secretory capacity induced by gastric mucosal atrophy.
As gastric mucosal atrophy is considered a key mechanism by which H. pylori infection prevents the occurrence or aggravation of GERD, several studies have assessed the relationship between atrophic gastritis and GERD. According to a few studies grading the severity of atrophic gastritis using endoscopic biopsies according to a modified updated Sydney classification, H. pylori infection alone may not influence the occurrence of reflux esophagitis. Meanwhile, the involvement and degree of atrophy in the gastric corpus are independent protective factors against GERD[9,14]. However, the histological diagnosis of atrophic gastritis based on endoscopic forcep biopsy is not always feasible in daily clinical settings because of its invasive nature. Pepsinogen is a validated serologic marker reflecting the acid secretory ability of the gastric gland; thus, it may predict the presence or absence of gastric atrophy[19]. In general, pepsinogen I levels ≤ 70 ng/mL and pepsinogen I/II ratios ≤ 3.0 are considered positive results for gastric atrophy[15]. A prospective case-control study in Korea found that the pepsinogen I/II ratio was higher in patients with ERD than in those without ERD[16]. Furthermore, the prevalence of reflux esophagitis decreased as the stage of H. pylori-related chronic gastritis assessed based on serum H. pylori antibody and pepsinogen levels progressed[17]. These studies showed that atrophic gastritis is a major preventive factor for GERD; however, the diagnosis of atrophic gastritis based on histological or serologic methods is not intuitive. In addition, these methods have limitations in terms of medical expenses, which cannot be easily applied in clinical trials.
Endoscopically, gastric atrophy is defined as a visible submucosal pattern in a non-overdistended stomach[18,19]. Previous epidemiological studies have revealed that the prevalence of endoscopic atrophic gastritis (EAG) and incidence of GERD show an inverse correlation[20-23]. However, these studies had several limitations; the extent of EAG was simplified into two types (closed and open types), and the definition of GERD was based solely on the presence of symptoms. Furthermore, these studies did not evaluate various confounding variables, such as demographic, metabolic, and lifestyle factors, which might interfere with the prevalence and grade of GERD.
In this study, we aimed to evaluate whether there is a quantitative correlation between the extent of atrophic gastritis and severity of GERD using endoscopic grading and serologic markers considering a variety of confounding variables in a large Korean population.
Individuals receiving health checkups who underwent upper gastrointestinal (GI) endoscopy at Seoul National University Healthcare System Gangnam Center between January 2015 and December 2016 were screened for inclusion in the present study. We excluded those who had prior history of esophageal or gastric cancer and who performed esophagectomy or gastrectomy, active or healing stage of benign gastric or duodenal ulcer, and recent proton pump inhibitor (PPI) medication within one month. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the International Review Board of Seoul National University Hospital (IRB No. H-1701-028-655).
Demographic data such as age, sex, height, weight, and waist circumference were collected. Physical examinations were performed using a written, systematic protocol with standardized instruments by trained personnel: The waist circumference were measured at midpoint between the lower border of rib cage and iliac crest[24]. Body mass index (BMI) was calculated using height and weight according to the formula: BMI = weight (kg)/height2 (m2). According to the modified World Health Organi-zation criteria from the Asia-Pacific guideline, BMI was categorized as follows: Normal (< 23 kg/m2), overweight (23-24.9 kg/m2) and obese (≥ 25 kg/m2)[25].
Structured self-administered questionnaires were used to collect information including alcohol consumption (≥ 140 g/wk), current smoking (at least one cigarette per day for the previous 12 mo), current use of PPI (history of taking PPI within the last month), medication of sedatives or hypnotics and prior eradication therapy for H. pylori infection. We also assessed the types of physical activity and the time spent for each exercise. The MET values were assigned to physical activity data: 3.3 for walking at a moderate pace, 4.0 for moderate intensity, and 8.0 for vigorous intensity. The MET-minutes per week were estimated by multiplying the reported time spent at each activity by the corresponding MET value. Insufficient physical activity was defined when the MET value was under 3500 per week. Pattern of daily diet was also evaluated for having large meal, high fat diet, high salt diet or high caffeine intake.
Blood samples were drawn for the measurement of serum glucose, triglyceride, high-density lipoprotein cholesterol (HDL-C), H. pylori immunoglobulin G (IgG) antibody and pepsinogen I and II, in the morning after participants had fasted for at least 8 h.
Blood pressure was measured using an automated blood pressure monitor (TM-2655P, A&D Company, Saitama, Japan) twice after at least 5 min of rest in a seated position, and the mean value was used. High blood pressure was defined as a systolic blood pressure ≥ 130 mmHg or a diastolic blood pressure ≥ 85mmHg or taking anti-hypertensive medication. Hyperglycemia was defined as a fasting glucose ≥ 100 mg/dL or taking glucose lowering agents. Metabolic syndrome was diagnosed according to the National Cholesterol Education Program Adult Treatment Panel III criteria[26], if there are three or more of the following characteristics: High blood pressure, hyperglycemia, abdominal obesity (waist circumference ≥ 90 cm in men and ≥ 80 cm in women), hypertriglyceridemia (serum triglyceride level ≥ 150 mg/dL), and low HDL-C (HDL-C level < 40 mg/dL in men and < 50 mg/dL in women).
Seventeen experienced board-certified gastroenterologists performed all endoscopic examinations using conventional white light videoscopes (GIFH260 or GIFH290; Olympus, Aizu, Japan/EG-450WR5). All the gastroenterologists had more than 5 years of endoscopy experience (mean 12.1 years, range 5-27 years) and performed at least 2000 cases esophagogastroduodenoscopies each year.
The reflux esophagitis was defined if mucosal breaks or mucosal change such as erythema and/or discoloration were present. The grade of reflux esophagitis was categorized from M to D according to the Los Angeles (LA) classification system with Japanese modification which is based on the length of the longest mucosal break, and confluence of erosions[27,28]: N, normal mucosa; M, minimal changes to mucosa, such as erythema and/or whitish turbidity; A, non-confluent mucosal breaks no longer than 5 mm in length; B, non-confluent mucosal breaks more than 5 mm in length; C, confluent mucosal breaks less than 75% circumferential; D, confluent mucosal breaks at least 75% circumferential. NERD was diagnosed when a subject had the symptom of acid regurgitation or heartburn at a frequency of at least once per week in the absence of reflux esophagitis.
Gastric atrophy was evaluated endoscopically according to the location of endoscopic atrophy border (EAB), which is characterized by differences in color, visible capillary network, and height of the gastric mucosa[18,29]: “Closed type” indicates that the EAB is on the lesser curvature of stomach. On the other hand, in “open type”, that is parallel to the vertical axis of stomach and extends along the anterior and posterior walls. The extent of EAG was categorized into six grades (C1 to O3): C0, no visible atrophy; C1, closed atrophy confined to the antrum; C2, closed atrophy confined to the antrum and lesser curvature of the distal gastric body; C3, closed atrophy involving the antrum and lesser curvature of the proximal gastric body; O1, open atrophy with the EAB placing between the lesser curvature and the anterior wall; O2, open atrophy with the EAB placing in the middle of the anterior wall; O3, open atrophy widely spread with the EAB between the anterior wall and the greater curvature.
A pepsinogen test was conducted for serological diagnosis of atrophic gastritis. Serum levels of pepsinogen I and pepsinogen II were measured by Latex Turbid immunoassay kits (HiSens Pepsinogen kit, HBI, Korea). Presence of serologic atrophic gastritis (SAG) was defined when pepsinogen I ≤ 70 ng/mL and pepsinogen I/II ratio ≤ 3[15].
H. pylori infection was measured by serum IgG antibodies using chemiluminescent microparticle immunoassay kits (Siemens, Germany). The values higher than 1.10 IU/mL were considered as a positive test for H. pylori infection.
For demographic and clinical characteristics of the study population, results were expressed as mean ± SD, median with interquartile range, or as counts with percentage. Pearson’s chi-square test was used to compare categorical variables and an independent t-test was performed for continuous variables.
The association between EAG and GERD was evaluated using univariate and multivariate logistic regression analyses. First, the extent of EAG was categorized into 7 groups (normal to O3) and multivariate logistic regression analysis was performed to evaluate the risk of GERD according to the extent of EAG. It could show whether the extent of EAG influence the occurrence of GERD. Second, the risk of each grade of GERD (normal to LA-C/D) was independently analyzed according to the extent of EAG (normal to O3). It could show the relationship between extent of EAG and severity of GERD.
Pepsinogen I, II, and I/II ratio were compared using Kruskal-Wallis test due to their non-normal distributions, and post-hoc analysis was done with Wilcoxon rank sum test. In post-hoc analysis, P-value less than 0.0033 was considered statistically significant according to Bonferroni correction. To figure out the impact of SAG on the risk of GERD, multivariate logistic regression analysis was done.
The data were analyzed using R software version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria). P-values less than 0.05 were considered statistically significant.
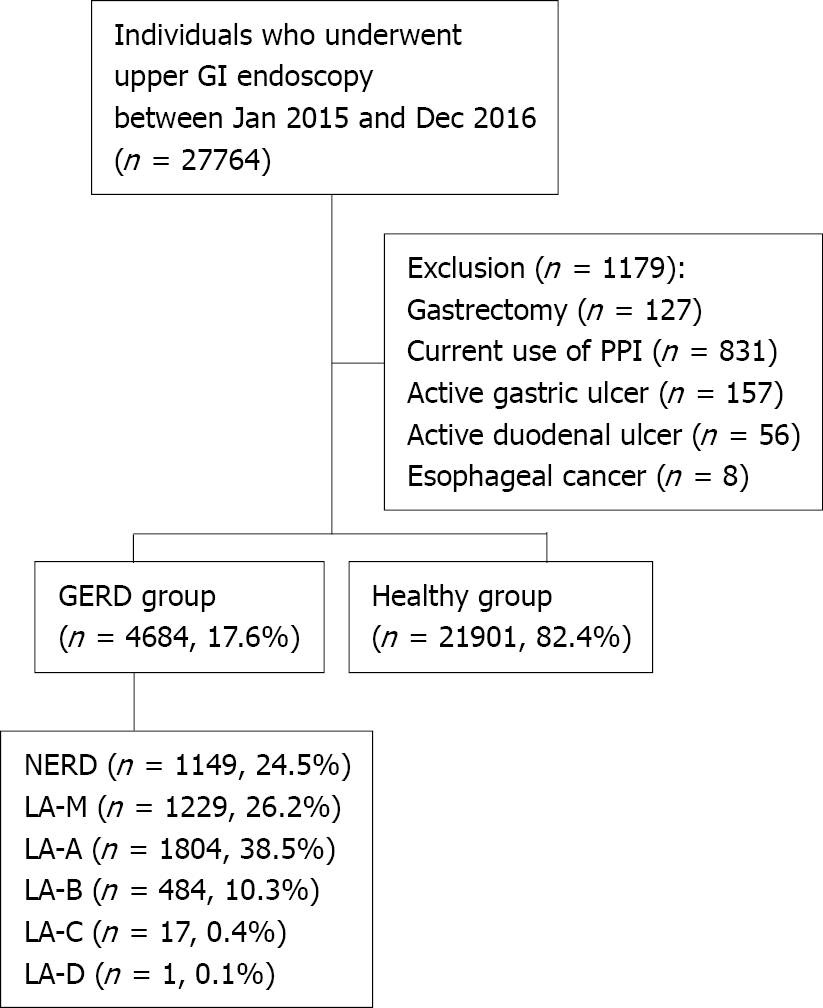
A total of 27764 individuals underwent health checkup including screening upper GI endoscopy at Seoul National University Healthcare System Gangnam Center between January 2015 and December 2016. Among them, a total of 1179 were excluded from the study; 127 with a history of gastrectomy, 831 with current use of PPI, 157 with active or healing stage of gastric ulcer, 56 with active or healing stage of duodenal ulcer and 8 with esophageal cancer. Finally, a total of 26585 subjects were enrolled in this study and were divided into two groups [4684 (17.6%) in GERD group and 21901 (82.4%) in healthy group] according to the presence or absence of reflux symptom and esophageal structural change. The severity of GERD was graded as follows: NERD (1149 patients, 24.5%), LA-M (1229 patients, 26.2%), LA-A (1804 patients, 38.5%), LA-B (484 patients, 10.3%), LA-C (17 patients, 0.4%) and LA-D (1 patient, < 0.1%) (Figure 1).
Demographic and clinical characteristics of the study population were compared between the GERD and healthy groups (Table 1). Higher proportion of male was observed in GERD group (72.1% vs 53.1%, P < 0.001). Patients with general obesity (BMI > 25 kg/m2) were more prevalent in GERD group (37.3% vs 25.0%, P < 0.001). Also, abdominal obesity (waist circumference ≥ 90 cm in men and ≥ 80 cm in women) were more common in GERD group (45.0% vs 35.6%, P < 0.001). Patients with GERD showed higher prevalence for high blood pressure (43.3% vs 35.6, P < 0.001), hyperglycemia (46.3% vs 38.6%, P < 0.001), hypertriglyceridemia (27.0% vs 18.9%, P < 0.001) and metabolic syndrome (30.7% vs 22.5%, P < 0.001). Excessive alcohol consumption and current smoking were more frequently observed in GERD group (21.6% vs 15.7%, P < 0.001 and 22.5% vs 15.4%, P < 0.001, respectively). Several life-style factors such as insufficient physical activity (33.7% vs 32.0%, P = 0.022), having large meal (12.3% vs 10.3%, P < 0.001), high fat diet (34.8% vs 31.3%, P < 0.001), high salt diet (13.3% vs 11.0%, P < 0.001), and high caffeine intake (30.5% vs 28.3%, P = 0.003) were more frequently accompanied in patients with GERD. Meanwhile, H. pylori antibody sero-positivity was less frequent in GERD group (25.3% vs 43.3%, P < 0.001).
| GERD group (n = 4684) | Healthy group (n = 21901) | P value | |
| Age | 50.9 ± 11.5 | 50.7 ± 11.1 | 0.330 |
| Sex | < 0.001 | ||
| Female | 1305 (27.9%) | 10265 (46.9%) | |
| Male | 3379 (72.1%) | 11636 (53.1%) | |
| BMI | < 0.001 | ||
| < 23 | 1657 (35.4%) | 11079 (50.6%) | |
| 23-25 | 1278 (27.3%) | 5346 (24.4%) | |
| ≥ 25 | 1748 (37.3%) | 5476 (25.0%) | |
| Abdominal obesity | 2109 (45.0%) | 7802 (35.6%) | < 0.001 |
| High B | 2028 (43.3%) | 7795 (35.6%) | < 0.001 |
| Hyperglycemia | 2168 (46.3%) | 8451 (38.6%) | < 0.001 |
| Hypertriglycemia | 1267 (27.0%) | 4136 (18.9%) | < 0.001 |
| Low-HDL | 853 (18.2%) | 4214 (19.2%) | 0.108 |
| Metabolic syndrome | 1437 (30.7%) | 4920 (22.5%) | < 0.001 |
| Medication of sedatives or hypnotics | 149 (3.2%) | 704 (3.2%) | 0.942 |
| Alcohol consumption | 1010 (21.6%) | 3449 (15.7%) | < 0.001 |
| Smoking history | < 0.001 | ||
| Never smoker | 2188 (46.7%) | 12759 (58.3%) | |
| Ex-smoker | 1444 (30.8%) | 5764 (26.3%) | |
| Current smoker | 1052 (22.5%) | 3378 (15.4%) | |
| Insufficient physical activity | 1578 (33.7%) | 6998 (32.0%) | 0.022 |
| Having large meal | 577 (12.3%) | 2250 (10.3%) | < 0.001 |
| High fat diet | 1631 (34.8%) | 6847 (31.3%) | < 0.001 |
| High salt diet | 624 (13.3%) | 2414 (11.0%) | < 0.001 |
| High caffeine intake | 1427 (30.5%) | 6200 (28.3%) | 0.003 |
| Seropositivity for H. Pylori IgG Ab | 1068 (25.3%) | 8453 (43.4%) | < 0.001 |
Several variables on the risk for GERD were analyzed by univariate and multivariate logistic regression analysis (Table 2). All the variables included in the univariate analysis were used in multivariate analysis. Advanced age and male sex were significant risk factors for GERD in multivariate analysis [Odds ratio (OR) = 1.10, 95% confidence interval (CI): 1.06-1.14, P < 0.001 and OR = 1.96, 95%CI: 1.79-2.15, P < 0.001, respectively]. As BMI increased, the risk of GERD also increased (OR = 1.27, 95%CI: 1.16-1.39, P < 0.001 for BMI 23-25; and OR = 1.51, 95%CI: 1.37-1.67, P < 0.001 for BMI > 25). Metabolic syndrome was a significant risk factor for GERD (OR = 1.12, 95%CI: 1.03-1.22, P = 0.008). Excessive alcohol intake and current smoking also significantly increased risk of GERD (OR = 1.10, 95%CI: 1.00-1.21, P = 0.043; and OR = 1.26, 95%CI: 1.14-1.39, P < 0.001, respectively). Among various life-style factors including exercise and dietary habits, there were no significant risk factors for GERD occurrence. On the other hand, sero-positivity for H. pylori IgG antibody lowered the risk for GERD by approximately half (OR = 0.49, 95%CI: 0.45-0.54, P < 0.001).
| Univariate analysis | Multivariate analysis | |||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age (10 yr) | 1.01 | 0.99-1.04 | 0.319 | 1.10 | 1.06-1.14 | < 0.001 |
| Male sex | 2.28 | 2.13-2.45 | < 0.001 | 1.96 | 1.79-2.15 | < 0.001 |
| BMI | ||||||
| < 23 | Reference | Reference | ||||
| 23-25 | 1.60 | 1.48-1.73 | < 0.001 | 1.27 | 1.16-1.39 | < 0.001 |
| > 25 | 2.13 | 1.98-2.30 | < 0.001 | 1.51 | 1.37-1.67 | < 0.001 |
| Metabolic syndrome | 1.53 | 1.42-1.64 | < 0.001 | 1.12 | 1.03-1.22 | 0.008 |
| Medication of sedatives or hypnotics | 0.99 | 0.83-1.18 | 0.906 | 1.01 | 0.83-1.22 | 0.919 |
| Alcohol intake | 1.47 | 1.36-1.59 | < 0.001 | 1.10 | 1.00-1.21 | 0.043 |
| Smoking history | ||||||
| Never smoker | Reference | Reference | ||||
| Ex-smoker | 1.46 | 1.36-1.57 | < 0.001 | 0.97 | 0.88-1.06 | 0.439 |
| Current smoker | 1.82 | 1.67-1.97 | < 0.001 | 1.26 | 1.14-1.39 | < 0.001 |
| Low level of physical activity | 1.08 | 1.01-1.16 | 0.021 | 1.06 | 0.98-1.14 | 0.132 |
| Having large meal | 1.23 | 1.11-1.35 | < 0.001 | 1.06 | 0.95-1.18 | 0.304 |
| High fat diet | 1.17 | 1.10-1.26 | < 0.001 | 1.00 | 0.93-1.08 | 0.992 |
| High salt diet | 1.24 | 1.13-1.36 | < 0.001 | 1.07 | 0.96-1.19 | 0.195 |
| High caffeine intake | 1.11 | 1.04-1.19 | 0.003 | 0.97 | 0.90-1.05 | 0.464 |
| Seropositivity for H. Pylori IgG Ab | 0.44 | 0.41-0.47 | < 0.001 | 0.49 | 0.45-0.54 | < 0.001 |
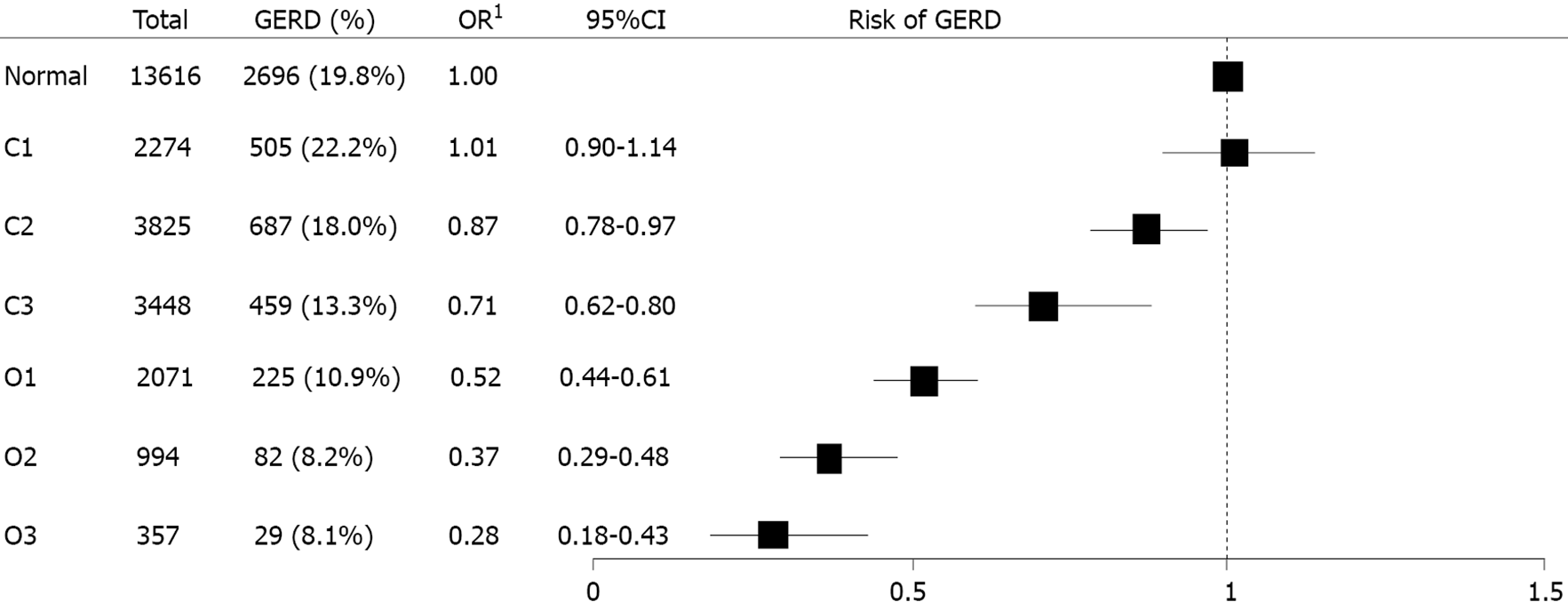
Interestingly, there was an inverse correlation between extent of EAG and occurrence of GERD: As the extent of EAG progressed from normal to O3, the prevalence of GERD decreased gradually. In the multivariate logistic regression analysis, EAG was a significant protective factor for GERD: OR, 1.01 (95%CI: 0.90-1.14) in C1, 0.87 (0.78-0.97) in C2, 0.71 (0.62-0.80) in C3, 0.52 (0.44-0.61) in O1, 0.37 (0.29-0.48) in O2, and 0.28 (0.18-0.43) in O3. (Figure 2).
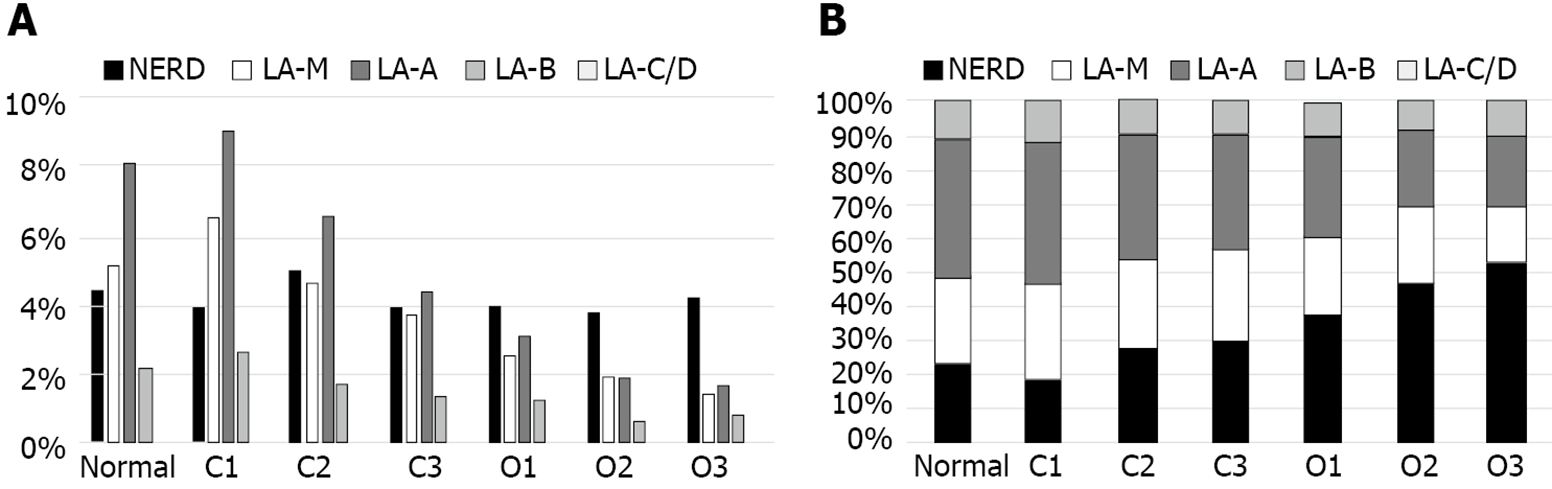
The grade of GERD in relation to the extent of EAG was evaluated and showed in Figure 3. The prevalence of GERD was highest in C1 and showed decreased tendency as extent of EAG progressed; 19.7% in normal, 22.1% in C1, 17.9% in C2, 13.3% in C3, 10.8% in O1, 8.2% in O2 and 8.1% in O3 (Figure 3A). As the extent of EAG progressed, the proportion of NERD gradually increased and proportion of ERD progressively decreased (Figure 3B).
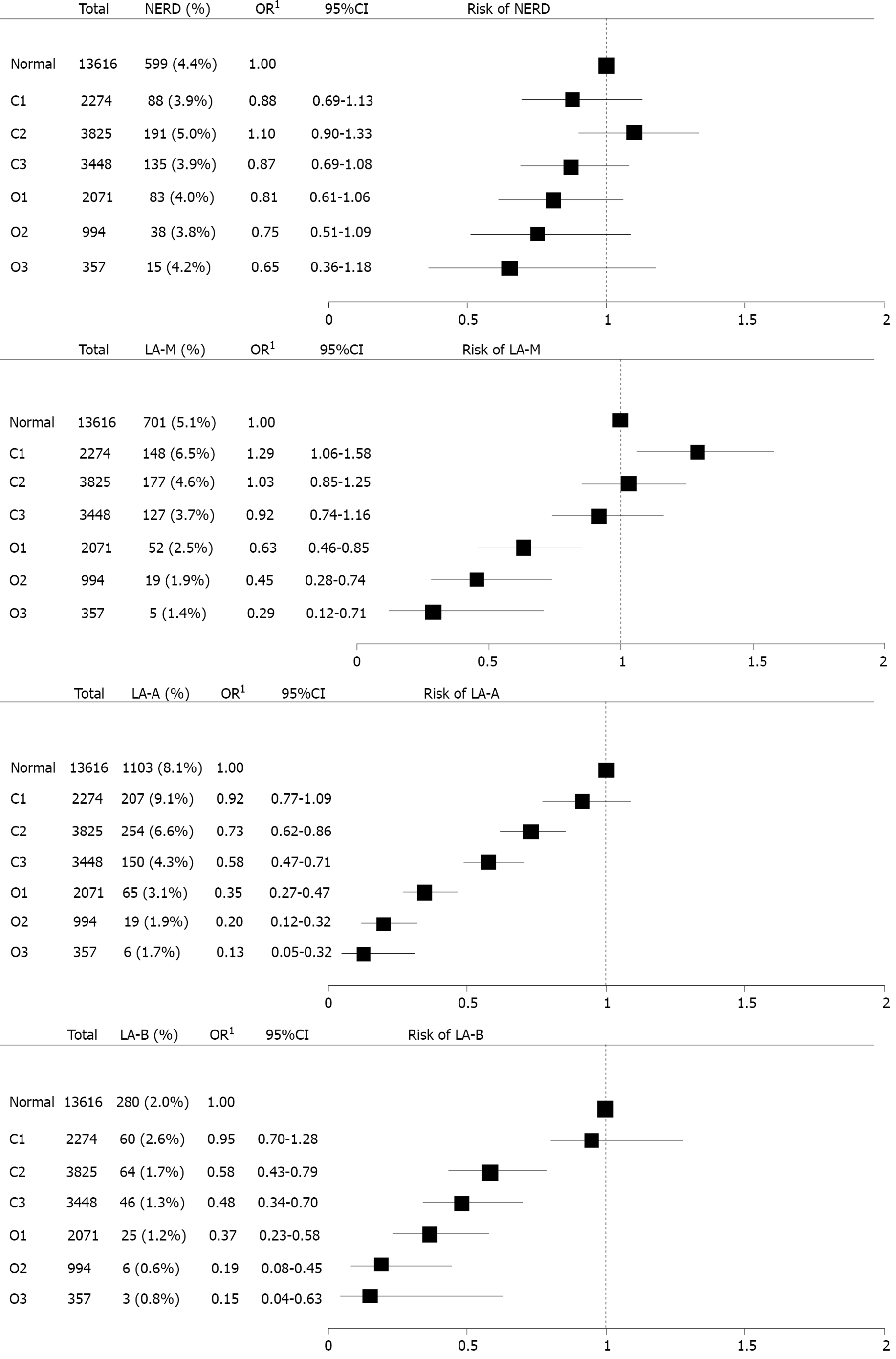
To evaluate the impact of extent of EAG on the severity of GERD, subgroup analysis was performed (Figure 4). Grossly, as extent of EAG advanced, the risk of GERD decreased. This decreased tendency of OR for GERD intensified as the severity of GERD progressed from NERD to LA-B. Because the number of patients with LA-C/D was too small, it was impossible to calculate OR for LA-C/D.
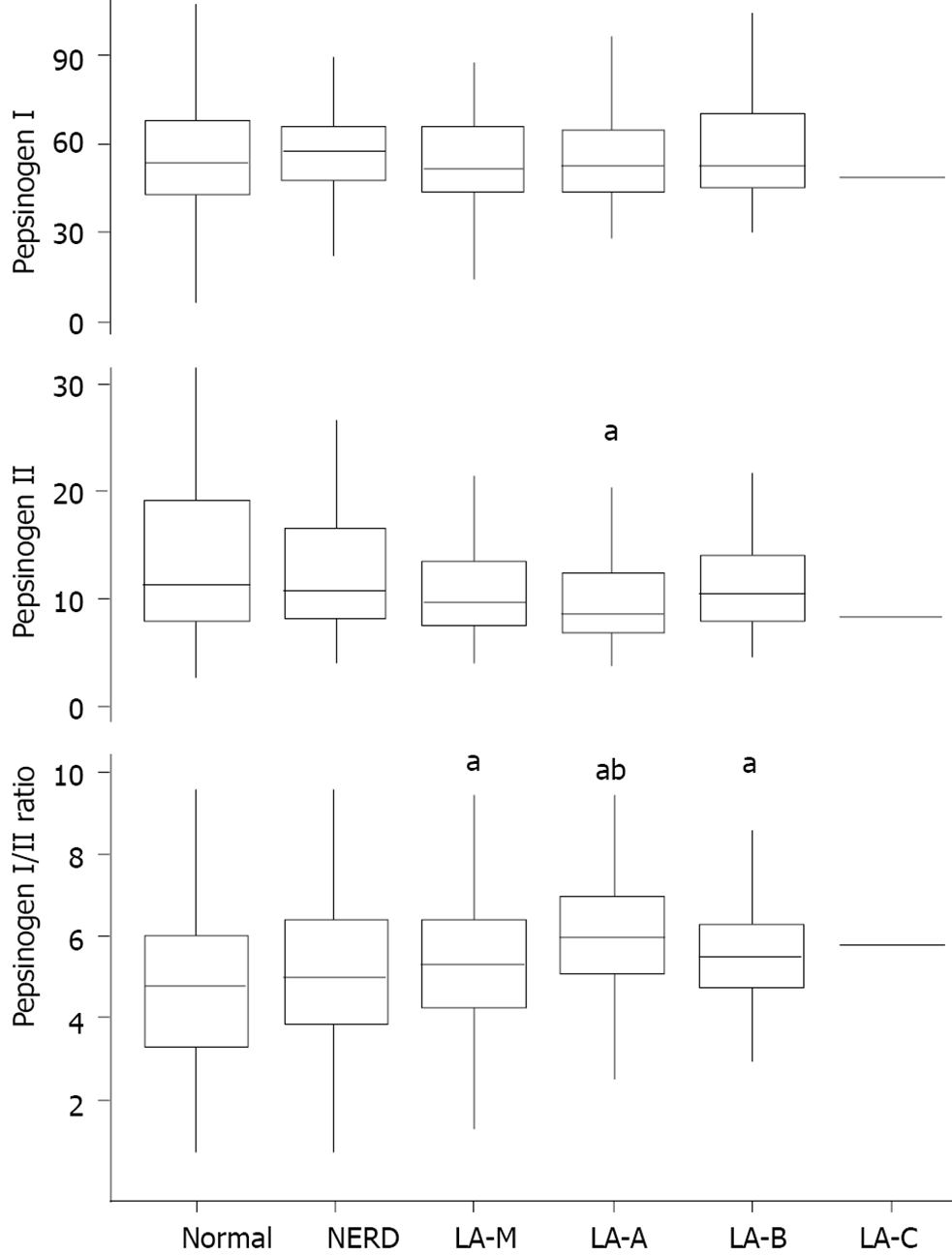
Among 26585 individuals included in this study, 2857 individuals (10.7%) underwent blood examination for pepsinogen test. There were minor and no significant differences in clinical characteristics including the prevalence of GERD between individuals with and without blood examination for pepsinogen test. Among them, 703 patients who underwent H. pylori eradication therapy were excluded and finally 2154 patients were included in the analysis. 358 patients (16.6%) had GERD and the severity of GERD was graded as follows: 78 patients (21.8%) with NERD, 79 patients (22.1%) with LA-M, 154 patients (43.0%) with LA-A, 46 patients (12.8%) with LA-B, and 1 patient (0.3%) with LA-C. Pepsinogen I level showed no significant association with the severity of GERD (P = 0.802). On the other hand, pepsinogen II level was significantly different according to GERD severity (P < 0.001). Post-hoc analysis revealed that LA-A showed significant lower level of pepsinogen II compared with normal group (normal vs LA-A, P < 0.001). In addition, pepsinogen I/II ratio was significantly higher in GERD group compared with normal group (normal vs LA-M, P =0.002; normal vs LA-A, P < 0.001; and normal vs LA-B, P = 0.002). LA-A group also showed significant higher pepsinogen I/II ratio than NERD (NERD vs LA-A, P < 0.001) (Figure 5).
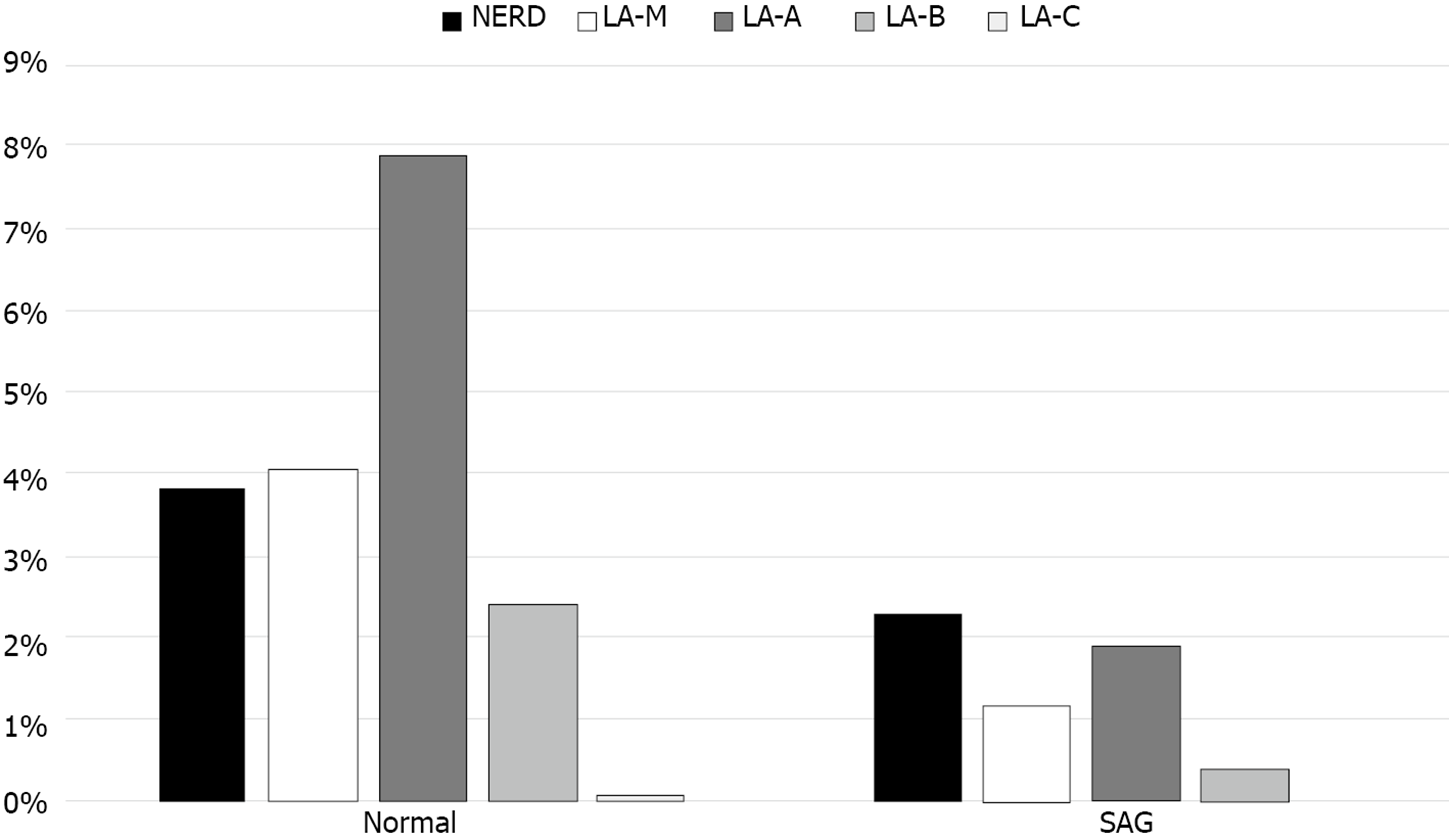
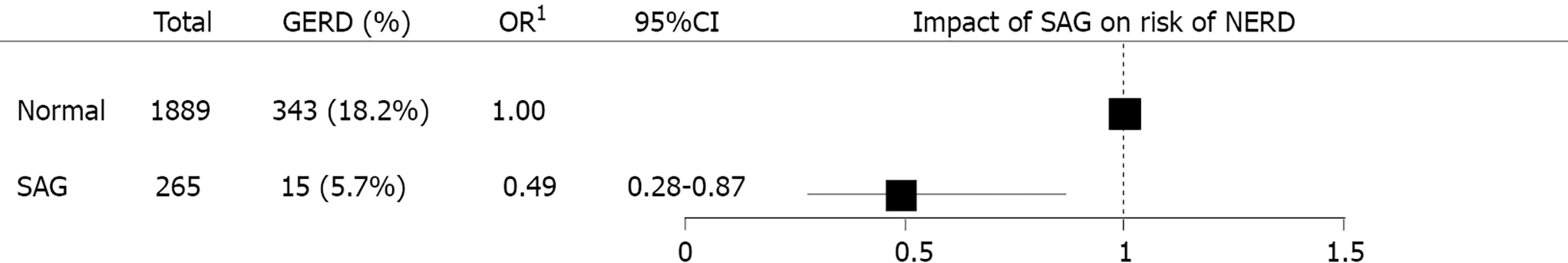
SAG group showed lower prevalence of GERD than normal group (Figure 6). Total 343 out of 1889 individuals without SAG (18.2%) had GERD, in other hands, 15 out of 265 individuals with SAG (5.7%) had GERD. In the multivariate logistic regression analysis, the risk of GERD was adjusted for age, sex, BMI, metabolic syndrome, medication of sedatives or hypnotics, alcohol intake, smoking history, physical activity, dietary factor, and H. pylori IgG. Presence of SAG was correlated with reduced risk of GERD (OR = 0.49, 95%CI: 0.28-0.87, P = 0.014) (Figure 7).
In this study, we aimed to evaluate the association between atrophic gastritis assessed using endoscopy and the occurrence and severity of GERD adjusting for many confounding variables in a large population of individuals receiving health checkups. As a result, the severity of GERD was inversely correlated with the extent of EAG. After adjusting for several known risk factors for GERD, EAG remained an independent protective factor for the occurrence of GERD. Furthermore, GERD also exhibited a negative association with SAG based on measurements of serum pepsinogen I and II levels. It has been postulated that a decreased acid secretory ability induced by gastric mucosal atrophy lowers the risk of GERD development and progression.
Several previous studies have evaluated the negative association between H. pylori infection and GERD[9-12]. Meanwhile, our study directly focused on atrophic gastritis as a protective factor for GERD, which is a more fundamental and systematic approach to show how H. pylori infection affects GERD. Furthermore, we performed stratified analysis based on the extent of EAG and endoscopic grade of GERD, while previous studies only provided limited information and simplified analyses based on the presence or absence of atrophic gastritis or GERD. We assessed atrophic gastritis using two independent methods: Endoscopy and serology. Both methods showed consistent protective effects of atrophic gastritis on the occurrence of GERD. We graded atrophic gastritis endoscopically, which is a non-invasive and relatively reliable method to evaluate the extent of gastric mucosal atrophy[29-33]. Meanwhile, histology-based assessment of gastric mucosal atrophy requires tissue sampling with biopsy forceps, which may induce complications such as bleeding, pain, and inflammation. Endoscopic diagnosis of atrophic gastritis is intuitive and applicable in daily clinical settings. We also assessed SAG by measuring serum pepsinogen I and II levels. We excluded patients who underwent H. pylori eradication therapy because pepsinogens normalize after successful H. pylori eradication. It would be incorrect to evaluate atrophic gastritis merely by pepsinogens in cases after H. pylori eradication therapy. We found that the risk of GERD decreased as SAG progressed. This suggests that not only endoscopy but also simple blood tests may provide information about the risk and severity of GERD. However, interpretation of our findings requires careful consideration from several aspects. Since GERD can only be diagnosed endoscopically, picking up patients without SAG as GERD high risk would only increase the burden of excessive endoscopy and seems unnecessary. It may be sufficient to recommend endoscopy to patients with GERD-related symptoms. In order to prove the clinical usefulness of serological tests in GERD risk assistance, more research data is needed in future studies.
We investigated a variety of possible risk factors for GERD based on structured questionnaires. According to our study, several factors also influence the occurrence of GERD. Advanced age, the male sex, a high BMI, and the presence of metabolic syndrome were shown to be significant risk factors for GERD. Alcohol consumption and smoking were also associated with an increased GERD risk. A recent meta-analysis revealed that advanced age, a high BMI, a low education level, living in an urban area, current smoking, a low income, the consumption of carbonated drinks, and coffee/tea intake increase the risk of GERD, which is comparable with our results[4].
Interestingly, the seropositivity of H. pylori IgG was negatively correlated with GERD. Several previous studies have revealed that the prevalence of H. pylori infection is significantly lower in patients with GERD[10,11]. It is assumed that chronic inflammation induced by H. pylori results in gastric atrophy, which further decreases the acid secretory capacity of gastric mucosa. Therefore, the distribution and type of gastritis related to H. pylori are more important than the H. pylori infection itself[34]. Antrum-predominant gastritis induces hypergastrinemia and increased acidity; consequently, the risk of GERD increases in patients with antral gastritis[35]. In contrast, in cases of severe corpus gastritis, decreased gastric acid production is considered the main pathogenesis by which H. pylori infection protects against GERD[36]. Based on this, we evaluated the risk of GERD according to the extent of EAG. Interestingly, the risk of GERD was highest in association with C1 and gradually decreased as the extent of EAG progressed. This supports that atrophic gastritis rather than H. pylori infection itself is a key risk factor for GERD.
Our study has several advantages over other studies. First, we defined the extent and severity of atrophic gastritis using endoscopic and serologic methods, which enabled us to evaluate the effect of atrophic gastritis on the occurrence and severity of GERD. Second, we enrolled participants from a health screening cohort that represented the general population. For this reason, there was a minimal risk of selection or referral bias. The subjects were limited to individuals who had no specific disease and underwent regular endoscopic screening, as these conditions facilitated the assessment of the true impact of the disease. Third, high-quality data were obtained based on structured questionnaires and fully computerized electronic medical records, including data on demographics, laboratory examinations, family history, lifestyle factors, and most importantly, disease-specific symptoms. Healthwatch version 2.0, the large database in our center, fully computerizes a broad range of exposures including a comprehensive drug history and makes it possible to control for potential confounders, thereby permitting a less biased estimate of the association.
The interpretation of our findings requires careful consideration from several perspectives. First, the cross-sectional design prevents any conclusions regarding causality among H. pylori infection, atrophic gastritis, and GERD. Second, this study was conducted in subjects at a single healthcare center; therefore, the pool of subjects might represent a relatively high socioeconomic status, and it is possible that the enrolled individuals were more concerned about health. However, this study design is more favorable to other studies performed in tertiary hospitals, and our study population approached the general population. Third, H. pylori infection status was evaluated solely using a serologic test. There was no clinical information available regarding current or past H. pylori infection status in the serologic test. Nevertheless, serology is the most frequently used method for epidemiological research and has been used to predict the prevalence of H. pylori infection in various populations with approved sensitivity and specificity. Fourth, it would be better if we had performed 24-h esophageal pH monitoring tests for the diagnosis of GERD, which is considered the gold-standard method. Twenty-four-hour esophageal pH monitoring tests require specific medical devices, and it is an uncomfortable and time-consuming procedure for patients. At our institute, 24-h esophageal pH monitoring is not generally available in most clinics; thus, the diagnosis of GERD is based on subjective reports of symptoms and endoscopic examination results. Therefore, we believe our data reflect real-world practice. Finally, gastric atrophy was assessed using conventional white-light endoscopy. The diagnostic accuracy of conventional white-light endoscopy is reported to be relatively low compared with that of autofluorescence imaging[37,38].
The primary goal of H. pylori eradication therapy is to improve atrophic gastritis and to reduce carcinogenic risk and associated mortality, and it is clear from previous studies that eradication therapy can reduce cancer deaths[39,40]. Even if eradication therapy is likely to exacerbate the symptoms and clinical course of GERD, it seems clear that eradication treatment should be prioritized over GERD prevention. Another implication of our study is that clinicians should pay attention to asymptomatic patients with atrophic gastritis. Patients with GERD may experience diverse symptoms such as heartburn or acid reflux; therefore, they might have a high chance of visiting clinics to manage the symptoms and undergo thorough testing, such as upper GI endoscopy. However, patients with severe atrophic gastritis, even with a higher risk of gastric cancer[8,30], have a lower risk of developing GERD. These individuals are less likely to visit a medical center on their own because they are free of GERD-related symptoms. Therefore, it is important to perform screening endoscopy in individuals without symptoms, especially in cases of extended EAG or SAG presented in previous examinations. Furthermore, if a patient with severe EAG complains of GERD-related symptoms such as heartburn or acid reflux, the possibility of other upper GI diseases including non-acid reflux or esophageal hypersensitivity should be considered because GERD is rarely accompanied by severe gastric atrophy. In the present patient, we considered a different approach than the routine prescrip-tion of PPIs.
Our findings must be confirmed through prospective clinical trials. The acid secretory activity of the gastric mucosa according to H. pylori status and extent of EAG should be further evaluated to determine the pathogenesis of GERD. In addition, an analysis of gastroesophageal motility based on H. pylori status and the extent of EAG would provide a better understanding of the mechanisms of GERD. Moreover, long-term interventional studies examining the effect of treating H. pylori infection on esophageal disease will provide clear information on which to base clinical decisions.
In summary, we found that the extent of EAG and SAG had strong inverse relationships with the occurrence and severity of GERD in a large sample of the Korean population after adjusting for multiple confounding factors. These data support the hypothesis that atrophic gastritis followed by H. pylori infection is an independent protective factor against GERD. As the number of patients with gastric atrophy may decrease based on the reduced H. pylori infection rate and broad application of H. pylori eradication therapy, the prevalence of GERD may increase. Appropriate diagnosis and treatment strategies are required based on the endoscopic findings and symptoms of patients.
Helicobacter pylori (H. pylori) infection is known to prevent the occurrence of gastroesophageal reflux disease (GERD) by inducing gastric mucosal atrophy. However, little is known about the relationship between atrophic gastritis (AG) and GERD.
Our study directly focused on AG as a protective factor of GERD, which was more fundamental and systematic approach to show how H. pylori infection affects GERD.
We aimed to confirm the inverse correlation between AG and the occurrence and severity of GERD.
We assessed AG in two independent methods, endoscopy and serology, furthermore, investigated variety of possible risk factors for GERD based on laboratory examination and structured questionnaires.
Advanced age, male sex, body mass index > 23 kg/m2, presence of metabolic synd-rome, current smoking, and alcohol consumption were associated with an increased risk of GERD. Seropositivity for H. pylori immunoglobulin G antibodies was associated with a decreased risk of GERD. There was an inverse correlation between the extent of endoscopic AG (EAG) and occurrence of GERD. Additionally, the extent of EAG showed an inverse correlation with the severity of GERD. The presence of serologic AG (SAG) was correlated with a reduced risk of GERD.
The extent of EAG and SAG exhibited strong inverse relationships with the occurrence and severity of GERD. AG followed by H. pylori infection may be independently protect against GERD.
As the Kimura-Takemoto visual endoscopic method used in our study might be subjective, it would be better to continue further study using the endoscopic morphological method - Updated Kimura-Takemoto classification of AG. Furthermore, to clarify the causality between AG and GERD, prospective studies are warranted to follow how the prevalence and severity of GERD change according to the progression or regression of AG.
The authors appreciate Gu-Cheol, Jung (Researcher, Healthcare Research Institute, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, South Korea) for his excellent statistical assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hirai R, Kishikawa H, Kotelevets SM S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-20; quiz 1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2368] [Cited by in RCA: 2455] [Article Influence: 129.2] [Reference Citation Analysis (2)] |
| 2. | Kim N, Lee SW, Cho SI, Park CG, Yang CH, Kim HS, Rew JS, Moon JS, Kim S, Park SH, Jung HC, Chung IS; H. pylori and Gerd Study Group of Korean College of Helicobacter and Upper Gastrointestinal Research. The prevalence of and risk factors for erosive oesophagitis and non-erosive reflux disease: a nationwide multicentre prospective study in Korea. Aliment Pharmacol Ther. 2008;27:173-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1265] [Article Influence: 115.0] [Reference Citation Analysis (2)] |
| 4. | Nirwan JS, Hasan SS, Babar ZU, Conway BR, Ghori MU. Global Prevalence and Risk Factors of Gastro-oesophageal Reflux Disease (GORD): Systematic Review with Meta-analysis. Sci Rep. 2020;10:5814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 184] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 5. | Youn YH, Kang YW, Ahn SH, Park SK. Prevalence alteration of reflux esophagitis in recent years. Korean J Gastrointest Endosc. 2001;23 (3):144-148. |
| 6. | El-Serag H. The association between obesity and GERD: a review of the epidemiological evidence. Dig Dis Sci. 2008;53:2307-2312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 188] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | Chung SJ, Kim D, Park MJ, Kim YS, Kim JS, Jung HC, Song IS. Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: a cross-sectional case-control study of 7078 Koreans undergoing health check-ups. Gut. 2008;57:1360-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Friedenberg FK, Xanthopoulos M, Foster GD, Richter JE. The association between gastroesophageal reflux disease and obesity. Am J Gastroenterol. 2008;103:2111-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Koike T, Ohara S, Sekine H, Iijima K, Kato K, Shimosegawa T, Toyota T. Helicobacter pylori infection inhibits reflux esophagitis by inducing atrophic gastritis. Am J Gastroenterol. 1999;94:3468-3472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Chung SJ, Lim SH, Choi J, Kim D, Kim YS, Park MJ, Yim JY, Kim JS, Cho SH, Jung HC, Song IS. Helicobacter pylori Serology Inversely Correlated With the Risk and Severity of Reflux Esophagitis in Helicobacter pylori Endemic Area: A Matched Case-Control Study of 5,616 Health Check-Up Koreans. J Neurogastroenterol Motil. 2011;17:267-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Raghunath A, Hungin AP, Wooff D, Childs S. Prevalence of Helicobacter pylori in patients with gastro-oesophageal reflux disease: systematic review. BMJ. 2003;326:737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 233] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 12. | Kandulski A, Malfertheiner P. Helicobacter pylori and gastroesophageal reflux disease. Curr Opin Gastroenterol. 2014;30:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Nam SY, Choi IJ, Ryu KH, Kim BC, Kim CG, Nam BH. Effect of Helicobacter pylori infection and its eradication on reflux esophagitis and reflux symptoms. Am J Gastroenterol. 2010;105:2153-2162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Queiroz DM, Rocha GA, Oliveira CA, Rocha AM, Santos A, Cabral MM, Nogueira AM. Role of corpus gastritis and cagA-positive Helicobacter pylori infection in reflux esophagitis. J Clin Microbiol. 2002;40:2849-2853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Hamashima C, Sasazuki S, Inoue M, Tsugane S; JPHC Study Group. Receiver operating characteristic analysis of prediction for gastric cancer development using serum pepsinogen and Helicobacter pylori antibody tests. BMC Cancer. 2017;17:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Kwon JH, Chung IS, Son HS, Park JM, Cho YK, Lee IS, Kim SW, Choi MG. [The relationship of gastrin, pepsinogen, and Helicobacter pylori in erosive reflux esophagitis]. Korean J Gastroenterol. 2008;51:159-166. [PubMed] |
| 17. | Enomoto S, Oka M, Ohata H, Mukoubayashi C, Watanabe M, Moribata K, Muraki Y, Shingaki N, Deguchi H, Ueda K, Inoue I, Maekita T, Iguchi M, Yanaoka K, Tamai H, Fujishiro M, Mohara O, Ichinose M. Assessment of gastroesophageal reflux disease by serodiagnosis of Helicobacter pylori-related chronic gastritis stage. World J Gastrointest Endosc. 2011;3:71-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 743] [Article Influence: 43.7] [Reference Citation Analysis (3)] |
| 19. | Tytgat GN. The Sydney System: endoscopic division. Endoscopic appearances in gastritis/duodenitis. J Gastroenterol Hepatol. 1991;6:223-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 148] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Kim DH, Kim GH, Kim JY, Cho HS, Park CW, Lee SM, Kim TO, Kang DH, Song GA. Endoscopic grading of atrophic gastritis is inversely associated with gastroesophageal reflux and gastropharyngeal reflux. Korean J Intern Med. 2007;22:231-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Kim GH, Song GA, Kim TO, Jo HJ, Kim DH, Heo J, Cho M, Kang DH. Endoscopic grading of gastroesophageal flap valve and atrophic gastritis is helpful to predict gastroesophageal reflux. J Gastroenterol Hepatol. 2008;23:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Fujiwara Y, Higuchi K, Shiba M, Watanabe T, Tominaga K, Oshitani N, Matsumoto T, Arakawa T. Association between gastroesophageal flap valve, reflux esophagitis, Barrett's epithelium, and atrophic gastritis assessed by endoscopy in Japanese patients. J Gastroenterol. 2003;38:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Kim TS, Park DI, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Chae SW, Kim DH. Association between atrophic gastritis and gastroesophageal reflux symptoms. Hepatogastroenterology. 2013;60:1583-1587. [PubMed] |
| 24. | Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1. [PubMed] |
| 25. | World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia, 2000: 15-17. |
| 26. | Lorenzo C, Williams K, Hunt KJ, Haffner SM. The National Cholesterol Education Program - Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 405] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 27. | Miwa H, Yokoyama T, Hori K, Sakagami T, Oshima T, Tomita T, Fujiwara Y, Saita H, Itou T, Ogawa H, Nakamura Y, Kishi K, Murayama Y, Hayashi E, Kobayashi K, Tano N, Matsushita K, Kawamoto H, Sawada Y, Ohkawa A, Arai E, Nagao K, Hamamoto N, Sugiyasu Y, Sugimoto K, Hara H, Tanimura M, Honda Y, Isozaki K, Noda S, Kubota S, Himeno S. Interobserver agreement in endoscopic evaluation of reflux esophagitis using a modified Los Angeles classification incorporating grades N and M: a validation study in a cohort of Japanese endoscopists. Dis Esophagus. 2008;21:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1518] [Cited by in RCA: 1653] [Article Influence: 63.6] [Reference Citation Analysis (1)] |
| 29. | Liu Y, Uemura N, Xiao SD, Tytgat GN, Kate FJ. Agreement between endoscopic and histological gastric atrophy scores. J Gastroenterol. 2005;40:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Jin EH, Chung SJ, Lim JH, Chung GE, Lee C, Yang JI, Kim JS. Training Effect on the Inter-observer Agreement in Endoscopic Diagnosis and Grading of Atrophic Gastritis according to Level of Endoscopic Experience. J Korean Med Sci. 2018;33:e117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Lee JY, Kim N, Lee HS, Oh JC, Kwon YH, Choi YJ, Yoon KC, Hwang JJ, Lee HJ, Lee A, Jeong Y, Jo HJ, Yoon H, Shin CM, Park YS, Lee DH. Correlations among endoscopic, histologic and serologic diagnoses for the assessment of atrophic gastritis. J Cancer Prev. 2014;19:47-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Takao T, Ishikawa T, Ando T, Takao M, Matsumoto T, Isozaki Y, Okita M, Nagao Y, Oyamada H, Yokoyama K, Tatebe A, Uchiyama K, Handa O, Takagi T, Yagi N, Kokura S, Naito Y, Yoshikawa T. Multifaceted Assessment of Chronic Gastritis: A Study of Correlations between Serological, Endoscopic, and Histological Diagnostics. Gastroenterol Res Pract. 2011;2011:631461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Lee SY. Endoscopic gastritis, serum pepsinogen assay, and Helicobacter pylori infection. Korean J Intern Med. 2016;31:835-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Yucel O. Interactions between Helicobacter pylori and gastroesophageal reflux disease. Esophagus. 2019;16:52-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Graham DY, Yamaoka Y. H. pylori and cagA: relationships with gastric cancer, duodenal ulcer, and reflux esophagitis and its complications. Helicobacter. 1998;3:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Ghoshal UC, Chourasia D. Gastroesophageal Reflux Disease and Helicobacter pylori: What May Be the Relationship? J Neurogastroenterol Motil. 2010;16:243-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Kanzaki H, Uedo N, Ishihara R, Nagai K, Matsui F, Ohta T, Hanafusa M, Hanaoka N, Takeuchi Y, Higashino K, Iishi H, Tomita Y, Tatsuta M, Yamamoto K. Comprehensive investigation of areae gastricae pattern in gastric corpus using magnifying narrow band imaging endoscopy in patients with chronic atrophic fundic gastritis. Helicobacter. 2012;17:224-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Buxbaum JL, Hormozdi D, Dinis-Ribeiro M, Lane C, Dias-Silva D, Sahakian A, Jayaram P, Pimentel-Nunes P, Shue D, Pepper M, Cho D, Laine L. Narrow-band imaging versus white light versus mapping biopsy for gastric intestinal metaplasia: a prospective blinded trial. Gastrointest Endosc. 2017;86:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | Li WQ, Zhang JY, Ma JL, Li ZX, Zhang L, Zhang Y, Guo Y, Zhou T, Li JY, Shen L, Liu WD, Han ZX, Blot WJ, Gail MH, Pan KF, You WC. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ. 2019;366:l5016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 40. | Take S, Mizuno M, Ishiki K, Kusumoto C, Imada T, Hamada F, Yoshida T, Yokota K, Mitsuhashi T, Okada H. Risk of gastric cancer in the second decade of follow-up after Helicobacter pylori eradication. J Gastroenterol. 2020;55:281-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |