Published online Dec 14, 2022. doi: 10.3748/wjg.v28.i46.6497
Peer-review started: September 13, 2022
First decision: October 19, 2022
Revised: October 28, 2022
Accepted: November 18, 2022
Article in press: November 18, 2022
Published online: December 14, 2022
Processing time: 85 Days and 15.8 Hours
Colorectal cancer (CRC) is the third most diagnosed cancer and the second leading cause of cancer-related mortality in the United States. Across the globe, people in the age group older than 50 are at a higher risk of CRC. Genetic and environmental risk factors play a significant role in the development of CRC. If detected early, CRC is preventable and treatable. Currently, available screening methods and therapies for CRC treatment reduce the incidence rate among the population, but the micrometastasis of cancer may lead to recurrence. Therefore, the challenge is to develop an alternative therapy to overcome this complication. Nanotechnology plays a vital role in cancer treatment and offers targeted chemotherapies directly and selectively to cancer cells, with enhanced therapeutic efficacy. Additionally, nanotechnology elevates the chances of patient survival in comparison to traditional chemotherapies. The potential of nanoparticles includes that they may be used simultaneously for diagnosis and treatment. These exciting properties of nanoparticles have enticed researchers worldwide to unveil their use in early CRC detection and as effective treatment. This review discusses contemporary methods of CRC screening and therapies for CRC treatment, while the primary focus is on the theranostic approach of nanotechnology in CRC treatment and its prospects. In addition, this review aims to provide knowledge on the advancement of nanotechnology in CRC and as a starting point for researchers to think about new therapeutic approaches using nanotechnology.
Core Tip: Colorectal cancer (CRC) is the third most diagnosed cancer and the second leading cause of cancer-related mortality in the United States. Genetic and environmental risk factors play a significant role in the development of CRC. This review discusses the recent insights into nanotechnology-based methods for screening, detection and treatment of CRC. This review aims to provide knowledge on the advancement of nanotechnology in CRC and as a starting point for researchers to think about new therapeutic approaches using nanotechnology.
- Citation: Gogoi P, Kaur G, Singh NK. Nanotechnology for colorectal cancer detection and treatment. World J Gastroenterol 2022; 28(46): 6497-6511
- URL: https://www.wjgnet.com/1007-9327/full/v28/i46/6497.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i46.6497
Colorectal cancer (CRC) is the second most common cause of cancer-related deaths in the United States, after lung cancer. The mortality rate of a cancer depends on its type and the sex, race and other social factors of the patient. Death from CRC affects more males than females[1]. While it is predicted that more than 1 million individuals will be diagnosed with CRC every year[2], there is a substantial geographical difference in the global distribution of CRC. The countries with the highest incidence rates of CRC are Australia, New Zealand, Canada, the United States and parts of Europe. The lowest-risk countries include China, India and parts of Africa and South America[3]. Risk factors of CRC are associated with body mass index, smoking cigarettes, red meat consumption, family history of CRC and irritable bowel disease[4], while published literature also suggests that risk of CRC decreases with physical activity, hormone therapy in postmenopausal women, aspirin/nonsteroidal anti-inflammatory drug use, fruit consumption and vegetable consumption. Thus, CRC is a disease that the management of our daily habits can prevent. Although a hereditary risk factor is involved with CRC, effective early diagnosis strategies enable earlier initiation of therapy, thereby reducing the cancer’s mortality rate.
Recently, nanotechnology has gained global consideration with its great potential for diagnosing and treating CRC. Nanotechnology utilizes nanoparticles (NPs) for specific identification of tumors and cancer biomarkers, biologically targeted contrast agents, drug delivery systems and novel treatment approaches[5]. This review discusses the recent insights into nanotechnology-based methods for screening, detection and treatment of CRC.
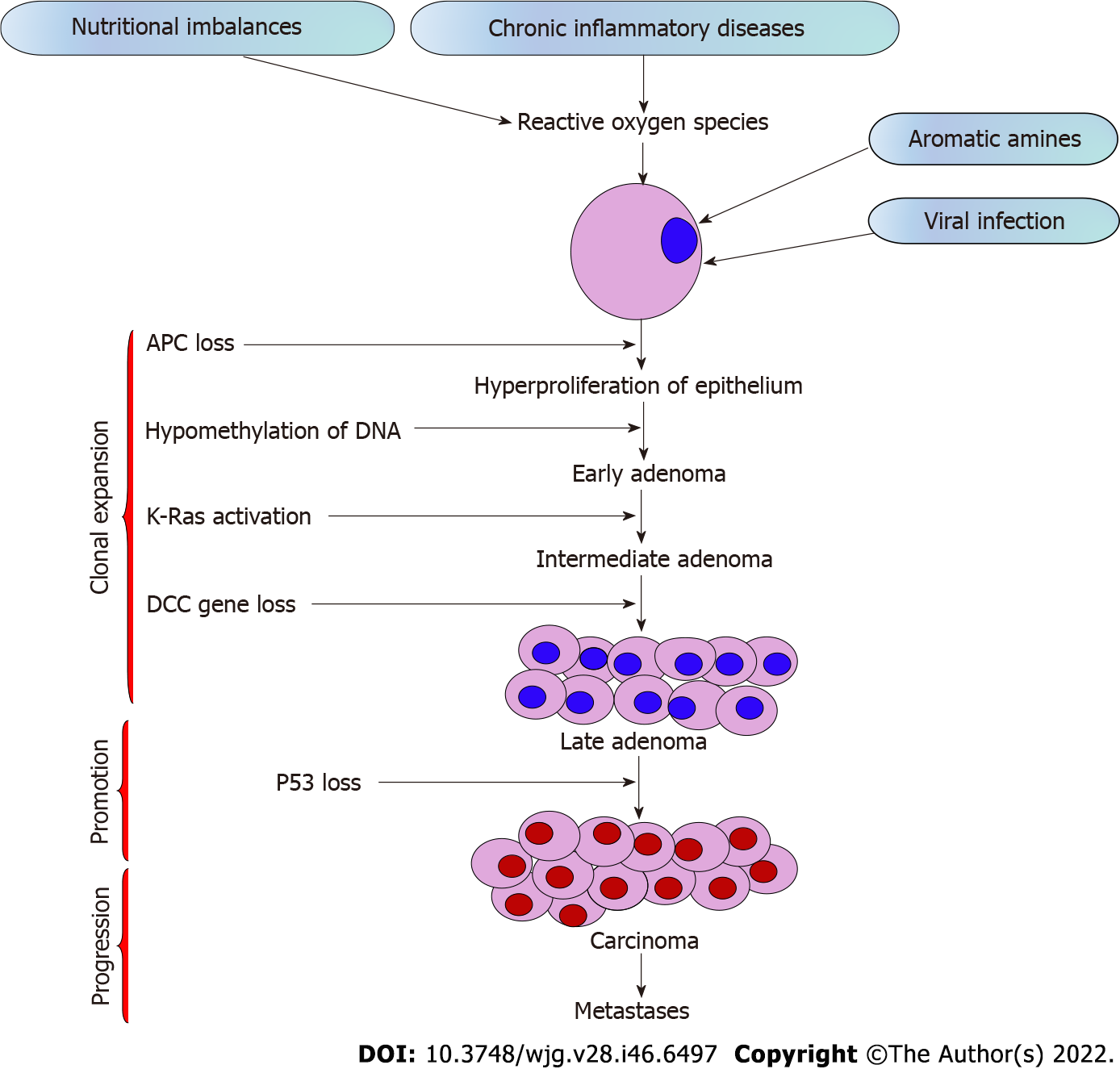
The growth of CRC incidence worldwide is related to genetic and environmental risk factors, in addition to sex, age, ethnicity, gut microbiota and socioeconomic factors[6]. Environmental risk factors include obesity, a sedentary lifestyle, poor diet, alcohol addiction, long-term smoking[7] and genetic factors due to inherited mutations or familial CRC[8]. Diet significantly impacts the microbiome environment and the risk of CRC development. Consumption of high-fiber diets and diet supplementation with polyunsaturated fatty acids, polyphenols and probiotics could potentially reduce the risk of CRC[9]. About 70% of CRC cases are sporadically seen among patients over age 50, mainly due to dietary and environmental factors[10]. In addition, a family history of CRC and a personal history of chronic inflammatory bowel disease, type 2 diabetes, or genetic diseases like familial adenomatous polyposis or Lynch syndrome are related to augmented risk[11]. Acquisition of genetic and epigenetic mutations in specific oncogenes and tumor suppressor genes in normal epithelial cells leads to the sequential transformation of the normal colorectal epithelial cell into adenocarcinoma[12]. Chromosomal instability, microsatellite instability and CpG island methylator phenotype are primary pathways influencing development of CRC[13].
The stages of CRC determine the survival rate. Around 90% of CRCs are stage I, while 10% of the cases are diagnosed with stage IV metastasis, implying that early diagnosis leads to high chances of survival[14]. The tumor stage determines whether the therapy is beneficial and how successful the treatment might be against CRC[15]. The stages of CRC range from stage 0 to IV depending on the progression of cancer across the layers of the colon and rectum, effect on lymph nodes or nearby tissues, or its metastasis to distant organs (Table 1)[16]. Out of all the stages, stage IV CRC is not often curable, but it can be managed based on the cancer’s growth and spread[17]. The sequential steps in CRC carcinogenesis are presented in Figure 1.
| Stage | Progression | Metastasis |
| 0 | Unusual abnormal cells arise from mucosa of the colonic wall, eventually becoming cancerous and known as carcinoma in situ or intramucosal carcinoma | No |
| I | Cancerous cells spread from the mucosa to submucosa and may also extended until the muscularis propria | No |
| II | ||
| IIA | Cancerous cells spread out from the submucosa to serosa (outermost layer) of the colon wall | No |
| IIB | Cancerous cells spread through serosa but are not extended to nearby organs | No |
| IIC | Cancerous cells spread to nearby organs | No |
| III | ||
| IIIA | In the first case, cancerous cells grow through the mucosa into submucosa and also may extended until the muscularis propria. The cancer spread to 1 to 3 nearby lymph nodes or into the areas of fat near lymph nodes but not to distant sites | No |
| In the second case, the cancer has spread through the mucosa into submucosa and has spread to 4 to 6 nearby lymph nodes | ||
| IIIB | In the first case, cancerous cells have spread through the muscularis propria of colon and/or rectum up to serosa or through the serosa to tissues and visceral peritoneum. They have also spread to 1 to 3 nearby lymph nodes or cancer cells are present in nearby tissues of lymph nodes but have not spread to distant sites | No |
| Second case, the cancer has spread into the muscularis propria or into the outermost layers of colon or rectum and has spread to 4 to 6 nearby lymph nodes but not to distant sites | ||
| Third case, the cancer has spread through the mucosa into submucosa and it might also have grown into the muscularis propria of colon and/or rectum and has spread to 7 or more nearby lymph nodes but not to distant sites | ||
| IIIC | First case, the cancer has grown through the serosa colon and/or rectum to visceral peritoneum but has not reached nearby organs. It has spread to 4 to 6 nearby lymph nodes but not to distant sites | No |
| Second case, the cancer has grown into the outermost layers of the colon and/or rectum or through visceral peritoneum but has not reached nearby organs. It has spread to 7 or more nearby lymph nodes but not to distant sites | ||
| Third case, the cancer has grown through the wall of the colon and/or rectum and is attached to or has grown into other nearby tissues or organs. It has spread to at least one nearby lymph node or into areas of fat near the lymph nodes but not to distant sites | ||
| IV | ||
| IVA | The cancer has spread to one area or organ that is not near the colon and/or rectum, may be liver, lung, ovary, or a distant lymph node | Yes |
| IVB | The cancer has spread to more than one area or organ that is not near the colon and/or rectum, such as the liver, lung, ovary, or a distant lymph node | Yes |
| IVC | The cancer has spread to the tissue that lines the wall of the abdomen and may have spread to other areas or organs | Yes |
The United States Preventive Services Task Force recommends colorectal screening for adults aged 45 to 75. Several techniques are used to detect polyps or CRC, but colonoscopy is a must to confirm the screening process and other screening tests. The most used methods for screening CRC are stool test, flexible sigmoidoscopy, colonoscopy, computed tomography colonography and double-contrast barium enema[18-28] in Table 2. Detection of DNA biomarkers is a promising technique for CRC screening. Stool-based DNA markers are convenient and easy to use. For early detection of CRC, biomarkers such as ITG4 methylation, SFRP2 methylation, miR-21, miR-92a and miR-135b, as well as CologuardÒ (Exact Sciences Corp., Madison, WI, United States), can be used. For malignancy detection, biomarkers such as SFRP2 methylation, VIM methylation, TFPI methylation, miR-21, miR-92a and miR-223 can be used[29].
| Ref. | Screening methods | |
| [18-20] | Stool tests | Kit-based technique; guaiac-based fecal occult blood test uses guaiac to detect heme; FIT uses antibody to detect hemoglobin in stool; FIT-DNA test detects hemoglobin and DNA biomarkers in the stool |
| Non-invasive, low cost, colon cleansing is not required, non-bleeding tumors cannot be detected | ||
| [21,22] | Flexible sigmoidoscopy | Flexible tube with light, lens for viewing and tool for removing tissue; rectum and lower third of the colon screened to detect polyps, cancer or other abnormalities |
| Sedation is required; accurate detection if polyps and cancers are present; unable to detect polyps or any abnormalities present in cecum, ascending colon hepatic flexure, or on transverse colon; colon cleansing is required | ||
| [23,24] | Colonoscopy | Flexible tube light with a lens for seeing and a tool for excising the abnormal tissue; entire colon and rectum are screened for cancer by inserting the colonoscope through anus |
| Sedation is required; visualization of entire inner lining of the colon and rectum; minute polyps can be detected; sedation may lead to bleeding or tear of the intestinal wall; sedation may lead to bleeding or tear of the intestinal wall | ||
| [25-27] | Computed tomography colonography | X-ray based computed tomography scanner captures two- and three-dimensional images of the entire colon; computer assembles these pictures into detailed images |
| Sedation is not required in this method; non-invasive; colon cleansing is not essential; may miss small polyps | ||
| [28] | Double-contrast barium enema | X-ray images are then captured by introducing barium sulphate enema |
| Examination of the whole colon and the rectum; sedation is not required; for the patient who cannot undergo standard colonscopy, this method is useful; colon cleansing is very necessary for this method; otherwise, it will give false positive results |
Clinical trials reported that sigmoidoscopy[30] and colonoscopy[31] reduce the chances of incidence and mortality of CRC. Sigmoidoscopy has a sensitivity and specificity of 92%-97% for the detection of polyps and expanded CRC and can be used to diagnose and treat CRC in patients who are ineligible for surgery (according to the severity of the tumor or comorbidities)[29]. The mentioned screening techniques detect either abnormal blood, antibodies, hemoglobin, DNA markers in the stool, or any polyps in the colon or rectum wall. Besides this, circulating tumor cell (CTC) detection-based diagnostic technology is also available, including CellSerch® assay (Janssen Diagnostics, LLC, South Raritan, NJ, United States), followed by faster and more efficient diagnostic systems like MagSweeper (Illumina, San Diego, CA, United States), Cynvenio (Cynvenio Biosystems Inc., Westlake Village, CA, United States), IsoFlux (Fluxion Biosciences, Alameda, CA, United States), VerIFASt, AdnaGen and magnetic sifters[5]. Most current screening methods of CRC are costly and not accessible at the point of care. Therefore, developing a more sensitive, fast, low-cost and specific screening test for CRC is essential.
CRC care entails a multidisciplinary approach as the treatment options depend on factors such as the patient’s age, comorbidities, overall health, type and stage of cancer, possible side effects of treatment regimens, etc[32]. Based on these characteristics and risk factors, patients are grouped into the following four categories for treatment guidance: (1) Group 0, no metastatic disease or relapse; (2) Group 1, potentially resectable metastatic disease; (3) Group 2, disseminated unresectable disease; and (4) Group 3, unresectable disease and unavailability of intensive or sequential treatment.
Surgery is considered the most common treatment for CRC. It demarcates the removal of the tumor clinically during an operation. This is also called surgical resection. Group 0 patients fall under this category. Depending upon the size and location of the tumor, the surgical options include laparoscopic surgery, colostomy and radiofrequency ablation or cryoablation. Laparoscopic surgery is an effective method that is carried out by passing several scopes into the abdomen via smaller incisions, whereas colostomy is a surgical opening for waste removal (into a pouch) by connecting the colon to the abdominal surface. When radiofrequency waves are used to heat or freeze the malignant tumors in the liver and lungs of CRC patients, then it is called radiofrequency ablation or cryoablation, respectively[33].
In radiation therapy, the size of the tumor is reduced and cancer cells are destroyed using high-energy radiations such as X-rays, radio waves and protons. This therapy is primarily used in patients at risk of tumor recurrence. Different types of radiation therapies include external-beam, stereotactic, intraoperative and brachytherapy[34]. The difference lies with the dose and the location of the cancer. For instance, external-beam radiation therapy is usually given 5 d a week for several weeks, whereas stereotactic radiation therapy delivers precise radiation to a small area. Stereotactic radiation therapy is given in malignant cancers that spread from the colorectal region to the liver or lungs. Further, intraoperative radiation therapy and brachytherapy are specialized radiation therapies applicable only in small areas of cancer that cannot be removed by surgery. In intraoperative radiation therapy, a single and high dose of radiation is given, whereas radioactive “seeds” are used and placed inside the body in brachytherapy. Lastly, neoadjuvant therapy shrinks the tumor for easier removal through surgery[35].
Medications are given as treatment plans to destroy cancer cells or reduce the tumor size. It can be delivered systemically via the bloodstream or directly through intravenous injections, pills, or capsules. Medications include chemotherapy, targeted therapy and immunotherapy[36]. Chemotherapy is generally given to group 1 patients. It is considered a central treatment strategy for all types of cancers. The United States Food and Drug Administration (FDA)-approved drugs to treat CRC are capecitabine (XelodaÒ), fluorouracil (5-FU), irinotecan (CamptosarÒ), oxaliplatin (EloxatinÒ) and trifluridine/ tipiracil (LonsurfÒ). For combined therapies, the combination of 5-FU with leucovorin (folinic acid), or leucovorin and oxaliplatin, or leucovorin and irinotecan, or capecitabine with irinotecan or oxaliplatin are used in group 3 patients for the treatment of CRC[37]. Different medications have different mechanistic pathways through which they act upon the cancer cells, viz., inhibition of DNA replication, interference in chromosomal separation in the cell cycle, or cytotoxicity. However, chemotherapy may attack healthy cells. Chemotherapy is often given with radiation therapy to increase the effectiveness of the treatment regimen. A combination of chemotherapy and radiation is known as chemoradiation therapy. Chemoradiation therapy is often given to reduce the risk of colostomy or cancer recurrence.
In targeted therapy, specific genes, proteins, or a niche is targeted to hinder the growth and survival of cancer cells. It limits damage to the healthy cells in group 1 and 2 patients. The targeted therapies for treating CRC include antiangiogenesis therapy and epidermal growth factor receptor inhibitors[38,39]. As the name suggests, the role of antiangiogenesis therapy is to stop angiogenesis, i.e., the formation of new blood vessels. In the tumor niche, the nutrients are provided by blood vessels for the growth of tumor cells. However, these antiangiogenesis therapies lead to the starvation of tumor cells. For example, bevacizumab (AvastinÒ), regorafenib (StivargaÒ), ziv-aflibercept (ZaltrapÒ) and ramucirumab (CyramzaÒ) are currently used targeted drugs. The FDA approved bevacizumab and chemotherapy as the first-line treatment for CRC in 2004[40].
On the other hand, epidermal growth factor receptor inhibitors such as cetuximab (ErbituxÒ) and panitumumab (VectibixÒ) are considered adequate for group 2 patients[41]. These drugs are specific for genes like Ras and Raf and are ineffective in cases with mutations[42,43]. Other nonspecific cancerous medications such as larotrectinib (VitrakviÒ) and entrectinib (RozlytrekÒ) are also used. These medications act on a specific genetic change called an neurotrophic tyrosine receptor kinase fusion. The currently available treatment methods for CRC and their side effects are detailed[32-34,36,38,39,44-47] in Table 3.
| Treatment methods | Types/medication details | Side-effects | Stages | Ref. |
| Surgery | Laparoscopic surgery | Pain; tenderness; irritation and itching; constipation or diarrhea | I, II, III, IV | [32,44] |
| Colostomy for rectal cancer | ||||
| Radiofrequency ablation or cryoablation | ||||
| Radiation therapy | External-beam radiation therapy | Fatigue; mild skin reactions; bleeding in stools; constipation or diarrhea; infertility | II, III, IV | [33,34] |
| Stereotactic radiation therapy | ||||
| Intraoperative radiation therapy | ||||
| Brachytherapy | ||||
| Chemotherapy | Capecitabine (Xeloda) | Vomiting; nausea; diarrhea; mouth sores; neuropathy; fatigue; hair loss | II, III, IV | [36,39] |
| Fluorouracil | ||||
| Irinotecan (Camptosar) | ||||
| Oxaliplatin (Eloxatin) | ||||
| Trifluridine/tipiracil (Lonsurf) | ||||
| Targeted therapy | Anti-angiogenesis therapy | Rashes | IV | [38,45] |
| Epidermal growth factor receptor inhibitors | ||||
| Combined targeted therapies | ||||
| Tumor-agnostic treatment | ||||
| Immunotherapy | Pembrolizumab (Keytruda) | Fatigue; rashes, pain and itching; diarrhea; nausea; fever; vomiting; shortness of breath | III, IV | [46,47] |
| Nivolumab (Opdivo) | ||||
| Dostarlimab (Jemperli) | ||||
| Nivolumab and ipilimumab (Yervoy) combination |
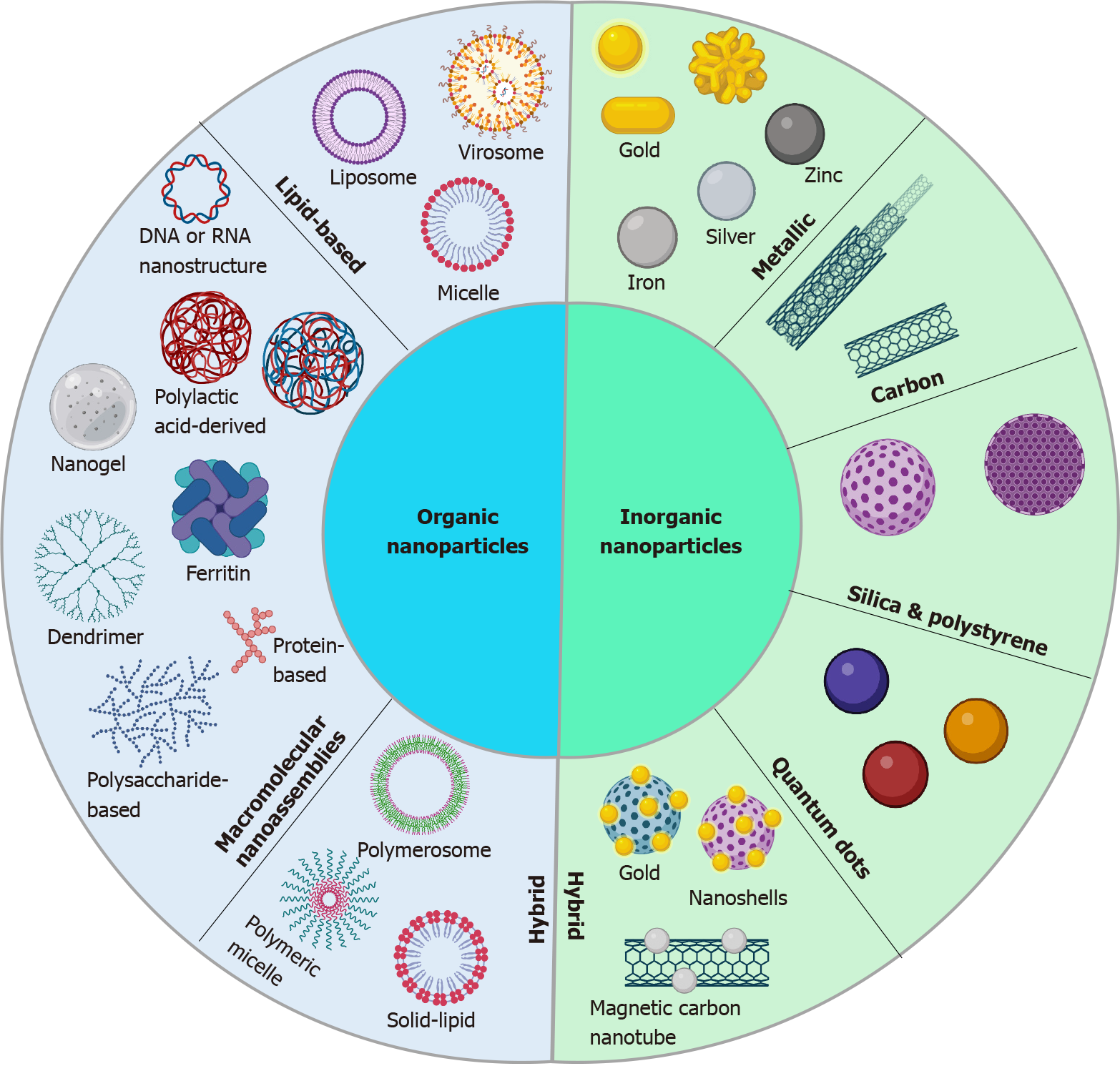
NPs were first reported as drug carriers in 1986 and it was shown that they accumulate in tumoral tissues. This passive accumulation of NPs, known as the ‘‘enhanced permeability and retention’’ effect, was one of the turning points of cancer treatment using NPs. Therefore, due to their high specificity, accumulation in tumor sites and prolonged blood circulation time, NPs are considered promising for cancer therapeutics[48,49] (Figure 2). Various families of organic and inorganic NPs are available today with a vast variety of sizes, structures and compositions[49] (Figure 3). Some of the common NPs that are used for CRC diagnosis and treatment are listed here.
Iron oxide is popular as a diagnostic and therapeutic tool for its use in oncology. The superparamagnetic properties of iron oxide NPs have attracted attention for their biomedical applications, arising due to their biocompatibility and non-toxicity[50,51]. The diameter ranges from 1-100 nm with a magnetic core to the iron oxide nanocrystals and a polymer covering containing the various medicinal molecules. These NPs have diverse biological properties due to their relatively small dimension, minimal deposition rate, efficient surface region and ease of cellular transport. Iron oxide NPs loaded with 5-FU with magnetic hyperthermia effectively reduce tumor growth in heterotopic human colon cancer in mice[52]. Epirubicin-5TR1 aptamer-superparamagnetic iron oxide NP (SPION) tertiary complex could efficiently deliver epirubicin to C26 murine colon carcinoma cells and allows tumor detection by magnetic resonance imaging (MRI)[51]. Chitosan-coated iron oxide enhanced reactive oxygen species’ production in a human colorectal carcinoma cell lines (HCT 116) and cell death was caused via caspase 9/3 activation[53].
Quantum dots are nanocrystal particles of semiconductors ranging from 2-10 nm, with fluorescence emission affected by particle size. With its optical and chemical advantages, quantum dot-based nanotechnology is a growing platform for cancer research, particularly CRC. Quantum dots are preferred for biomedical imaging due to their high quantum efficiency, photostability, extended excitation wavelengths and narrow emission band[54,55]. Various in vitro and in vivo studies have used quantum dots as fluorescent markers for cancer, with their in vivo use limited to nontargeted or xenograft labeling[56,57]. Vascular endothelial growth factor receptor 2 (VEGFR2) is upregulated in CRCs and QDot655 targeted to VEGFR2 (QD655-VEGFR2) showed its ability to detect VEGFR2-expressing tumors in vivo[58].
Amongst the natural and synthetic polymeric NPs, poly (lactic-co-glycolic acid) (PLGA) has been used for oral drug delivery applications approved by the United States FDA. PLGA NPs are physically and chemically stable, have higher stability in biological fluids and protect encapsulated drugs from enzymatic degradation. In addition, PLGA NPs can entrap small macromolecules, enhance thermal stability of the molecules and help in sustained release[59,60]. Due to its biodegradability and biocompatibility characteristics and its ability to encapsulate hydrophobic and hydrophilic drugs, PLGA is often used as a drug carrier[61,62]. PEGylated-PLGA nanocapsules loaded with docetaxel and SPIONs treated tumor growth in CT26 colon cancer[63]. Encapsulated 5-FU into PLGA NPs reduced the proliferation rate of the colon cancer cell line HT-29 by increasing the intracellular concentration of drugs in cancer cells[64]. EGF-functionalized PLGA NPs loaded with 5-FU and perfluorocarbons inhibited colon tumor growth[65]. The single-step surface-functionalizing technique was used to prepare PLGA/PLA-PEG-FA NPs to incorporate 17-AAG (NP-PEG-FA/17-AAG), which improved the oral bioavailability of 17-AAG and effectively treated ulcerative colitis and associated cancer[66]. 5-FU-loaded PHBV/PLGA NPs are a promising nanodrug delivery system for the treatment of colon cancer[67].
Dendrimers are highly branched spherical molecules having three-dimensional chemical structures, being enormously useful in nanopharmaceuticals due to their biodegradable backbones. The versatility of dendrimers in the delivery of anticancer drugs and theranostic applications in cancer therapy has been well established. Anticancer conjugated dendrimers can deliver the drug intracellularly, bypassing the efflux transporter and improving the bioavailability of loaded molecular cargo. They are also used for delivering diagnostic agents for tumor-targeted imaging[68,69].
The chemotherapeutic approach to treat CRC is not proven to be effective, as only a tiny fraction of the drug reaches the tumor target site at an effective concentration[70]. The CTCs are cancer cells that migrate and are primarily responsible for tumor metastasis. Given the importance of CTCs as an indicator of poor prognosis, several technologies (such as size-based filtration, microfluidics-based, etc) were utilized to isolate and capture CTCs from large populations of interfering cells but were not truly successful. Various methods were employed to detect colorectal CTC using dendrimers conjugated with antibodies, such as PAMAM dendrimers conjugated with Sialyl Lewis X antibodies to capture colon cancer HT29 cells[71].
Apart from diagnostic use, dendrimers are also reported for use in anticancer therapy in vitro. G4 PAMAM dendrimers conjugated with capecitabine reported decreased tumor size and reduced side effects of capecitabine[72]. Gold NPs inside PAMAM dendrimer conjugated with curcumin showed higher cellular uptake, internalization and cytotoxicity in C26 and HT29 colorectal cells[73]. Camptothecin-loaded PEGylated PAMAM dendrimer functionalized with AS1411 (anti-nucleolin aptamers) for site-specific targeting of CRC cells[74] and L-lysine dendrimers with polyoxazoline conjugated with SN-38 (the active metabolite of irinotecan) increased efficacy and it minimized adverse side effects[75]. PAMAM G4 dendrimers with oxaliplatin enhanced targeting efficacy towards folic acid receptor-expressing CRC cells in vitro[76]. Gemcitabine-loaded YIGSR-CMCht/PAMAM dendrimer NPs induced targeted mortality on HCT-116 cancer cells[48].
Carbon nanotubes (CNTs) are carbon nanomaterials with multifaceted roles in diagnosis, gene therapy, immunotherapy and a carrier in the drug delivery system. They have excellent optical properties, thermal conductivity, chemical stability and functionalization. CNTs are tiny tubular carbon atoms ordered to form a honeycomb nanostructure with exceptional physicochemical properties[77]. Based on the sheets of carbon atom numbers, CNTs are divided into single-wall CNTs or multi-wall CNTs. Various researchers have reported the effectiveness of CNT in cancer treatment and diagnosis. CpG-conjugated CNTs elevate the CpG uptake in mouse colon cancer cells and activates nuclear transcription factor-kappa B signals. CpG-CNT conjugation successfully attenuated local xenograft tumor growth and liver metastasis[78]. Fluorescein functionalized single-walled CNT/II-NCC hybrids showed higher intrinsic activity against colon cancer cells (Caco-2) compared to the non-functionalized counterpart[79]. Single-wall CNT-conjugated antibody C225 binds to epidermal growth factor receptor-expressed CRC cells via receptor-mediated endocytosis[80]. Photodynamic therapy enhanced the ability to kill colon cancer cells by single-walled CNT nanobiocomposites[81].
Liposomes are artificial and non-toxic lipid-based vesicular carriers. Historically, liposomes were first introduced as nanocarriers in 1961 as an FDA-approved drug delivery system. It comprises a phospholipid bilayer structure with a small and spherical aqueous core[82]. The main characteristic and advantage of NPs is their smaller size, which favors the particle for effective and targeted delivery of the drug used to diagnose and treat the disease[83]. Moreover, these particles have minor side effects. Among several NPs, liposomes are primarily used for the delivery system for nucleic acids, proteins and peptides. Depending on the acting properties of liposomes, there are three types, as follows: (1) Stealth or long-circulating; (2) Active targeting; and (3) Thermo-sensitive/pH-sensitive/magnetic liposomes[84]. For example, doxorubicin (Doxil), DaunoXome and Marqibo® are FDA-approved liposomal drugs and Thermodox® is a thermo-sensitive liposome used to treat CRC[85].
Silica NPs possess a beehive-like porous structure with adjustable sizes of cavities ranging from 50-300 nm and 2-6 nm[86]. The advantageous characteristics of silica NPs include a highly porous framework, less toxicity, biocompatibility, pH sensitivity and easy functionalization[87]. It is highly recommended for precise drug delivery for anticancer activity. For instance, a mesoporous silica NP-protamine hybrid system and conjugated hyaluronic acid to silica NPs load more medicine and increase the drug’s efficacy in the tumor niche. For CRC, silica NPs, along with photosensitizer chemicals, are used. The photosensitizers become activated when exposed to light, releasing reactive oxygen molecules to kill the cancer cells[88].
Gold NPs are considered the most stable among all noble NPs. They can be nanostructured in the form of cubes, spheres, rods, flowers, branches, wires, pyramids, shells and cages. With accurate surface coating, the gold NPs are safer, efficient for drug delivery and specific for cancer targeting[89]. Gold NPs enhanced cisplatin delivery efficacy and effectively decompressed CRC vessels[90]. Like nanoemulsion, gold NPs can also be conjugated with several molecules, such as antibodies, nucleic acids, proteins, enzymes and fluorescent dyes. These factors augment the properties of gold NPs, including stability, biocompatibility and functionalization in the medical field[91].
As the name suggests, a nanoemulsion system consists of oil, water and surfactant, formulating a transparent colloidal solution. It possesses the characteristics of less toxicity, high stability, thermosensitivity, pH-sensitivity and better efficacy[82]. Nowadays, antiangiogenic drugs have been used to target CRC cells. However, these agents cause toxicity and resistance and are affected by barriers. In the tumor niche, vascularized tissue is a barrier that nanoemulsions can easily overcome to target cancer cells[92]. Nanoemulsion systems are used to deliver water-insoluble drugs with a hydrophobic core. For effective drug delivery and treatment activity, nanoemulsions are conjugated with different antibodies for selective and specific targeting. Other than antibodies, nanoemulsions are also conjugated with polyethylene glycol. Polyelectrolyte complex micelles and DNA complexes enhance their ability as cancer therapeutics. Tween-80 is a widely accepted and used emulsifier[93].
In traditional cancer treatments, the therapeutic agents affect the immune system adversely, eliciting several side effects. Nanodrug delivery systems encapsulate the therapeutic agents, followed by targeted drug delivery in the tumor niche, which decreases the side effects. These systems not only lower the toxicity in the body but are highly stable, biocompatible and efficient [e.g., polypeptide-based copolymers, poly(trimethylene carbonate)-block-poly(L-glutamic acid) derived polymersomes, plitidepsin-unloaded polymersomes, near-infrared fluorescent proteinoid-poly(L-lactic acid), P(EF-proteinoid-poly(L-lactic acid)] random copolymer, proteinoid-proteinoid-poly(L-lactic acid) copolymer, etc. Several copolymers are effective against CRC cells and do not elicit any side effects. Nanoformulation of curcumin in micelles, nanogels, liposomes, NPs and cyclodextrins has been documented for treating CRC[94].
With the advent of nanotechnology-based approaches, cancer detection and treatment strategies have changed in recent years. However, the clinical applications of nanotechnology-based formulations are limited due to their complex pharmacokinetics. The following are the combinatorial usage of nanotechnology-based approaches in the theranostics of CRC.
Nanotechnology-based contrasting agents are used to enhance the capability of cancer diagnostic imaging modalities such as positron emission tomography (PET), MRI and other optical imaging techniques. The primary aim of this new technology is to detect the smallest possible number of cancer cells. PET is a powerful tool in diagnosing cancer due to its high sensitivity. A variety of relevant biomarkers are used for molecular and metabolic imaging with PET, such as fluorodeoxyglucose, 9mTc-labeled polyethylene glycol, [18F] DCFPyL, etc. Recently, NP-based contrasting agents including 56Fe and 14C conjugated with SPIONs, 99mTc-radiolabeled nanosilica system conjugated with a trastuzumab half-chain and MUC1 aptamer conjugated mesoporous silica NP have been used as radiolabeled tracers for PET. PET scan does not provide anatomical information; thus, other modalities such as computed tomography or MRI are used or have been merged with PET. In the case of MRI, SPIONs, gadolinium-encapsulated silicon microparticles and gadolinium-ion-doped upconversion NPs are mainly used as contrast agents. At the same time, 2-deoxy-d-glucose labeled gold NPs and iodinated gold nanoclusters (AuNCs-BSA-I) are used in computed tomography. The advent of nanotechnology has made advancements in the imaging modalities for early detection of cancer and thus timely treatment for cancer[95].
The primary functional role of nanotechnology in the cancer field is drug delivery. The issues of multidrug resistance, stability, efficacy and biocompatibility have been improved by formulating the drug delivery system using NPs. Traditionally, chemotherapy and other therapies have significant side effects, which are also reduced with the implementation of the nanotechnology-based formulation. For instance, 5-FU nanoencapsulation, forming a combinatorial nanomedicine agent with thiolated chitosan, is non-toxic and has enhanced chemotherapeutic efficacy in CRC patients. The use of nanomedicine has also led to a reduction in the dose quantity. In conventional therapy of 5-FU, the dose was much higher and toxic compared to nanoencapsulation of 5-FU[96].
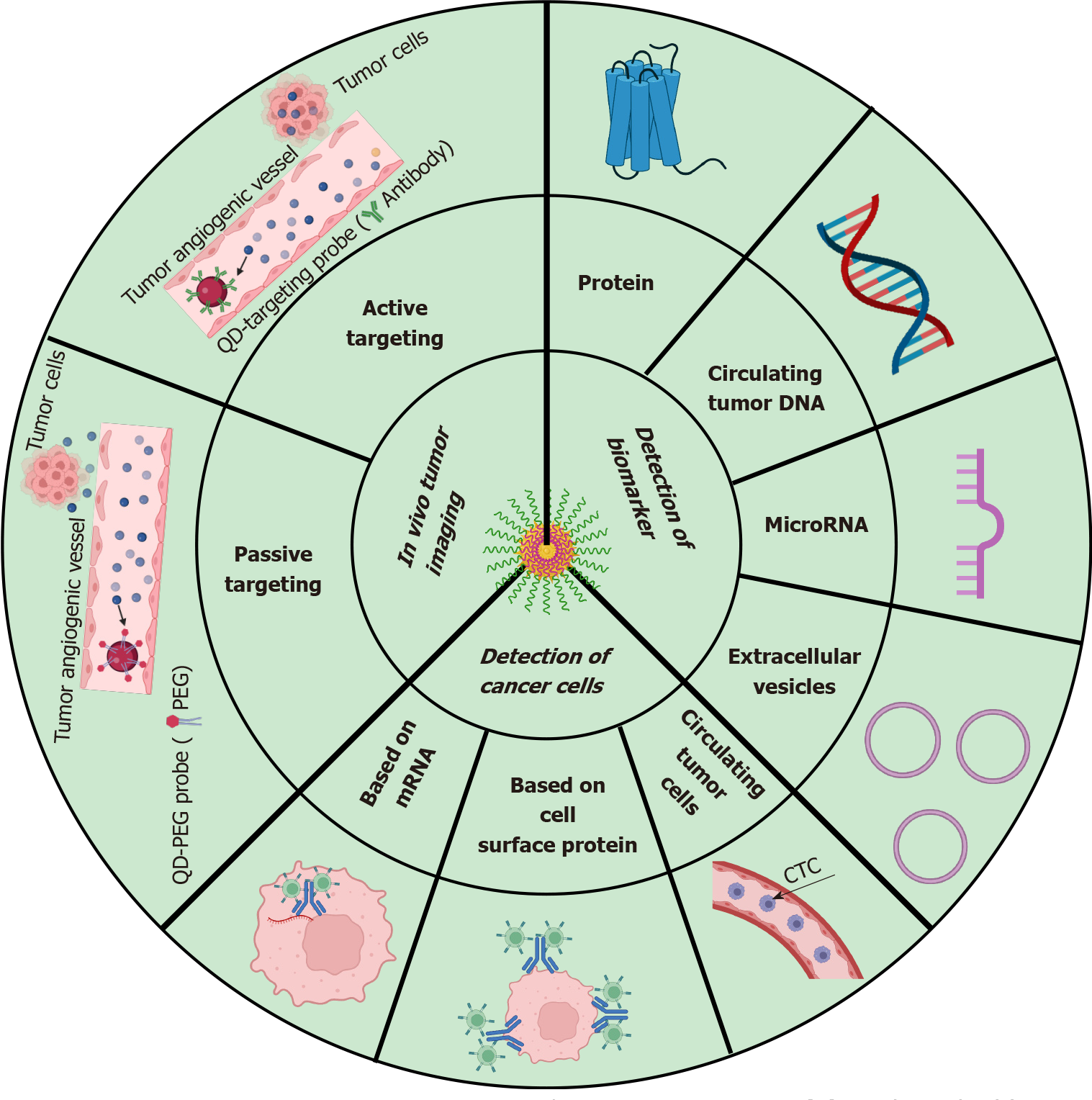
There has been significant advancement in the field of nanomedicine over the last decade. Nanotechnology is gaining immense importance because of its highly efficient delivery system. The enormous use of nanomaterials for disease diagnosis and treatment is on-trend currently. The NP-based approaches for colon cancer diagnosis are summarized[97] in Figure 4. The combined therapy with diagnosis, i.e., theranostic application of nanomaterials, makes treatment easier and faster. CRC is mainly associated with lifestyle, sex and race, suggesting that certain groups are highly vulnerable. Thus, regular screening of CRC seems to be highly recommended to prevent the occurrence of CRC. Early diagnosis of CRC increases the chances of curing cancer and survival. Theranostic application of nanomaterials against CRC is still in development. Several researchers have already reported the successful treatment of CRC in vitro in cancer cell lines and in vivo in various CRC model animals. However, new strategies are required to improve the pre-existing therapeutics and hopefully novel therapeutics using nanomaterials to treat CRC will be available soon for clinical application.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jin C, China; Wei G, China; Xiao BT, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Li N, Lu B, Luo C, Cai J, Lu M, Zhang Y, Chen H, Dai M. Incidence, mortality, survival, risk factor and screening of colorectal cancer: A comparison among China, Europe and northern America. Cancer Lett. 2021;522:255-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 235] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 2. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13558] [Article Influence: 677.9] [Reference Citation Analysis (1)] |
| 3. | Boyle P, Langman JS. ABC of colorectal cancer: Epidemiology. BMJ. 2000;321:805-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 325] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival and risk factors. Prz Gastroenterol. 2019;14:89-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 1068] [Article Influence: 178.0] [Reference Citation Analysis (1)] |
| 5. | Viswanath B, Kim S, Lee K. Recent insights into nanotechnology development for detection and treatment of colorectal cancer. Int J Nanomedicine. 2016;11:2491-2504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Sawicki T, Ruszkowska M, Danielewicz A, Niedźwiedzka E, Arłukowicz T, Przybyłowicz KE. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 460] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 7. | Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1584] [Article Influence: 264.0] [Reference Citation Analysis (2)] |
| 8. | Rustgi AK. The genetics of hereditary colon cancer. Genes Dev. 2007;21:2525-2538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 361] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 9. | Sánchez-Alcoholado L, Ramos-Molina B, Otero A, Laborda-Illanes A, Ordóñez R, Medina JA, Gómez-Millán J, Queipo-Ortuño MI. The Role of the Gut Microbiome in Colorectal Cancer Development and Therapy Response. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 10. | Dobre M, Salvi A, Pelisenco IA, Vasilescu F, De Petro G, Herlea V, Milanesi E. Crosstalk Between DNA Methylation and Gene Mutations in Colorectal Cancer. Front Oncol. 2021;11:697409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Debesa-Tur G, Pérez-Brocal V, Ruiz-Ruiz S, Castillejo A, Latorre A, Soto JL, Moya A. Metagenomic analysis of formalin-fixed paraffin-embedded tumor and normal mucosa reveals differences in the microbiome of colorectal cancer patients. Sci Rep. 2021;11:391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Parmar S, Easwaran H. Genetic and epigenetic dependencies in colorectal cancer development. Gastroenterol Rep (Oxf). 2022;10:goac035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 13. | Malki A, ElRuz RA, Gupta I, Allouch A, Vranic S, Al Moustafa AE. Molecular Mechanisms of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int J Mol Sci. 2020;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 14. | Van der Jeught K, Xu HC, Li YJ, Lu XB, Ji G. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol. 2018;24:3834-3848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 448] [Cited by in RCA: 413] [Article Influence: 59.0] [Reference Citation Analysis (5)] |
| 15. | Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 905] [Cited by in RCA: 1032] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 16. | American Joint Committee on Cancer. Colon and Rectum. 8th ed. New York: Springer. |
| 17. | Sun Y, Liu Y, Cogdell D, Calin GA, Sun B, Kopetz S, Hamilton SR, Zhang W. Examining plasma microRNA markers for colorectal cancer at different stages. Oncotarget. 2016;7:11434-11449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Ansa BE, Coughlin SS, Alema-Mensah E, Smith SA. Evaluation of Colorectal Cancer Incidence Trends in the United States (2000-2014). J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr, García FAR, Gillman MW, Harper DM, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Owens DK, Phillips WR, Phipps MG, Pignone MP, Siu AL. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:2564-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1387] [Article Influence: 154.1] [Reference Citation Analysis (1)] |
| 20. | Gutierrez-Stampa MA, Aguilar V, Sarasqueta C, Cubiella J, Portillo I, Bujanda L. Impact of the faecal immunochemical test on colorectal cancer survival. BMC Cancer. 2020;20:616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Holme Ø, Løberg M, Kalager M, Bretthauer M, Hernán MA, Aas E, Eide TJ, Skovlund E, Schneede J, Tveit KM, Hoff G. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA. 2014;312:606-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 310] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 22. | Cross AJ, Wooldrage K, Robbins EC, Pack K, Brown JP, Hamilton W, Thompson MR, Flashman KG, Halligan S, Thomas-Gibson S, Vance M, Saunders BP, Atkin W. Whole-colon investigation vs. flexible sigmoidoscopy for suspected colorectal cancer based on presenting symptoms and signs: a multicentre cohort study. Br J Cancer. 2019;120:154-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Manfredi L. Endorobots for Colonoscopy: Design Challenges and Available Technologies. Front Robot AI. 2021;8:705454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Kahi CJ, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Lieberman D, Levin TR, Robertson DJ, Rex DK; United States Multi-Society Task Force on Colorectal Cancer. Colonoscopy Surveillance After Colorectal Cancer Resection: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2016;150:758-768.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 25. | Pickhardt PJ, Graffy PM, Weigman B, Deiss-Yehiely N, Hassan C, Weiss JM. Diagnostic Performance of Multitarget Stool DNA and CT Colonography for Noninvasive Colorectal Cancer Screening. Radiology. 2020;297:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Kuipers EJ, Spaander MC. Colorectal Cancer Screening by Colonoscopy, CT-Colonography, or Fecal Immunochemical Test. J Natl Cancer Inst. 2016;108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Pickhardt PJ. Missed lesions at CT colonography: lessons learned. Abdom Imaging. 2013;38:82-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Lohsiriwat V, Prapasrivorakul S, Suthikeeree W. Colorectal cancer screening by double contrast barium enema in Thai people. Asian Pac J Cancer Prev. 2012;13:1273-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Al-Joufi FA, Setia A, Salem-Bekhit MM, Sahu RK, Alqahtani FY, Widyowati R, Aleanizy FS. Molecular Pathogenesis of Colorectal Cancer with an Emphasis on Recent Advances in Biomarkers, as Well as Nanotechnology-Based Diagnostic and Therapeutic Approaches. Nanomaterials (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 30. | Selby JV, Friedman GD, Quesenberry CP Jr, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 1076] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 31. | Ko CW, Doria-Rose VP, Barrett MJ, Kamineni A, Enewold L, Weiss NS. Screening flexible sigmoidoscopy versus colonoscopy for reduction of colorectal cancer mortality. Int J Colorectal Dis. 2019;34:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 520] [Cited by in RCA: 907] [Article Influence: 113.4] [Reference Citation Analysis (2)] |
| 33. | Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, Carrafiello G, Azoulay D, Petrillo A, Curley SA. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist. 2019;24:e990-e1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 360] [Article Influence: 60.0] [Reference Citation Analysis (1)] |
| 34. | Chen Y, Dai J, Jiang Y, Ji Z, Jiang P, Sun H, Xu F, Wang J. Long-Term Outcomes of Personalized Stereotactic Ablative Brachytherapy for Recurrent Head and Neck Adenoid Cystic Carcinoma after Surgery or External Beam Radiotherapy: A 9-Year Study. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Ludmir EB, Palta M, Willett CG, Czito BG. Total neoadjuvant therapy for rectal cancer: An emerging option. Cancer. 2017;123:1497-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 137] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 36. | Watanabe T, Wu TT, Catalano PJ, Ueki T, Satriano R, Haller DG, Benson AB 3rd, Hamilton SR. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 639] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 37. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7730] [Article Influence: 368.1] [Reference Citation Analysis (1)] |
| 38. | Hansen TF, Qvortrup C, Pfeiffer P. Angiogenesis Inhibitors for Colorectal Cancer. A Review of the Clinical Data. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Li QH, Wang YZ, Tu J, Liu CW, Yuan YJ, Lin R, He WL, Cai SR, He YL, Ye JN. Anti-EGFR therapy in metastatic colorectal cancer: mechanisms and potential regimens of drug resistance. Gastroenterol Rep (Oxf). 2020;8:179-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 40. | Riechelmann R, Grothey A. Antiangiogenic therapy for refractory colorectal cancer: current options and future strategies. Ther Adv Med Oncol. 2017;9:106-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F. Cetuximab plus irinotecan, fluorouracil and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1452] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 42. | Siena S, Sartore-Bianchi A, Di Nicolantonio F, Balfour J, Bardelli A. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J Natl Cancer Inst. 2009;101:1308-1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 400] [Cited by in RCA: 413] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 43. | Kafatos G, Niepel D, Lowe K, Jenkins-Anderson S, Westhead H, Garawin T, Traugottová Z, Bilalis A, Molnar E, Timar J, Toth E, Gouvas N, Papaxoinis G, Murray S, Mokhtar N, Vosmikova H, Fabian P, Skalova A, Wójcik P, Tysarowski A, Barugel M, van Krieken JH, Trojan J. RAS mutation prevalence among patients with metastatic colorectal cancer: a meta-analysis of real-world data. Biomark Med. 2017;11:751-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Yang YP, Qu JH, Chang XJ, Lu YY, Bai WL, Dong Z, Wang H, An LJ, Xu ZX, Wang CP, Zeng Z, Hu KQ. High intratumoral metastasis-associated in colon cancer-1 expression predicts poor outcomes of cryoablation therapy for advanced hepatocellular carcinoma. J Transl Med. 2013;11:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Peng CL, Lin HC, Chiang WL, Shih YH, Chiang PF, Luo TY, Cheng CC, Shieh MJ. Anti-angiogenic treatment (Bevacizumab) improves the responsiveness of photodynamic therapy in colorectal cancer. Photodiagnosis Photodyn Ther. 2018;23:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Golshani G, Zhang Y. Advances in immunotherapy for colorectal cancer: a review. Therap Adv Gastroenterol. 2020;13:1756284820917527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 47. | Elez E, Baraibar I. Immunotherapy in colorectal cancer: an unmet need deserving of change. Lancet Oncol. 2022;23:830-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Carvalho MR, Carvalho CR, Maia FR, Caballero D, Kundu SC, Reis RL, Oliveira JM. Peptide-Modified Dendrimer Nanoparticles for Targeted Therapy of Colorectal Cancer. Adv Ther. 2019;2:1900132. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Briolay T, Petithomme T, Fouet M, Nguyen-Pham N, Blanquart C, Boisgerault N. Delivery of cancer therapies by synthetic and bio-inspired nanovectors. Mol Cancer. 2021;20:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 50. | Palzer J, Eckstein L, Slabu I, Reisen O, Neumann UP, Roeth AA. Iron Oxide Nanoparticle-Based Hyperthermia as a Treatment Option in Various Gastrointestinal Malignancies. Nanomaterials (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 51. | Suciu M, Ionescu CM, Ciorita A, Tripon SC, Nica D, Al-Salami H, Barbu-Tudoran L. Applications of superparamagnetic iron oxide nanoparticles in drug and therapeutic delivery and biotechnological advancements. Beilstein J Nanotechnol. 2020;11:1092-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 52. | Dabaghi M, Rasa SMM, Cirri E, Ori A, Neri F, Quaas R, Hilger I. Iron Oxide Nanoparticles Carrying 5-Fluorouracil in Combination with Magnetic Hyperthermia Induce Thrombogenic Collagen Fibers, Cellular Stress and Immune Responses in Heterotopic Human Colon Cancer in Mice. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Alkahtane AA, Alghamdi HA, Aljasham AT, Alkahtani S. A possible theranostic approach of chitosan-coated iron oxide nanoparticles against human colorectal carcinoma (HCT-116) cell line. Saudi J Biol Sci. 2022;29:154-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Gil HM, Price TW, Chelani K, Bouillard JG, Calaminus SDJ, Stasiuk GJ. NIR-quantum dots in biomedical imaging and their future. iScience. 2021;24:102189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 55. | Molaei MJ. Carbon quantum dots and their biomedical and therapeutic applications: a review. RSC Adv. 2019;9:6460-6481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 225] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 56. | Jaiswal JK, Mattoussi H, Mauro JM, Simon SM. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat Biotechnol. 2003;21:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1638] [Cited by in RCA: 1231] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 57. | Lidke DS, Lidke KA, Rieger B, Jovin TM, Arndt-Jovin DJ. Reaching out for signals: filopodia sense EGF and respond by directed retrograde transport of activated receptors. J Cell Biol. 2005;170:619-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 58. | Carbary-Ganz JL, Welge WA, Barton JK, Utzinger U. In vivo molecular imaging of colorectal cancer using quantum dots targeted to vascular endothelial growth factor receptor 2 and optical coherence tomography/laser-induced fluorescence dual-modality imaging. J Biomed Opt. 2015;20:096015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Lee PW, Pokorski JK. Poly(lactic-co-glycolic acid) devices: Production and applications for sustained protein delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018;10:e1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 60. | Emami F, Seyed JMY, Dong HN. Poly(lactic acid)/poly(lactic-co-glycolic acid) particulate carriers for pulmonary drug delivery. J Pharma Investig. 2019;49:427-442. [RCA] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 61. | Jain AK, Swarnakar NK, Godugu C, Singh RP, Jain S. The effect of the oral administration of polymeric nanoparticles on the efficacy and toxicity of tamoxifen. Biomaterials. 2011;32:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 62. | Rezvantalab S, Drude NI, Moraveji MK, Güvener N, Koons EK, Shi Y, Lammers T, Kiessling F. PLGA-Based Nanoparticles in Cancer Treatment. Front Pharmacol. 2018;9:1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 343] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 63. | Al-Jamal KT, Bai J, Wang JT, Protti A, Southern P, Bogart L, Heidari H, Li X, Cakebread A, Asker D, Al-Jamal WT, Shah A, Bals S, Sosabowski J, Pankhurst QA. Magnetic Drug Targeting: Preclinical in Vivo Studies, Mathematical Modeling and Extrapolation to Humans. Nano Lett. 2016;16:5652-5660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 64. | Eynali S, Khoei S, Khoee S, Esmaelbeygi E. Evaluation of the cytotoxic effects of hyperthermia and 5-fluorouracil-loaded magnetic nanoparticles on human colon cancer cell line HT-29. Int J Hyperthermia. 2017;33:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Wu P, Zhou Q, Zhu H, Zhuang Y, Bao J. Enhanced antitumor efficacy in colon cancer using EGF functionalized PLGA nanoparticles loaded with 5-Fluorouracil and perfluorocarbon. BMC Cancer. 2020;20:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 66. | Yang M, Zhang F, Yang C, Wang L, Sung J, Garg P, Zhang M, Merlin D. Oral Targeted Delivery by Nanoparticles Enhances Efficacy of an Hsp90 Inhibitor by Reducing Systemic Exposure in Murine Models of Colitis and Colitis-Associated Cancer. J Crohns Colitis. 2020;14:130-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 67. | Handali S, Moghimipour E, Rezaei M, Ramezani Z, Dorkoosh FA. PHBV/PLGA nanoparticles for enhanced delivery of 5-fluorouracil as promising treatment of colon cancer. Pharm Dev Technol. 2020;25:206-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Li J, Yu F, Chen Y, Oupický D. Polymeric drugs: Advances in the development of pharmacologically active polymers. J Control Release. 2015;219:369-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 69. | Li T, Smet M, Dehaen W, Xu H. Selenium-Platinum Coordination Dendrimers with Controlled Anti-Cancer Activity. ACS Appl Mater Interfaces. 2016;8:3609-3614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 70. | Yan W, Tao M, Jiang B, Yao M, Jun Y, Dai W, Tang Z, Gao Y, Zhang L, Chen X, Wang QL. Overcoming Drug Resistance in Colon Cancer by Aptamer-Mediated Targeted Co-Delivery of Drug and siRNA Using Grapefruit-Derived Nanovectors. Cell Physiol Biochem. 2018;50:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 71. | Xie J, Wang J, Chen H, Shen W, Sinko PJ, Dong H, Zhao R, Lu Y, Zhu Y, Jia L. Multivalent conjugation of antibody to dendrimers for the enhanced capture and regulation on colon cancer cells. Sci Rep. 2015;5:9445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 72. | Nabavizadeh F, Fanaei H, Imani A, Vahedian J, Asadi Amoli F, Ghorbi J, Sohanaki H, Mohammadi SM, Golchoobian R. Evaluation of Nanocarrier Targeted Drug Delivery of Capecitabine-PAMAM Dendrimer Complex in a Mice Colorectal Cancer Model. Acta Med Iran. 2016;54:485-493. [PubMed] |
| 73. | Alibolandi M, Hoseini F, Mohammadi M, Ramezani P, Einafshar E, Taghdisi SM, Ramezani M, Abnous K. Curcumin-entrapped MUC-1 aptamer targeted dendrimer-gold hybrid nanostructure as a theranostic system for colon adenocarcinoma. Int J Pharm. 2018;549:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 74. | Alibolandi M, Taghdisi SM, Ramezani P, Hosseini Shamili F, Farzad SA, Abnous K, Ramezani M. Smart AS1411-aptamer conjugated pegylated PAMAM dendrimer for the superior delivery of camptothecin to colon adenocarcinoma in vitro and in vivo. Int J Pharm. 2017;519:352-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 75. | England RM, Hare JI, Barnes J, Wilson J, Smith A, Strittmatter N, Kemmitt PD, Waring MJ, Barry ST, Alexander C, Ashford MB. Tumour regression and improved gastrointestinal tolerability from controlled release of SN-38 from novel polyoxazoline-modified dendrimers. J Control Release. 2017;247:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 76. | Narmani A, Kamali M, Amini B, Salimi A, Panahi Y. Targeting delivery of oxaliplatin with smart PEG-modified PAMAM G4 to colorectal cell line: In vitro studies. Process Biochem. 2018;69:178-187. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 77. | Liu X, Ying Y, Ping J. Structure, synthesis and sensing applications of single-walled carbon nanohorns. Biosens Bioelectron. 2020;167:112495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 78. | Jin H, Gao S, Song D, Liu Y, Chen X. Intratumorally CpG immunotherapy with carbon nanotubes inhibits local tumor growth and liver metastasis by suppressing the epithelial-mesenchymal transition of colon cancer cells. Anticancer Drugs. 2021;32:278-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 79. | González-Domínguez JM, Grasa L, Frontiñán-Rubio J, Abás E, Domínguez-Alfaro A, Mesonero JE, Criado A, Ansón-Casaos A. Intrinsic and selective activity of functionalized carbon nanotube/nanocellulose platforms against colon cancer cells. Colloids Surf B Biointerfaces. 2022;212:112363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 80. | Lee PC, Chiou YC, Wong JM, Peng CL, Shieh MJ. Targeting colorectal cancer cells with single-walled carbon nanotubes conjugated to anticancer agent SN-38 and EGFR antibody. Biomaterials. 2013;34:8756-8765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 81. | Sundaram P, Abrahamse H. Effective Photodynamic Therapy for Colon Cancer Cells Using Chlorin e6 Coated Hyaluronic Acid-Based Carbon Nanotubes. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 82. | Silva R, Ferreira H, Cavaco-Paulo A. Sonoproduction of liposomes and protein particles as templates for delivery purposes. Biomacromolecules. 2011;12:3353-3368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 83. | Patil YP, Jadhav S. Novel methods for liposome preparation. Chem Phys Lipids. 2014;177:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 356] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 84. | Noble GT, Stefanick JF, Ashley JD, Kiziltepe T, Bilgicer B. Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol. 2014;32:32-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 358] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 85. | Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2871] [Cited by in RCA: 3149] [Article Influence: 262.4] [Reference Citation Analysis (0)] |
| 86. | Stang J, Haynes M, Carson P, Moghaddam M. A preclinical system prototype for focused microwave thermal therapy of the breast. IEEE Trans Biomed Eng. 2012;59:2431-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 87. | Bharti C, Nagaich U, Pal AK, Gulati N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int J Pharm Investig. 2015;5:124-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 411] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 88. | National Institutes of Health. Nanotechnology to Improve Early Detection and Treatment of Colorectal Cancer. [cited 22 August 2022]. Available from: https://www.nih.gov/research-training/nanotechnology-improve-early-detection-treatment-colorectal-cancer. |
| 89. | Siddique S, Chow JCL. Gold Nanoparticles for Drug Delivery and Cancer Therapy. Appl Sci. 2020;10:3824. [RCA] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 90. | Zhao X, Pan J, Li W, Yang W, Qin L, Pan Y. Gold nanoparticles enhance cisplatin delivery and potentiate chemotherapy by decompressing colorectal cancer vessels. Int J Nanomedicine. 2018;13:6207-6221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 91. | Pissuwan D, Gazzana C, Mongkolsuk S, Cortie MB. Single and multiple detections of foodborne pathogens by gold nanoparticle assays. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12:e1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 92. | Ganta S, Talekar M, Singh A, Coleman TP, Amiji MM. Nanoemulsions in translational research-opportunities and challenges in targeted cancer therapy. AAPS PharmSciTech. 2014;15:694-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 93. | Sánchez-López E, Guerra M, Dias-Ferreira J, Lopez-Machado A, Ettcheto M, Cano A, Espina M, Camins A, Garcia ML, Souto EB. Current Applications of Nanoemulsions in Cancer Therapeutics. Nanomaterials (Basel). 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 94. | Brar B, Ranjan K, Palria A, Kumar R, Ghosh M, Sihag S, Minakshi P. Nanotechnology in Colorectal Cancer for Precision Diagnosis and Therapy. Front Nanotechnol. 2021;3:699266. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 95. | Shahbazi-Gahrouei D, Moradi Khaniabadi P, Moradi Khaniabadi B, Shahbazi-Gahrouei S. Medical imaging modalities using nanoprobes for cancer diagnosis: A literature review on recent findings. J Res Med Sci. 2019;24:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 96. | Linton SS, Sherwood SG, Drews KC, Kester M. Targeting cancer cells in the tumor microenvironment: opportunities and challenges in combinatorial nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:208-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 97. | Zhang Y, Li M, Gao X, Chen Y, Liu T. Nanotechnology in cancer diagnosis: progress, challenges and opportunities. J Hematol Oncol. 2019;12:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 261] [Article Influence: 43.5] [Reference Citation Analysis (0)] |