Published online Nov 7, 2022. doi: 10.3748/wjg.v28.i41.5993
Peer-review started: July 18, 2022
First decision: September 8, 2022
Revised: September 21, 2022
Accepted: October 19, 2022
Article in press: October 19, 2022
Published online: November 7, 2022
Processing time: 108 Days and 12.2 Hours
Collagenous gastritis (CG) is a rare condition whose pathogenesis may be related to immune abnormalities. We report a case of CG from China.
A 24-year-old woman presented with recurrent abdominal distension and discomfort for 3 mo. Upper gastrointestinal endoscopy found diffuse nodular elevation-depression changes in the mucosa of the entire gastric corpus. Endoscopic ultrasound showed predominant involvement of the lamina propria and submucosa, and computed tomography imaging showed mild enhancement of the gastric wall. Pathological histology revealed that the thickness of the subepithelial collagen band was about 40 μm, and the Masson trichrome staining result was positive and the Congo red staining result was negative. This case is consistent with the child-adolescent type of CG.
Serum pepsinogen I, pepsinogen II, pepsinogen I/II ratio, and gastrin-17 may be potential non-invasive monitoring markers. Currently, treatments for CG vary, and the likely prognosis is unknown. Individual cases of gastric cancer in patients with CG have been reported.
Core Tip: We report a case of collagenous gastritis. We introduce the clinical features, findings of esophagogastroduodenoscopy and endoscopic ultrasound, and response to treatment in this young female patient.
- Citation: Zheng QH, Hu J, Yi XY, Xiao XH, Zhou LN, Li B, Bo XT. Collagenous gastritis in a young Chinese woman: A case report. World J Gastroenterol 2022; 28(41): 5993-6001
- URL: https://www.wjgnet.com/1007-9327/full/v28/i41/5993.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i41.5993
Collagenous gastritis (CG) is a rare condition characterized histopathologically by the deposition of collagenous bands under the mucosal epithelium and infiltration of inflammatory cells within the lamina propria of the mucosa. It is more prevalent in children and adolescents. The pathogenesis and clinical outcome remain unclear.
The first case of CG was reported in 1989 by Colletti and Trainer in the United States[1]. Since then, the literature on CG has predominantly included case reports. A search of PubMed using the keyword “collagenous gastritis” revealed < 100 reported cases. The first and second cases of CG in China were respectively reported in 2010[2] and 2018[3]. We now report the third Chinese case of CG diagnosed in 2020 at the Affiliated Hospital of Guilin Medical University.
A 24-year-old female patient presented to our outpatient service in June 2020 with the complaints of recurrent abdominal distension and discomfort for 3 mo.
The patient reported feeling epigastric distension after eating that could last anywhere from 30 min to 12 h, with no other significant discomfort.
Following hospital admission, the patient denied a history of allergies, asthma, and pet exposure. Both her father and mother were living and healthy.
The physical examination on admission revealed stable vital signs, no yellow staining of the skin or sclera, and no enlargement of superficial lymph nodes. Cardiopulmonary and abdominal examinations showed no abnormalities.
Laboratory tests revealed a normal blood count, liver function, and renal function and normal carcinoembryonic antigen, cancer antigen 125, cancer antigen 19-9, and alpha-fetoprotein values. Her liver fibrosis test was normal, and she had normal levels of immunoglobulins immunoglobulin (Ig) A, IgM, and IgG, as well as serum type III procollagen, type IV collagen, laminin, and hyaluronic acid. Her serum pepsinogen (PG) I value was 133.41 μm/L, her PG II value was elevated at 25.99 μm/L, her pepsinogen I/II ratio (PGR) value was reduced at 5.13, and her gastrin-17 value was 54.01 pmol/L. The 13C urea breath test negative, the allergen assay was negative, and her extractable nuclear antigen antibodies were negative.
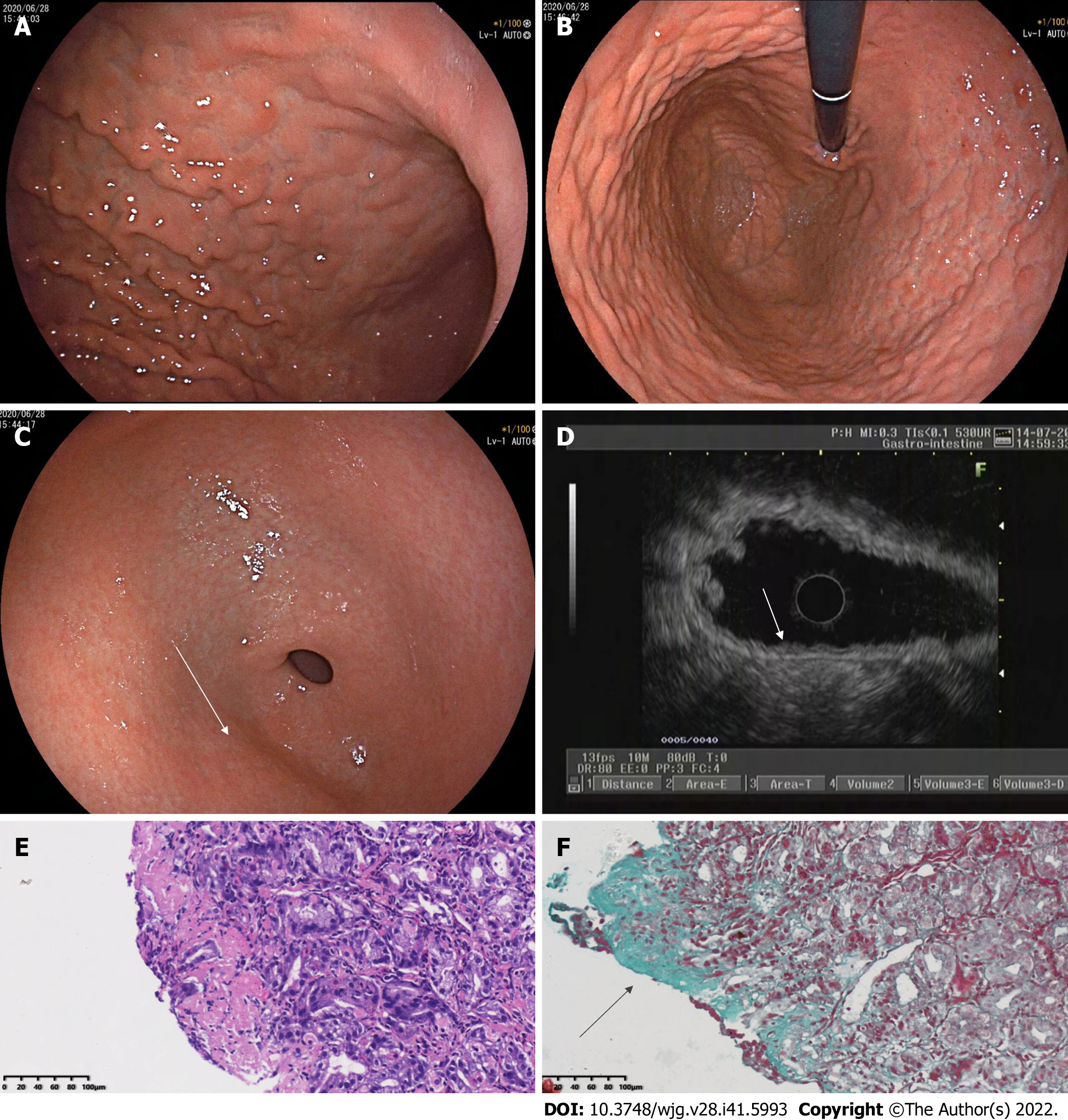
Eesophagogastroduodenoscopy (EGD) performed on June 28, 2020 revealed diffuse nodular elevation-depression changes in the mucosa of the entire gastric corpus (Figure 1A and B).
Nodular reddening-like changes were seen in the anterior wall of the gastric antrum (Figure 1C). Then, endoscopic ultrasound (EUS) completed in July 2020 suggested an intact 5-layer structure of the gastric corpus wall with hypoechoic changes in the mucosal layer (Figure 1D). Histopathology reported moderate chronic inflammation with erosion and mild activity (Figure 1E).
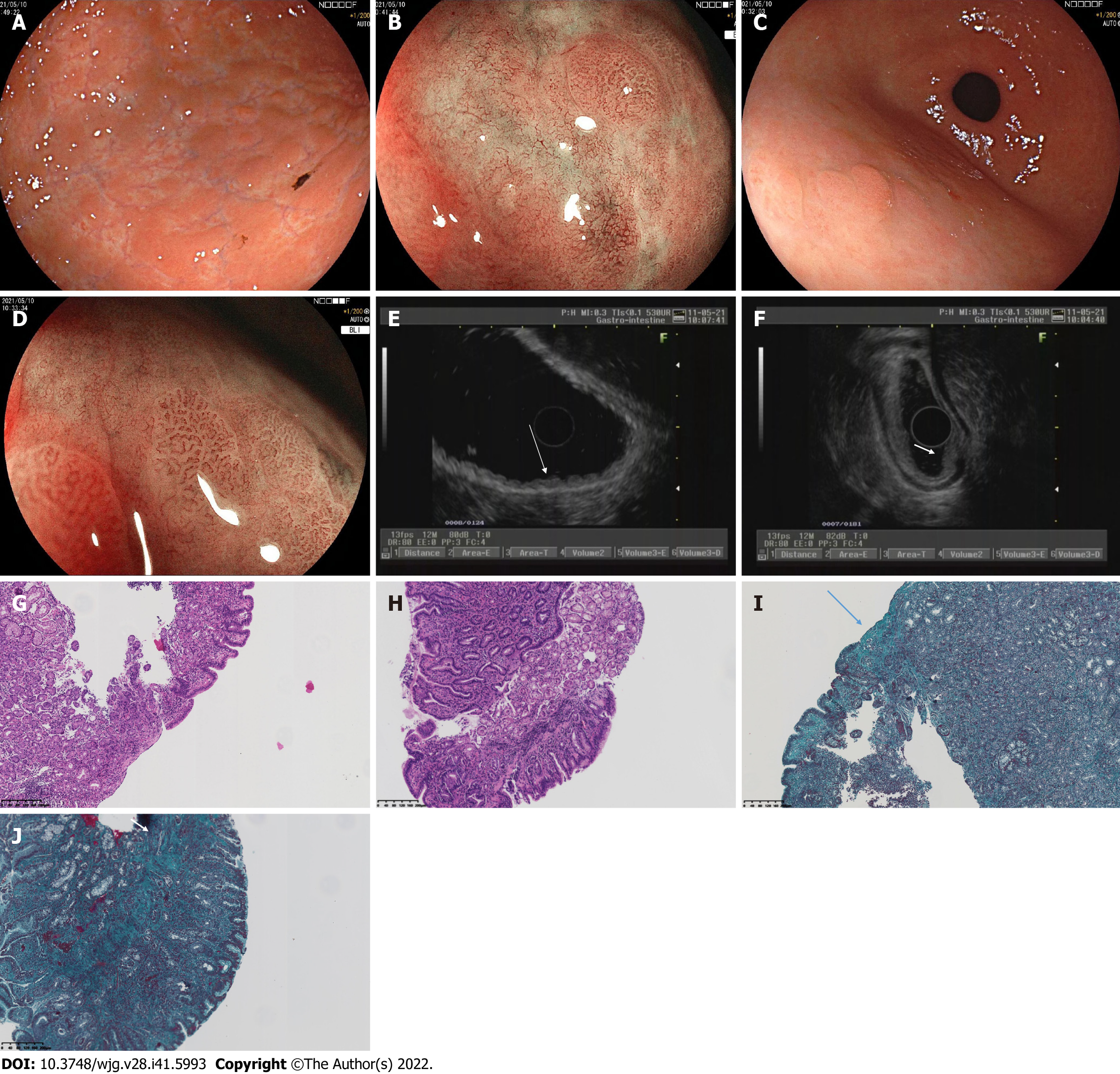
In May 10, 2021, EGD confirmed that no mass or ulcer could be found in the esophagus or duodenum. The mucosa of the angulus was smooth, while the mucosa of the gastric corpus and fundus appeared uneven, with elevation-depression changes (Figure 2A).
Magnifying endoscopy with blue laser imaging (ME-BLI) showed clear borders of the elevation, regular arrangement of the marginal crypt epithelium (MCE), and widening of the microvascular vessels. The MCE in the depression was indistinct, and the irregular microvascular pattern showed dendritic changes with a constant ductal diameter, and the microvessels here were thinner than those of the elevated area of the gastric antrum (Figure 2B). Nodular reddening-like changes were seen in the anterior wall of the gastric antrum (Figure 2C). The findings of ME-BLI were similar to those from the gastric corpus (Figure 2D). EUS showed that the mucosal layer of the gastric corpus was thickened, appearing slightly hypoechoic, with no thickening of its posterior submucosal layer (Figure 2E).
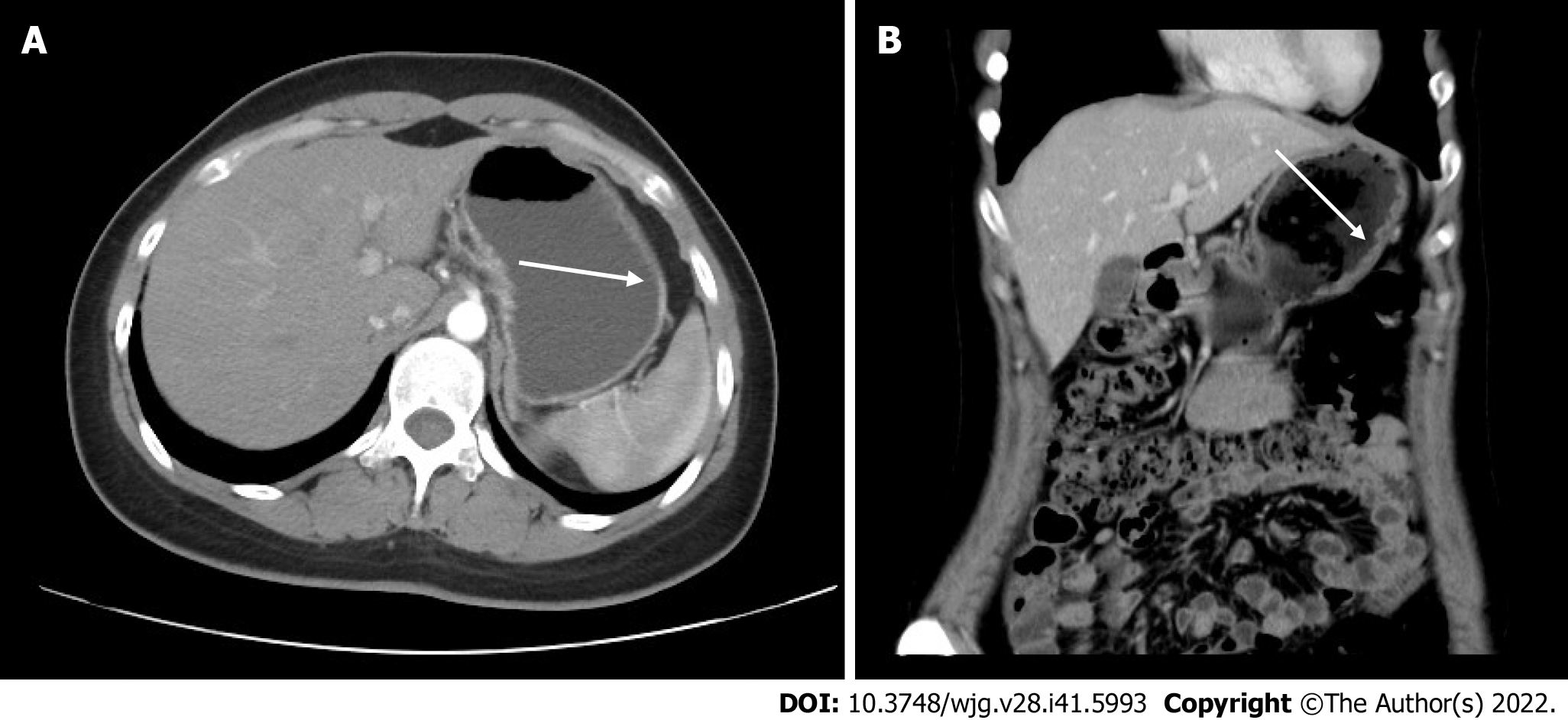
The normal structures of the mucosa, muscularis mucosa, and submucosa at the elevation of the gastric antrum had disappeared and were replaced by inhomogeneous hypoechoic changes, with the muscularis propria and serosa still intact (Figure 2F). Colonoscopy confirmed that the mucosa of the whole colon, rectum, and terminal ileum was smooth, and no mass or ulcer was visible. Computed tomography showed a relative thickening of the gastric wall in the gastric corpus and antrum, and mild enhancement was seen in the arterial phase (Figure 3A and B).
Histopathological findings of the gastric fundus, body, and antrum biopsies included moderate chronic inflammation with erosion, mild activity, a higher number of lymphocytes, a few neutrophils and eosinophils [20-30/high-powered field (HPF)] infiltrating the interstitium, multifocal lymphoid tissue hyperplasia, and negative Congo red staining (Figure 2G and H). Collagenous bands deposited under the mucosal epithelium (about 40 μm) were seen (Figure 2I and J). Angulus biopsy revealed moderate chronic inflammation with erosion and positive Masson’s trichome staining. The bulbous, terminal ileum, and ascending colon biopsies showed moderate mucosal chronic inflammation with erosion, mild activity, focal lymphocytosis, and positive Masson’s trichome staining. The rectal biopsy showed moderate mucosal chronic inflammation with erosion and positive Masson’s trichome staining. Furthermore, complementary Masson staining of the patient’s gastric corpus biopsy specimen from June 28, 2020 revealed the presence of collagenous band deposited under the epithelium (approximately 40 μm) (Figure 1F).
The patient was diagnosed as having CG.
Esomeprazole (20 mg every day, taken 30 min before breakfast) and mosapride (5 mg three times a day, i.e., once before each of all 3 meals) were given orally for 2 wk, and the patient’s abdominal distension was subsequently relieved. However, the patient continued to experience intermittent abdominal distension for > 6 mo thereafter, with episodes lasting for the same duration as before, while taking esomeprazole and mosapride irregularly. The patient was then hospitalized at the Affiliated Hospital of Guilin Medical University from May 9 to May 12, 2021.
Treatment with oral glucocorticoids was initiated for the patient in June 2021. Thirty milligrams daily of prednisone acetate was given for 2 wk, and then reduced by 5 mg every 2 wk thereafter and finally discontinued when the dose reached 5 mg daily and was maintained as such for 2 wk. During the 8 wk of treatment, the patient continued to present with intermittent abdominal distention, lasting 12 h in severe episodes, and she was given 5 mg of oral mosapride three times daily 30 min before meals. A re-examination showed that her PG I value was 105.21 μm/L, her PG II value was elevated to 24.3 μm/L, her PGR value was decreased to 4.33, and her gastrin-17 value was 46.99 pmol/L.
After treatment, the patient’s abdominal distension was relieved for 2-3 d every week. Laboratory tests performed in October 2021 revealed that her PG I value was 118 μm/L, her PG II value was elevated to 33.74 μm/L, her PGR value was decreased to 3.5, and her gastrin-17 value was elevated to 39.69 pmol/L. As of November 2021, the patient was still undergoing follow-up (Table 1).
| Date | Description |
| June 2020 | First visit, chief complaints: Recurrent abdominal distension and discomfort for 3 mo |
| June 2020 | 1st EGD + pathological histology |
| July 2020 | 1st EUS |
| July 2020 | Esomeprazole and mosapride for 2 wk; abdominal distension subsequently relieved |
| Late 2020 to first half of 2021 | Continued intermittent abdominal distension for > 6 mo |
| May 9 to May 12, 2021 | Hospitalized; PG I, PG II, and PGR tested; 2nd EGD + pathological histology; ME-BLI; 2nd EUS |
| June 2021 | Oral glucocorticoids for 8 wk and tapered; PG I, PG II, and PGR retested |
| October 2021 | PG I, PG II, and PGR retested |
The pathology is characterized by a deposited collagen band with a thickness of > 10 μm under the epithelium, with an average thickness of 30 μm and a maximum thickness of up to 120 μm[4]. Inflammatory cell infiltration is seen in the lamina propria of the mucosa. Infiltrating inflammatory cells include lymphocytes, plasma cells, monocytes, and eosinophils. Collagenous gastritis can be classified as eosinophilic, lymphocytic, and atrophic according to the type of infiltrated cells. The main criteria for these types are as follows: Eosinophils in the lamina propria > 30/HPF (eosinophilic type); lymphocytes > 25/HPF (lymphocytic type); and a reduction in glands, a reduction in specialized cells such as parietal cells and chief cells, pyloric gland metaplasia, and hyperplasia of smooth muscle in the lamina propria of the mucosa (atrophic type). The same case may contain just one or all of these types. Considering special staining in addition to Masson’s trichome staining, tenascin positivity in immunohistochemistry may also be a characteristic indicator[5]. In addition to the stomach, the disease may include colla
The pathogenesis of CG is unclear. Some pathological findings suggest an association with immune abnormalities. For example, signs of local immune activation were detected in some specimens, such as overexpression of human leucocyte antigen DR in epithelial cells, increased CD3+ intradermal lymphocytes, and CD25+ cells found in the lamina propria[6], as well as a large number of IgG4-positive plasma cells that failed to confirm an association with IgG4-related disease[5]. The histological changes in collagenous gastritis may be caused by a local immune response. In a few patient specimens, collagen bands can be isolated from type III and type IV collagen fibers. During the repair process, type III collagen is released by subepithelial fibroblasts[5]. Specimens were also found to be positive for tenascin, a marker suggestive of cell proliferation and migration[5]. Therefore, collagen synthesis in collagenous gastritis is a reparative response. In the pathogenesis of collagenous gastritis, an association with reduced serum IgA levels has also been reported[7].
Most of the CG studies reported so far are single case reports and retrospective analyses, lacking large sample data. Cases of CG have been documented mainly in Europe, the United States, and Japan. Women are more commonly afflicted than men, and the age of patients ranges from 7-85 years[8,9]. Clinical symptoms include abdominal pain, abdominal distension, diarrhea, nausea, vomiting, gastrointestinal bleeding, weight loss, anemia, fatigue, retrosternal pain, dyspepsia, perforation[10], dysphagia[11], and constipation.
Lagorce-Pages et al[4] classified CG into child-adolescent type and adult type. The child-adolescent type occurs mainly in early adolescence, where inflammation is usually limited to the stomach and anemia and abdominal pain are the main symptoms. The adult type often combines with collagenous enteritis, which is characterized by chronic watery diarrhea. CG and collagenous colitis exist on a clinical spectrum, with the difference being the site of involvement of the GI tract.
The typical endoscopic feature of collagen gastritis is the presence of mucosal nodules. These nodules vary in size and are often diffuse in the gastric corpus and antrum, with their size and number depending upon the severity of the inflammation. Endoscopic manifestations also include mucosal erythema, erosion, and exudation. The endoscopic presentation differs between pediatric and adult patients. Pediatric and adolescent patients usually present with gastric nodules, whereas adult patients often present with mucosal erythema, atrophy, and relatively uncommon nodules[9]. In image-enhanced endoscopy, glandular duct structures are seen on the surface of the nodules under magnifying narrow-band imaging, and microvascular thinning and tortuosity are seen in the structureless area[12,13].
Patients can also have other co-morbidities, including Helicobacter pylori infection[9], human immunodeficiency virus infection combined with gastric Kaposi’s sarcoma[14], and Sjögren’s syndrome[15].
Our case is a young female patient who presented with abdominal distension as the main manifestation, without obvious symptoms of anemia, abdominal pain, and diarrhea. Pathological findings supported the idea that the inflammation was limited to her stomach only, and colonoscopy and biopsy showed no intestinal involvement, consistent with the child-adolescent type. The pathological histology of this case in our hospital showed the presence of a higher number of eosinophilic infiltrates in the lamina propria, consistent with the eosinophilic type. Endoscopy showed a depressed lesion surrounding the elevation of the gastric corpus and fundus greater curvature, with a structureless area in the depression under image-enhanced endoscopy and thinning microvessels, similar to the findings observed by Kawasaki et al[12] using magnifying narrow-band imaging in the gastric corpus.
EGD at both our hospital and Hangzhou No. 1 Hospital[3] showed redness on the elevation of the gastric antrum, and biopsy suggested a collagen deposition band of > 10 μm. A routine biopsy of the gastric angulus with a smooth surface mucosa failed to find a band of collagen deposition at our hospital, and it was hypothesized that the CG inflammation might be multifocal and discontinuously distributed. The EUS findings in this case are not exactly the same as those reported previously in China[3]. The similarity between these cases is that the lesion of the gastric antrum is a hypoechoic replacement of normal structures; however, the difference is that a rough and swollen mucosa with nodular elevation of the gastric corpus was seen under white-light in the case at Hangzhou First Hospital[3], which corresponds to thickening of the muscularis mucosa layer to the submucosal layer with an unclear boundary and low echoes, while, using EGD at our hospital, we found depressed lesions surrounding the elevated ones in the gastric corpus, which corresponds to the presence of five layers of the gastric wall with clear boundaries, as seen by EUS. The mucosal elevation under white-light endoscopy in the present case showed slightly hypoechoic thickening changes under EUS with no thickening of the posterior submucosa.
The white-light endoscopic findings in 2020 and 2021 were similar, both in the gastric corpus and in the antrum. In 2021, the white-light endoscopic findings were different in the gastric corpus and antrum, with elevation-depression changes in the gastric corpus and nodular reddening-like changes in the gastric antrum. EUS findings were also different in the gastric corpus and the gastric antrum. Only the mucosa was involved in the gastric corpus, while the mucosa, muscularis mucosa, and submucosa were all involved in the gastric antrum.
Considering the white-light endoscopic and EUS images, it was presumed that the development of CG inflammation might be progressively from the mucosal layer to the submucosal layer, and the progression of lesions in the gastric corpus and antrum might not parallel each other. The fact that the lesion is hypoechoic might be a EUS change of CG.
There are a number of treatments available for CG, some of which are merely symptomatic. H2 receptor antagonists or proton pump inhibitors, aluminum, iron supplementation in patients with anemia, a gluten-free diet in patients with celiac disease[16], glucocorticoids (prednisone[2,8,17], budesonide), other drugs including salicylic acid preparations such as mesalazine and salazosulfapyridine, and parenteral nutrition[9] have been used with varying degrees of efficacy according to different reports.
We gave our patient esomeprazole and mosapride successively, but she had no relief from her symptoms. Then, she was given prednisone acetate for 8 wk according to the treatment protocol for eosinophilic gastritis, and there was no significant relief of her abdominal distension.
The prognosis of CG at follow-up varies significantly, with some patients having reduced or even no collagen deposition and some experiencing recurrent symptoms[9]. The first reported CG case had unremarkable changes in collagen deposition during 12 years of follow-up[18] and showed mild dysplasia. One case of gastric adenocarcinoma positive for Epstein-Barr virus was confirmed after EGD follow-up 8 years after the diagnosis of collagenous gastritis in a patient with IgM reduction[19]. The duration of the follow-up interval and the endpoint are currently unclear.
At present, most facilities use EGD as a follow-up tool, but EUS can clearly identify all layers of the gastric wall, which helps to determine the depth of lesion involvement and is superior to EGD. The hypoechoic lesion may be an EUS change of CG. However, the availability of other non-invasive tests as tools of follow-up has not been clinically reported. The biochemical indices of PG I, PG II, PGR, and gastrin-17 in this patient in our hospital were consistent with atrophic gastritis, but the blood eosinophil and lymphocyte counts, IgA, type III procollagen, and collagen type IV findings were not abnormal. The patient continued to have episodes of abdominal distension after treatment with glucocorticoids, and the results of PG I, PG II, PGR, and gastrin-17 on several retests were abnormal, suggesting that the values obtained by this test may parallel the condition. PG I, PG II, PGR, and gastrin-17 may be used as biochemical indicators for disease treatment and follow-up. As of November 2021, the patient is still being followed up with.
In addition to histopathology, diffuse nodular elevation-depression under white-light endoscopy, hypoechoic changes in the lamina propria and submucosa under EUS, and tests of serum pepsinogen I, pepsinogen II, PGR, and gastrin-17 may be helpful in the diagnosis of CG. There is a lack of specific treatment for CG.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ko J, South Korea; Okasha H, Egypt S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Freeman HJ, Piercy JRA, Raine RJ. Collagenous Gastritis. Cana J of Gastroenterol. 1989;3:171-174. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Zhang H, Jin Z, Lin S, Ding S, Bai P, Cui R, Han Y, Zhang Y, Shang H. Collagenous gastritis: a case report and literature review. Zhonghua Neike Zazhi. 2010;49:688-690. |
| 3. | Huang H, Wang H, Xiang J, Lv W. A case of collagenous gastritis. Zhejiang Yixue Zazhi. 2018;40:297-299. |
| 4. | Lagorce-Pages C, Fabiani B, Bouvier R, Scoazec JY, Durand L, Flejou JF. Collagenous gastritis: a report of six cases. Am J Surg Pathol. 2001;25:1174-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Arnason T, Brown IS, Goldsmith JD, Anderson W, O'Brien BH, Wilson C, Winter H, Lauwers GY. Collagenous gastritis: a morphologic and immunohistochemical study of 40 patients. Mod Pathol. 2015;28:533-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Côté JF, Hankard GF, Faure C, Mougenot JF, Holvoet L, Cézard JP, Navarro J, Peuchmaur M. Collagenous gastritis revealed by severe anemia in a child. Hum Pathol. 1998;29:883-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Anwar MS, Aamar A, Marhaba A, Sidhu JS. Collagenous Gastritis in a Young Female With IgA Deficiency. Gastroenterology Res. 2017;10:126-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Lim HW, Wong BY, Elkowitz D, Sultan K. An elderly patient's complete response to steroid therapy for collagenous gastritis. Ther Adv Chronic Dis. 2018;9:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Kamimura K, Kobayashi M, Sato Y, Aoyagi Y, Terai S. Collagenous gastritis: Review. World J Gastrointest Endosc. 2015;7:265-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Appelman MH, de Meij TG, Neefjes-Borst EA, Kneepkens CM. Spontaneous Gastric Perforation in a Case of Collagenous Gastritis. APSP J Case Rep. 2016;7:7. [PubMed] |
| 11. | Liao CH, Saddik M, Ip S. An Unusual Case of Collagenous Gastritis: Incidental Finding in a Patient Presenting with Dysphagia. Case Rep Gastrointest Med. 2019;2019:5427085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Kawasaki K, Kurahara K, Oshiro Y, Ohtsu K, Nakamura S, Fuchigami T, Matsumoto T. Gastrointestinal: Idiopathic granulomatous gastritis observed by magnifying narrow-band imaging endoscopy. J Gastroenterol Hepatol. 2017;32:947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Masaaki K, Yuichi S, Ken’ya K, Rintaro N, Atsuo S, Yutaka A, Yoichi A, Hidenobu W. Collagenous Gastritis, a Counterpart of Collagenous Colitis: Review of Japanese Case Reports. Stomach and Intestine. 2009;44:2019-2028. [DOI] [Full Text] |
| 14. | Kiryukhin AP, Tertychnyy AS, Shcherbakov PL, Inozemtsev AS, Kolokolnikova OA. Isolated gastric Kaposi's sarcoma in a non-AIDS/HIV patient with coexisting collagenous gastritis and microscopic colitis. Korean J Intern Med. 2021;36:1530-1531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Vesoulis Z, Lozanski G, Ravichandran P, Esber E. Collagenous gastritis: a case report, morphologic evaluation, and review. Mod Pathol. 2000;13:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Bajwa RU, Joshi A, Heikenen JB. Successful Treatment of Collagenous Gastritis in a Child with a Gluten-Free Diet. WMJ. 2015;114:271-273. |
| 17. | Akkari I, Skandrani K, Abdelkader AB, Mrabet S, Jazia EB. Anemia revealing a collagenous gastritis in a young Tunisian man. Pan Afr Med J. 2018;30:231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Winslow JL, Trainer TD, Colletti RB. Collagenous gastritis: a long-term follow-up with the development of endocrine cell hyperplasia, intestinal metaplasia, and epithelial changes indeterminate for dysplasia. Am J Clin Pathol. 2001;116:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Narsai T, Su H, Braxton D, Gupta S. Collagenous Gastritis in Primary Selective IgM Deficiency: Transition to EBV+ Gastric Adenocarcinoma. Case Reports Immunol. 2021;2021:5574944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |