Published online Nov 7, 2022. doi: 10.3748/wjg.v28.i41.5893
Peer-review started: July 28, 2022
First decision: August 31, 2022
Revised: September 17, 2022
Accepted: October 27, 2022
Article in press: October 27, 2022
Published online: November 7, 2022
Processing time: 98 Days and 23.5 Hours
Lichen planus (LP) is a frequent, chronic inflammatory disease involving the skin, mucous membranes and/or skin appendages. Esophageal involvement in lichen planus (ELP) is a clinically important albeit underdiagnosed inflammatory condition. This narrative review aims to give an overview of the current know
Core Tip: Lichen planus (LP) is a frequent, chronic inflammatory disease involving the skin, mucous membranes and/or skin appendages. Esophageal involvement in lichen planus (ELP) is an underdiagnosed inflammatory condition. ELP mainly affects middle-aged women. The principal symptom is dysphagia. Aymptomatic cases may occur. An immune-mediated pathogenesis is probable. Endoscopy shows mucosal denudation and tearing, trachealization, and hyperkeratosis. Scarring esophageal stenosis occurs. Histology includes mucosal detachment, T-lymphocytic infiltrations, epithelial apoptosis, dyskeratosis, and hyperkeratosis. Direct immuno-fluorescence shows fibrinogen deposits along the basement membrane zone. Treatment with topical steroids or immunosuppression may induce symptomatic and histologic improvement. ELP can be regarded as a precancerous condition.
- Citation: Decker A, Schauer F, Lazaro A, Monasterio C, Schmidt AR, Schmitt-Graeff A, Kreisel W. Esophageal lichen planus: Current knowledge, challenges and future perspectives. World J Gastroenterol 2022; 28(41): 5893-5909
- URL: https://www.wjgnet.com/1007-9327/full/v28/i41/5893.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i41.5893
Inflammatory esophageal diseases comprise a broad spectrum of differential diagnoses [1-3]out of which reflux esophagitis is the most frequent condition[4]. Infectious etiologies include Candida or viral esophagitis which are mainly linked to compromised immune function[5]. Esophageal disorders based on immunological background include Crohn’s disease[6], Behçet’s disease[7], graft-versus-host disease after allogeneic stem cell transplantation[8], and eosinophilic esophagitis (EoE)[9-12]. The spectrum of differential diagnoses ranges to less defined subtypes such as lymphocytic[13] or sloughing esophagitis[14]. These differential diagnoses as summed up in Table 1 encompass additional manifestation of autoimmune bullous diseases such as mucous membrane pemphigoid or pemphigus vulgaris[2,3,15] as well as lichen planus (LP). Esophageal lichen planus (ELP), a mucocutanous manifestation of LP, should be considered in patients with signs and symptoms corresponding to esophageal inflammation. Since many aspects of this disease are still poorly understood, ELP tends to be underreported and often misdiagnosed. However, in the last decade, gastroenterologists and researchers provided more emphasis to this condition. Likewise, proposals for macroscopic and histopathologic diagnostic criteria were made and data on therapy has been increasingly available[16-20].
| Chemical or physical damages |
| Reflux esophagitis |
| Chemical esophagitis (acids, leach) |
| Radiation induced esophagitis |
| Drug-induced esophagitis e.g. NSAID, bisphosphonates, tetracyclines, KCl, ferric sulfate, ascorbinic acid |
| Infectious esophagitis |
| Candida spp. |
| Viruses, e.g. Cytomegalovirus, Herpes simplex, HIV |
| Immune-mediated esophagitis |
| EoE |
| Crohn’s disease |
| GVHD |
| Behçet’s disease |
| Systemic sclerosis |
| Lymphocytic esophagitis |
| Lichen planus |
| Mucus membrane pemphigoid |
| Pemphigus |
| Congenital skin disease |
| Esophageal involvement in epidermolysis bullosa |
| Others |
| EIPD |
| Sloughing esophagitis |
This narrative review aims to summarize current knowledge on ELP in order to increase awareness about this clinically important esophageal inflammatory disease and make it more accessible in clinical practice.
LP is a frequent mucocutaneous disease whose pathogenesis is only partly understood[21-24]. It affects 0.5%–2% of the general population and has female predominance (65%)[21,23,24]. Lesions of skin, oral, and genital mucosa are the most frequent manifestations, however involvement of nails, scalp, genitoanal mucosa, eyes, ears, urinary bladder, or nasal mucosa are also seen. Classic exanthematic, cutaneous LP manifests as flat, reddish, itching papules in the face, arms, wrists, with a tendency to develop postinflammatory hyperpigmentation. In two-thirds of patients, an oral manifestation is observed with reticular, erythematous, and erosive subtypes. Patients with oral LP complain of oral discomfort or pain, exhibit characteristic fine white buccal lines (Wickham striae) and often have visible ulcerations on gingiva and palate, tongue and/or labial mucosa. Genital LP may cause itching lesions on glans penis, prepuce or scrotum in men, and on vulva or vagina in women. Involvement of genital mucosa may show all stages of inflammation, starting with erythema, progressing to erosions, plaque formation, and scarring. LP pemphigoides is a rare, mostly IgG-mediated autoimmune variant of LP, exhibiting characteristics of bullous pemphigoid (reactivity against collagen XVII)[25]. As LP may involve multiple organ systems, this disease requires multidisciplinary approach involving dermatologists, dentists, gynaecologists, and gastroenterologists[26-29]. The European guidelines for therapy of LP have recently been published[30,31].
A T-cell mediated inflammatory reaction involving antigen-specific and antigen-unspecific mechanisms is regarded as the basic mechanism of pathogenesis[21,28,32]. A recent review about the immunogenetics of LP reported that multiple imbalances of cytokines or interleukins are involved[33]. In addition, genetic influences and MHC associations were found. Micro-RNAs might also be implicated in LP. Antigen-specific mechanisms include antigen presentation of an unknown trigger by basal keratinocytes, activation of CD4+ Th1-helper cells, cytokine production, and CD8-positive cytotoxic reaction against basal epidermal cells. On the other hand, antigen-unspecific mechanisms could involve upregulation of proinflammatory mediators such as interferon-γ, tumour necrosis factor-alpha, interleukins, and matrix metalloproteases, leading to T-cell infiltration in the epidermal cell layer.
The cytokine profile suggests a Th1/Th2-imbalance, whereas B-cells, plasma cells, or antibodies may play a minor role[33]. Similar to psoriasis or pemphigus[34-36], a disturbance in the IL17/IL23 axis was observed[37,38]. Bacterial or viral antigens may trigger LP. An association with chronic hepatitis C was described, however data remains controversial[39,40]. An association with IgG4-related disease is possible[41]. LP may be triggered by several drugs, e.g. NSAIDs, beta-blockers, ACE-inhibitors, and check-point inhibitors[42]. Amalgam or mercury are regarded as trigger for oral LP[43], while concomitant diabetes or smoking influence the clinical severity[44]. There are associations with systemic diseases and autoimmune disorders such as primary biliary cholangitis, autoimmune thyroiditis, myasthenia, alopecia areata, vitiligo, thymoma, and autoimmune polyendocrinopathy[28,45-47]. As in other immune-mediated diseases, psychological component may influence the disease progression[48-50].
Involvement of the esophagus in LP as another possible site of mucosal affection was first described in 1982[51,52], followed by case reports and small case series presenting this new type of esophageal inflammatory disease with lichenoid features[53-64]. ELP was regarded then as a rarity, likely because its clinical, endoscopic, and histologic features were not yet clearly understood. In recent years, interest about this “new” disease was growing and consequently, larger case series and studies[16-20,58,60,65-69],as well as two comprehensive reviews were published[70,71]. For this narrative review, studies were collected using PubMed/Medline and single case reports were excluded. Table 2 presents an overview of these studies and their key findings.
| Ref. | Study design | Number of ELP cases | Further manifestation sites of LP | Macroscopic findings as described in the manuscript | Histologic findings as described in the manuscript | Signs and symptoms | Therapy |
| Keate et al[53], 2003 | Case series | 3 (all) | Cutaneous oral genital | Mucosal sloughing stenosis | Band-like infiltrate hyperkeratosis acanthosis | Dysphagia | Tacrolimus intralesional Pred. Response 3/3; Etretinate (no effect) |
| Donnellan et al[116], 2011 | Case series | 5 (all) | Oral (all), genital (2), cutaneous (1) | Ulcerations, strictures | Band-like lymphocytic infiltrate, civatte bodies | Dysphagia (all) | Dilation (4) Flut; Response 3/5 |
| Franco et al[69], 2015 | Case series | 6 (83%) | Cutaneous and oral (4) | Ulcerations, strictures (5) | Band-like lymph infiltrate, civatte bodies, fibrinogen + in DIF | Dysphagia (all), food impaction (2) | Dilation (3) Flut/Pred/Triam. Response 5/5 |
| Dickens et al[74], 1990 | 19 LP patients | 5 | Cutaneous (19), oral (4) | Papular lesions, mucosal detachment on biopsy, erosions | Submucosal lymphocytic infiltrate | Dysphagia (1) | |
| Harewood et al[65], 1999 | Retrospective search in patient register | 6 (100%) | Oral (5), genital (3), cutaneous (2), ELP as initial manifestation (5) | Proximal strictures (4) | Lymphocytic infiltration (4) | Dysphagia (6); odynophagia (2) | Dilation of strictures (6); Prednisone (40-60mg). Response 3/4 |
| Quispel et al[16], 2009 | 24 LP patients | 12 | Oral and/or cutaneous (all) | Whitish papules (10), hyperemic lesions (3), mucosal detachment (2), submucosal plaques (3) | Lymphohistiocytic infiltrations para-/hyperkeratosis, hyperplasia, civatte bodies, glycogen akanthosis | Dysphagia (4), odynophagia (3), heart burn (3), regurgitation (2) | |
| Katzka et al[17], 2010 | Retrospecitve review (10 years) of data base/ esophageal biopsies from patients with dysphagia | 27 (92%) | Oral (19), genital (13), cutaneous (3), ELP as initial manifestation (13) | Strictures (18): Proximal (11), distal (3), both (4), mucosal detachment (11), erythema, plaques, whitish mucosa, superficial ulcerations, Koebner effect after dilat | Lichenoid lymphocytic infiltration, damage of ephithelial basal layer civatte bodies squamous cell carcinoma (1) | Dysphagia (27); odynophagia (2) | Dilation of strictures (17). Dilation + Fluticasone Response 10/11. No dilation plus intralesional corticosteroids (2) or swallowed Futicason/ Budesonide (2). Response 6/6 |
| Fox et al[77], 2011 | Review of published ELP cases until 2009 (including 4 own cases) | 72 (87%) | Oral (89%), genital (42%), cutaneous (38%), scalp (7%); nails (3%), eyes (1%), ELP as initial manifestation (14) | Pseudomembranes, bleeding, fragility, inflammation; proximal (64%); distal (11%); Both (26%); Stenosis (47%) | Lichenoid lymphocytic infiltrates; dysplasia/squamous cell carcinoma (6%) | Dysphagia (81%); odynophagia (24%); weight loss (14%); heart burn (8%); regurgitation (3%); hoarseness (1%); asymptomatic (17%) | |
| Linton et al[66], 2013 | Retrospective analysis of esophageal biopsies from 273 patients out of a large cohort | 1 typical ELP; 6 possible ELP | No data | Inflammation (7); stricture (5); trachealization (4) mucosal fragility (1); ulcerations (3); nodules (3) | Lymphocytic infiltration (7); Civatte bodies (1); parakeratosis (6); mixed infiltration (6) elongation of lamina propria papillae (7) hyperplasia of basal cells (4); widened intercellular space (3); neutrophilic inflamm (1) | Dysphagia (7); odynophagia (4) | Dilation of stenosis (3). Topical Fluticasone (2). Response 2/2. Proton pump inhibitors (7). Sucralfate (2). 5-HT4-RA (1) |
| Podboy et al[19], 2017 | Retrospective analysis of a cohort of ELP-patients | 40 (80%) | Cutaneous (4), oral (19), genital (15), ELP as only; manifestation (13) | Strictures (29), ring formation (29), ulcerations (8), mucosal detachment (6), other mucosal, lesions (14), squameous cell carcinoma (2) | Common findings (> 5): Esophagitis (20), focal ulcerations (13), mucosal hyperplasia (10), intraepithelial lymphocytic infiltrate (13), eosinophilia < 5 (13) dyskeratosis (11). DIF positive: Lichenoid (2) equivocal (5) not evaluable because of mucosal detachment (13) | Dysphagia for solid food (32) even for fluids (8); odynophagia (6); reflux (1) | Topical corticosteroids: Budesonide in honey 2 x 3mg (32). Fluticasone spray 880 µg 2x/d (8). Response rate: Endoscopic (72,5%), clinical (62%) |
| Ravi et al[101], 2019 | Retrospective analysis of ELP patients | 132 (80%) | Clinical diagnosis (77) | “Specific histology” (55); Esophageal carcinoma (8) | Response to topical steroids 84/132 63.6%. Immunosuppressive; therapy necessary 38/132. Response: No data | ||
| Kern et al[18], 2016; Schauer et al[20], 2019 | 52 patients.with proven LP on other site (75%) | 34mild (18); severe (16) | Oral 78-100% (vs 78% in non-ELP), genital 44-61% (vs 6% non-ELP). Cutaneous 25-44% (vs 28% non-ELP) | Mucosal detachment iatrogenic (12); spontaneous (16); hyperkeratosis (7); trachealization (10); stenosis/strictures (7) | Epithelial detachment, lymphocytic infiltration, Civatte bodies, dyskeratosis; DIF: Fibrinogen deposits (17); (85% in severe ELP) | Dysphagia. severe ELP: 15; mild ELP: 8 | Topical corticosteroids (12). Budesonide gel 3x0.5mg. Fluticasone. Response 11/12. Stenosis: Topical corticosteroids dilation |
The population-based prevalence of LP was estimated to reach an average of 1.3%[21,73]. Oral LP is considered the most predominant mucosal manifestation affecting two-thirds of patients with cutaneous LP[26-28]. A recent metaanalysis showed a varying global prevalence of oral LP (0.57% in Asia, 1.68% in Europe, and 1,39% in South America)[72,73]. Esophageal involvement was initially regarded as a rarity, however further studies showed an esophageal manifestation in up to 50% of patients presenting with cutaneous or oral LP[16,74]. Since the number of cases in these studies were limited and the patient groups non-randomized, the true prevalence of ELP might be overestimated. Surprisingly, ELP does not necessarily correlate with oral disease[20]. However, oral LP is found in most of the cases of severe ELP. Esophageal manifestation also correlates with the occurrence of other mucosal involvement such as genital LP. The median age at presentation is 60 years and 80% of patients are female[71,75]. Determining the true prevalence of ELP remains a challenge, as it would require endoscopic screening in a large group of patients with LP regardless of localization and symptoms. Focusing on patients with esophageal symptoms only, e.g. dysphagia, would underestimate the true prevalence of ELP. A previous study showed that more than 50% of patients with mild ELP did not report dysphagia[20]. Moreover, cases where the esophagus is the only affected site of LP could still be missed. Hence, the prevalence of ELP on a population-based level can only be roughly estimated thus far. Furthermore, assuming that about 10% of all LP patients would have an esophageal involvement, the prevalence could be as high as 0.1% in the general population, thus outnumbering the prevalence of eosinophilic esophagitis which has been reported to reach 0.04%–0.05% in Western countries[76].
Clinical symptoms: Dysphagia is the leading symptom found in 80%–100% of patients with ELP. Other symptoms include odynophagia, heart burn, regurgitation, weight loss, hoarseness, and chronic unproductive cough. In some studies, approximately 20% of patients with ELP did not manifest any esophageal symptoms[77]. Development of esophageal symptoms might be influenced by severity of disease. In a previously published study, 94% of patients with endoscopically severe ELP presented with dysphagia. However, only 44.4% of patients with mild ELP complained about dysphagia[20]. On the other hand, up to 6% of LP patients had symptoms of dysphagia without esophageal involvement. In clinical practice, ELP should be investigated in patients presenting with the above-mentioned symptoms, especially in patients with known LP. Moreover, ELP should be considered in all patients where other common causes of esophagitis (see Table 1) have been ruled out.
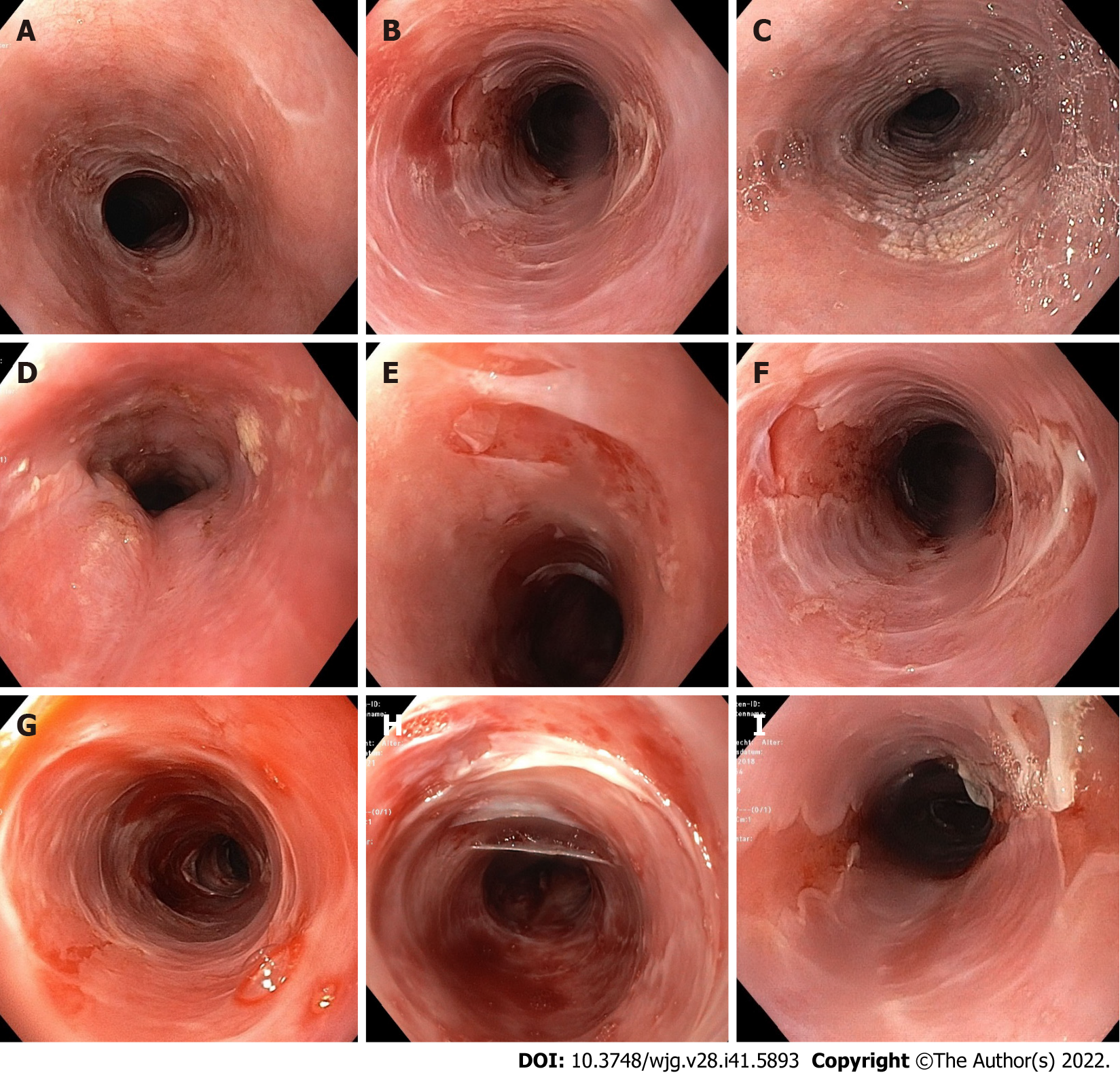
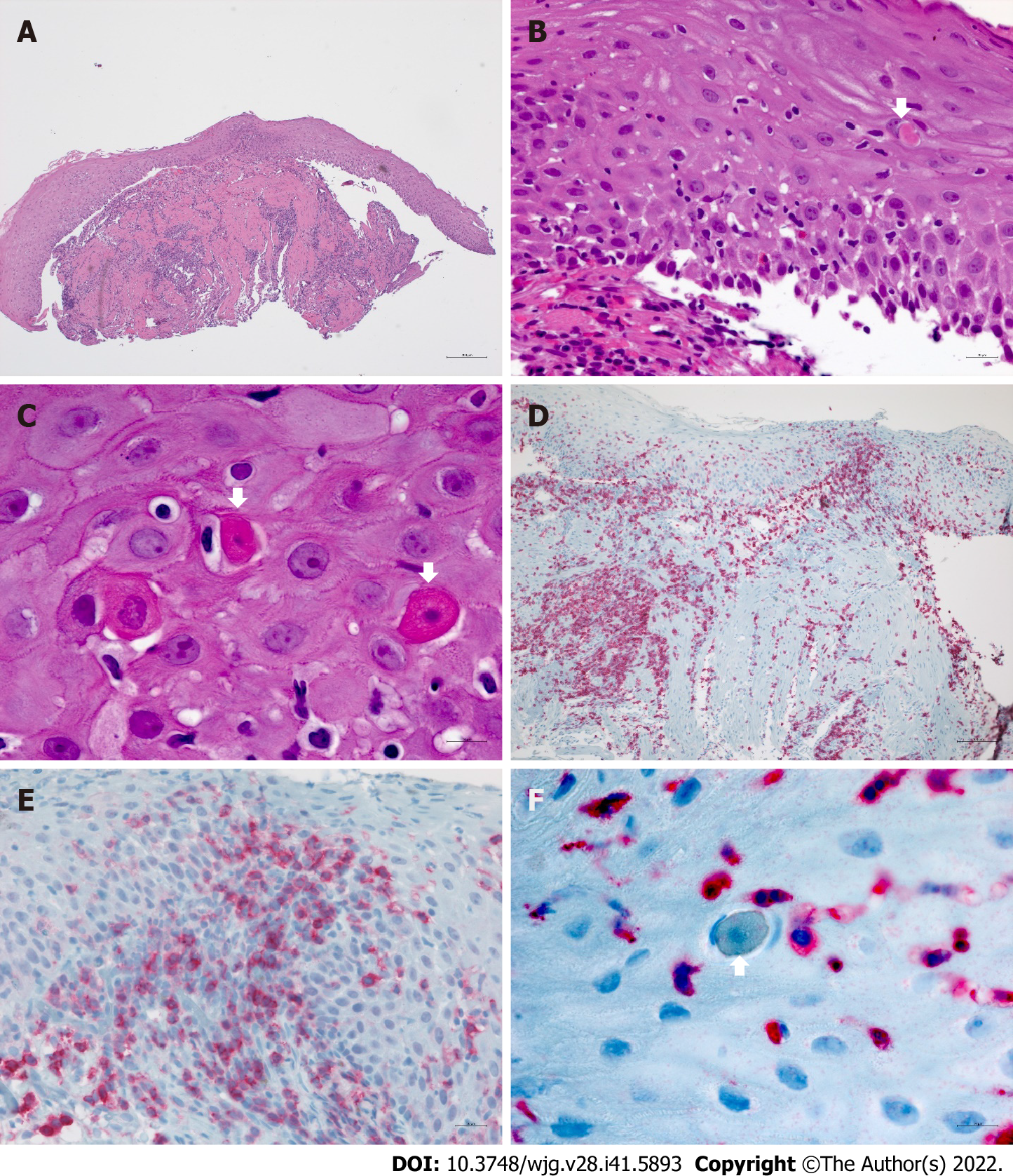
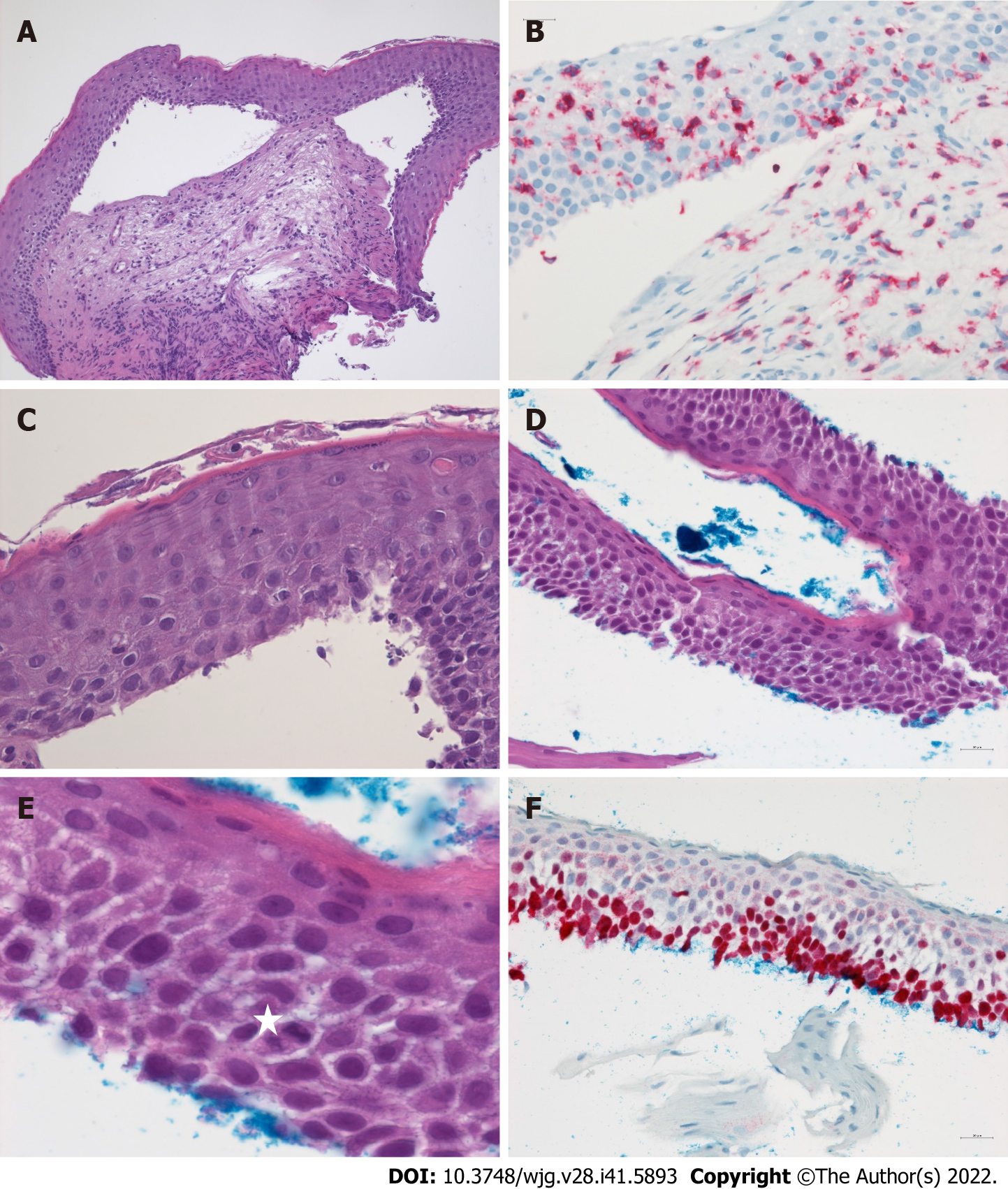
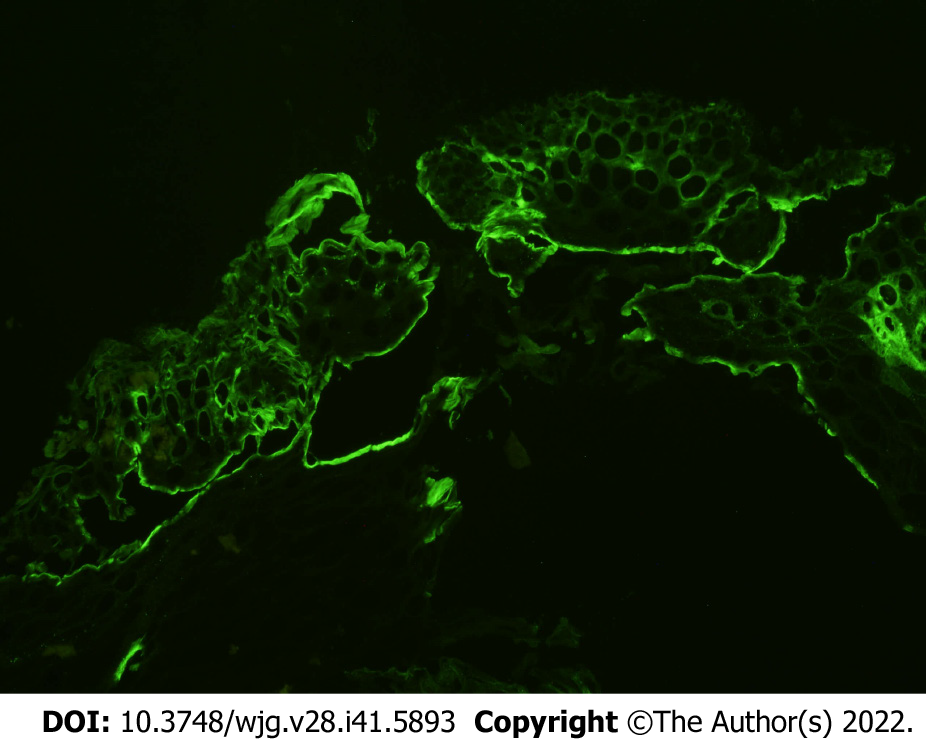
Diagnosis: Similar set of macroscopic and histologic features of ELP has been repeatedly described in literature (see Table 2). Alongside some findings which can be considered typical of ELP, some similarities with other esophageal disorders such as eosinophilic esophagitis, lymphocytic esophagitis, and sloughing esophagitis can be found[3,9-11,13,78-82], hence, making the diagnosis challenging. Based on published data and experience from our cohort of patients, a diagnostic score combining endoscopic and histopathologic findings, as well as direct immunofluorescence (DIF), and a severity grading (no ELP, mild ELP, and severe ELP) has been previously proposed by our group[20]. These criteria are not completely new, however existing criteria and our own findings were integrated into a comprehensive and reproducible scoring system. Examples for endoscopic, histopathologic, and DIF findings are shown in Figures 1-4.
The endoscopic hallmark in nearly all studies analysed (see Table 2) is denudation or sloughing of the esophageal mucosa. It may occur spontaneously or during the endoscopic procedure. Less specific indicators of ELP are “trachealization” (an endoscopic sign well known in EoE) and presence of a rough and whitish surface of the mucosa which is the macroscopic correlate of hyperkeratosis as seen in histology. Stenoses or strictures may occur as sequelae of chronic inflammation in ELP as in other chronic inflammatory esophageal disorders. Endoscopic images of mucosal alterations are shown in Figure 1. Endoscopic changes may be observed in all parts of the esophagus, but mainly in the middle third. As reflux esophagitis often occurs simultaneously, macroscopic and histologic alterations directly above the gastroesophageal junction may be ambiguous. Thus, biopsies should be taken at least 5 cm above the gastroesophageal junction. To evaluate microscopic changes in patients with known LP, we recommend to perform at least two biopsies (in the lower and upper third of the esophagus) regardless if the above-mentioned endoscopic signs are not present.
Esophageal biopsies provide a reliable assessment of mucosal lesions characteristic of ELP (Figure 2). Band-like inflammatory infiltrates are observed at the interface between the squamous epithelium and the lamina propria corresponding to a lichenoid esophagitis pattern. The predominant cell type in the inflammatory infiltrate of ELP are CD3+ T cells which spill over into the adjacent epithelium involving the lower third or lower half of the epithelial thickness. CD4+ cells are the main T-cell subset reported in cutaneous LP while ELP also frequently harbors abundant intraepithelial CD8+ lymphocytes. Intraepithelial lymphocytosis is associated with scattered squamous cell apoptosis designated as Civatte bodies. The epithelium may become partially or completely detached from the tunica propria or show intraepithelial splitting reminiscent of sloughing esophagitis. However, superficial necrosis and neutrophilic aggregates seen in sloughing esophagitis are not a feature of ELP. The squamous epithelium may be hyperplastic and exhibit acanthosis similar to the saw-toothed rete ridges of cutaneous LP especially in long-standing esophageal involvement. In contrast to the normal esophageal epithelium, hyper
In ELP, DIF often highlights fibrinogen deposits along the basal membrane as another important criterion (Figure 4). This is based on the data on oral LP, where linear fibrinogen deposition (or granular IgG and IgM deposits) in DIF could discriminate the diagnosis from other lichenoid lesions[83] and mucus membrane pemphigoid[15,27]. Therefore, positive results in DIF support the diagnosis of ELP yielded by conventional histopathology and, in turn, differentiate the findings from diseases like mucous membrane pemphigoid or pemphigus vulgaris in erosive stages.
In contrast to cutaneous and oral LP[30,31], there are no generally accepted guidelines for therapy of ELP. Conventional management of cutaneous LP with retinoids does not seem to prevent the emergence of ELP, nor is it suitable for therapy of ELP[20,53,84,85]. However, a few case reports described successful therapy using alitretinoin[62] . Good therapeutic response was reported with topical corticosteroids such as fluticasone or budesonide leading to clinical and/or endoscopic response rate of 62% up to 74% in ELP[17-20]. The type of budesonide preparation might play an important role for its efficacy. Viscous syrups or gels offer better adherence to the esophageal mucosa than swallowed sprays, and led to good response rates[20]. However, for a comparison of response rates based on specific preparation, case numbers in literature are too limited (see Table 2). Orodispersible tablets designed for eosinophilic esophagitis might play an interesting role but have not yet been studied in ELP. Intralesional injection of triamcinolone has also been described in literature[53,69,86]. Systemic corticosteroids have been proposed to induce rapid response in severe cases[66]. However, they are not suitable for maintenance therapy and tapering may lead to reoccurrence of symptoms. Therefore, more severe cases not responding to topical corticosteroids require therapy with systemic immunosuppressants. Different types of immunosupressors such as adalimumab, hydroxychloroquine, mycophenolate, azathioprin, cyclosporine, tacrolimus or rituximab have been used[24,53,54,63,67,68,87,88]. In one of our patients, cyclophosphamide was the only drug which effectively induced at least a partial remission. Refractory cases also exist[64].
Since ELP mainly occurs as part of a systemic or multilocular LP, treatment should always be initiated in a multidisciplinary approach involving at least gastroenterologists and dermatologists, especially when topical therapy is not effective and systemic immunosuppressive therapy is necessary.
Esophageal stenosis/Food impaction: As with other inflammatory esophageal diseases, inflammatory or scarring stenosis can be a sequela of chronic untreated or refractory course leading to typical complications such as dysphagia, odynophagia, food impaction, and weight loss[17]. Therefore, ELP should be considered as one of the potential causes of food impaction[89], together with achalasia or eosinophilic esophagitis, or of unexplained esophageal stenosis[90-92]. This applies, not only, but especially to patients with known LP on other site or to patients presenting with signs of undiagnosed mucocutaneous disease.
In symptomatic esophageal stenosis, endoscopic dilation may be necessary and has been successfully performed in multiple cases[17,93]. The possibility of considerable mucosal denudation, the main feature of florid ELP, prompted some authors to advice against endoscopic dilation in the past. However, this can be overcome by simultaneously treating the underlying inflammation as recommended in other esophageal inflammatory conditions. Anti-inflammatory treatment can reduce mucosal fragility, making it more resistant to physical stress, consequently preventing the reoccurrence of stenosis and inducing remission. The need for endoscopic dilation has been reported to decrease under anti-inflammatory therapy[71] and in a few cases, budesonide alone led to relief of symptomatic stenosis [20]. However, vis-a-vis therapy of stenosis in Crohn’s disease, this may only apply for inflammatory and not for scarring stenosis.
Several factors may limit the life expectancy of patients with LP[94,95]. Oral squamous cell carcinoma is one of them, as oral LP is widely regarded as a precancerous condition, even though the exact rate of malignant transformation is a matter of debate[55,96-99].
Accordingly, correlation between ELP and development of esophageal squamous cell carcinoma (ESCC) has been well documented. The number of case reports has been increasing in which esophageal inflammatory and hyperkeratotic lesions have progressed to squamous cell dysplasia/IEN and even to invasive ESCC. In some studies, development of ESCC has been reported in up to 4.5% of ELP patients[100,101].
ELP-associated esophageal precancerous squamous lesions are generally detected in areas of EEM[102-104]. In low-grade dysplasia, cytologic and structural epithelial abnormalities are confined to the lower half of the esophageal epithelium, while high-grade dysplasia involves more than half of the epithelial cell layers with lack of surface maturation. Therefore, endoscopically detected areas of EEM/leukoplakia should be systematically sampled for histologic evaluation since these constitute a hallmark of orthokeratotic dysplasia (Figure 3). It should be noted that invasive ESCC may be detected underneath or adjacent to EEM. Our experience showed uncomplicated hyperkeratosis/EEM in a considerable number of patients with severe ELP (37.5%), while predominantly low-grade orthokeratotic dysplasia was rare (6%) and the transition to an early invasive ESCC was diagnosed in only one patient[20]. Anti-inflammatory therapy did not lead to regression of hyperkeratotic areas in this cohort. New therapeutic strategies should aim to either slow down or arrest the development of EEM.
According to Singhi et al[103], mutation in TP53 correlates with occurrence of or progression to ESCC in ELP. p53 overexpression in immunohistochemistry has been frequently observed in our cohort. Additional molecular analyses have yet to be performed to gain more knowledge on risk stratification. Future advances in identifying the molecular landscape which drives the development of precancerous lesions and overt invasive carcinoma may help establish prognostic biomarkers for early detection of ELP cases at high risk of progression to overt ESCC.
Translating this knowledge to clinical practice, we recommend regular endoscopic surveillance of ELP patients for development of dysplasia. Detection of suspicious areas may be assisted by chromoendoscopy. Patients with known hyperkeratotic regions or florid inflammation should be assessed more often. In cases of low grade dysplasia, we recommend further endoscopy every six months; in cases of transition to high grade dysplasia, endoscopic mucosal ablation should be performed similar to patients developing dysplasia in Barrett’s esophagus. Furthermore, other known risk factors for development of ESCC such as nicotine or alcohol intake should be discouraged.
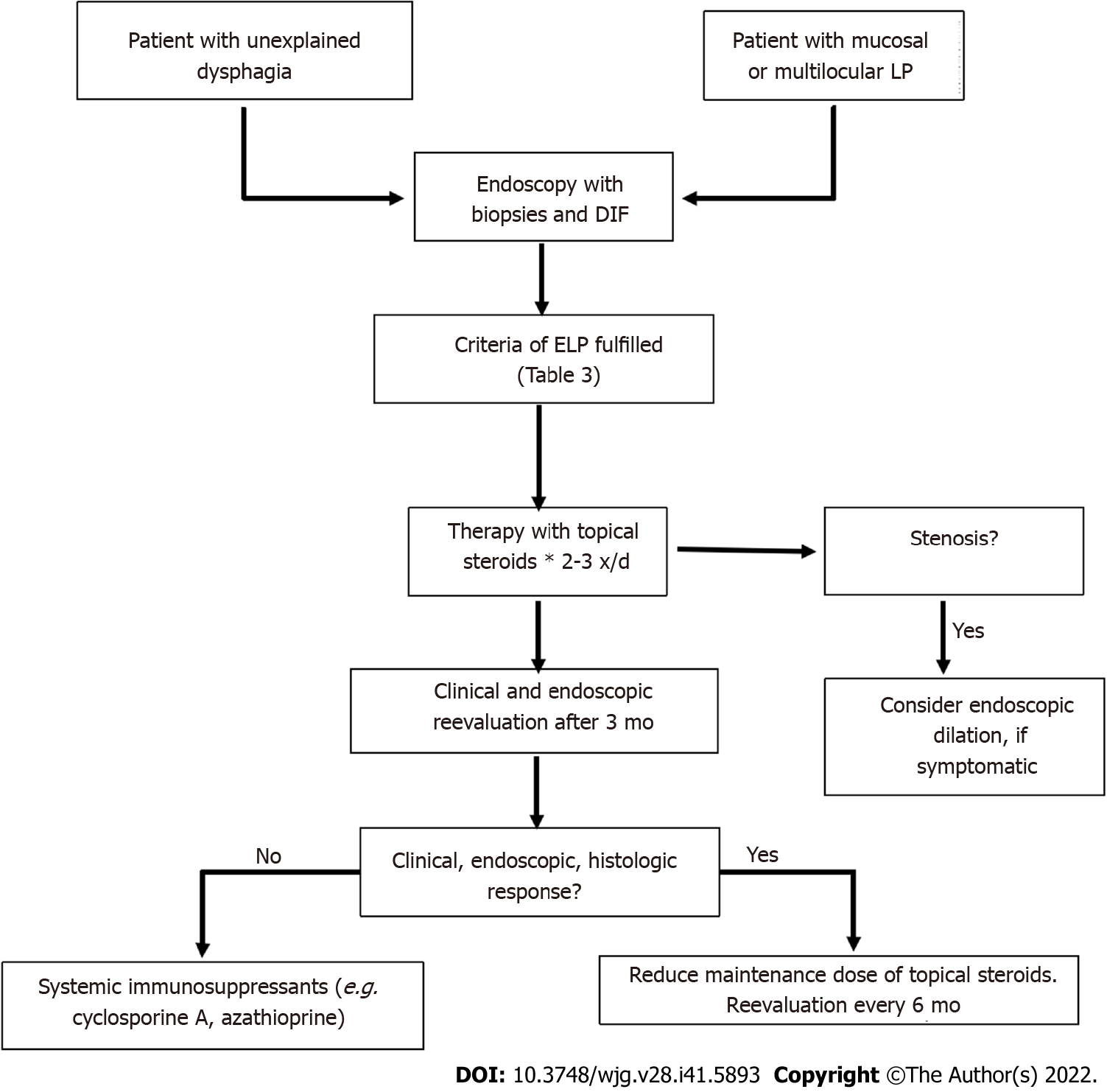
Figure 5 presents a proposal for clinical management of ELP. We recommend esophago-gastro-duodenoscopy (EGD) in every patient with known LP (skin or mucosal manifestation) and with any associated esophageal symptoms as described above. Diagnosis can be established using the above-mentioned criteria (Table 3). We recommend to treat every newly diagnosed ELP initially with topical steroids and then to reevaluate therapeutic response after a certain time interval (e.g. three months). In our clinical experience, 0.5 mg budesonide in 5 mL viscous solution three time a day for the initial treatment period is used. Further therapy would depend on whether a clinical and/or histological remission has been established. Otherwise, systemic immunosuppressive therapy may be necessary as described above. At present, there is not enough data on recommended immunosuppressant. Every patient diagnosed with ELP with no known LP on other sites should also be assessed by a dermatologist.
| Macroscopic-endoscopic criteria | |
| Specific signs | |
| D | Denudation/sloughing of the mucosa |
| D1 | Iatrogenic denudation (caused by biopsies) |
| D2 | Spontaneous localized denudation < 1 cm2 |
| D3 | Spontaneous spacious denudation > 1 cm2 |
| Possible signs | |
| S | Stenosis/stricture |
| S1 | Passable with standard endoscope |
| S2 | Not passable with standard endoscope |
| H | Hyperkeratosis (whitish, rough mucosa) |
| T | Trachealization |
| N | None of the criteria fulfilled |
| Microscopic criteria- histopathology and direct immunofluorescence | |
| HP | Sloughing of the epithelia (subepithelial, intraepithelial) |
| Lymphocytic infiltrate, mainly T-lymphocytes subepithelial, intraepithelial, junctional (region of the basal membrane) | |
| Intraepithelial apoptosis of keratinocytes (Civatte bodies) | |
| Dyskeratosis | |
| HP0 | Negative |
| HP1 | Weakly positive |
| HP2 | Positive |
| HP3 | Strong positive |
| F | Fibrinogen deposition along the basal membrane |
| F0 | No visible reaction |
| F1 | Weak positive, discrete depositions visible |
| F2 | Marked fibrinogen depositions along the basal membrane |
| Severity grading | |
| Severe ELP | ≥ D2 and HP ≥ 1 and/or F ≥ 1 |
| Mild ELP | D1 and HP ≥ 1 and/or F ≥ 1; S, H, T, N and HP ≥ 1 and F ≥ 1 |
| No ELP | Criteria not fulfilled in a patient with LP on other localization |
To date, there is still no consensus on how to identify and treat asymptomatic ELP patients, specifically patients with asymptomatic hyperkeratosis, a potential precursor of ESCC. A wait-and-see strategy seems to be warranted[20,70]. However, in patients with EEM, we recommend EGD every six months to screen the emergence of dysplasia.
Investigation of pathogenesis and search for targeted therapy: Current data on the pathogenesis of LP suggest an (auto)-immunological background with T-cells as key players. As in other diseases triggered by overactive immune system, environmental or lifestyle factors may play an important role, as well as psychological circumstances. Further investigation of mucosal lymphocyte populations in ELP might yield more insights on pathogenesis and establish new options for targeted therapies. Evaluation of environmental factors might lead to identification of triggers (e.g. dental fillings with gold or amalgam).
As no therapeutic option has been universally approved for ELP so far, there is a need for further investigation in larger cohort of patients. Although several studies had demonstrated beneficial effects of topical glucocorticoids, duration and maintenance of treatment still need to be defined. In terms of galenics, an orodispersible preparation of budesonide has recently been licensed for eosinophilic esophagitis[105-107] and should be evaluated in ELP.
New therapeutic approaches may be chosen vis-a-vis contemporary therapy of inflammatory bowel disease[108]. A favorable candidate could be ozanimod, an SP-1-modulator recently licensed for therapy of ulcerative colitis[109,110]. Available data suggest a disturbance in the IL12/23 cytokines and/or IL-17 axis in ELP quite similar to psoriasis[34-38], promising possible targeting of these regulatory factors[24]. A candidate influencing the interleukin 12 and 23 pathways would be tyrosine-kinase 2-inhibitor deucravacitinib[111] which has been already used in other diseases with an autoimmune background (e.g. Crohn's disease, ulcerative colitis) and localized or systemic lupus erythematosus[112-116]. In patients with precancerous lesions, new endoscopic mucosal resection techniques can prevent progression to invasive carcinoma.
ELP is an underdiagnosed yet clinically important inflammatory disease of the esophagus which should be considered in patients with unclear dysphagia or esophagitis, especially but not limited to those with history of mucocutaneous LP. Its diagnosis may be based on endoscopic features and typical findings in histopathology and immunofluorescence. Management and treatment of ELP patients is a multidisciplinary challenge. Further understanding of the pathogenesis and new options for targeted therapies need to be established.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Garcia-Pola M, Spain; Waingade M, India S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Jansson-Knodell CL, Codipilly DC, Leggett CL. Making Dysphagia Easier to Swallow: A Review for the Practicing Clinician. Mayo Clin Proc. 2017;92:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Almashat SJ, Duan L, Goldsmith JD. Non-reflux esophagitis: a review of inflammatory diseases of the esophagus exclusive of reflux esophagitis. Semin Diagn Pathol. 2014;31:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Malone V, Sheahan K. Novel and rare forms of oesophagitis. Histopathology. 2021;78:4-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Richter JE, Rubenstein JH. Presentation and Epidemiology of Gastroesophageal Reflux Disease. Gastroenterology. 2018;154:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 362] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 5. | Ahuja NK, Clarke JO. Evaluation and Management of Infectious Esophagitis in Immunocompromised and Immunocompetent Individuals. Curr Treat Options Gastroenterol. 2016;14:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | De Felice KM, Katzka DA, Raffals LE. Crohn's Disease of the Esophagus: Clinical Features and Treatment Outcomes in the Biologic Era. Inflamm Bowel Dis. 2015;21:2106-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Watanabe K, Tanida S, Inoue N, Kunisaki R, Kobayashi K, Nagahori M, Arai K, Uchino M, Koganei K, Kobayashi T, Takeno M, Ueno F, Matsumoto T, Mizuki N, Suzuki Y, Hisamatsu T. Evidence-based diagnosis and clinical practice guidelines for intestinal Behçet's disease 2020 edited by Intractable Diseases, the Health and Labour Sciences Research Grants. J Gastroenterol. 2020;55:679-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Zeiser R, Blazar BR. Acute Graft-versus-Host Disease - Biologic Process, Prevention, and Therapy. N Engl J Med. 2017;377:2167-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 910] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 9. | Straumann A, Spichtin HP, Bernoulli R, Loosli J, Vögtlin J. [Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings]. Schweiz Med Wochenschr. 1994;124:1419-1429. [PubMed] |
| 10. | Dellon ES. Eosinophilic esophagitis: diagnostic tests and criteria. Curr Opin Gastroenterol. 2012;28:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, Spechler SJ, Attwood SE, Straumann A, Aceves SS, Alexander JA, Atkins D, Arva NC, Blanchard C, Bonis PA, Book WM, Capocelli KE, Chehade M, Cheng E, Collins MH, Davis CM, Dias JA, Di Lorenzo C, Dohil R, Dupont C, Falk GW, Ferreira CT, Fox A, Gonsalves NP, Gupta SK, Katzka DA, Kinoshita Y, Menard-Katcher C, Kodroff E, Metz DC, Miehlke S, Muir AB, Mukkada VA, Murch S, Nurko S, Ohtsuka Y, Orel R, Papadopoulou A, Peterson KA, Philpott H, Putnam PE, Richter JE, Rosen R, Rothenberg ME, Schoepfer A, Scott MM, Shah N, Sheikh J, Souza RF, Strobel MJ, Talley NJ, Vaezi MF, Vandenplas Y, Vieira MC, Walker MM, Wechsler JB, Wershil BK, Wen T, Yang GY, Hirano I, Bredenoord AJ. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology. 2018;155:1022-1033.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 813] [Article Influence: 116.1] [Reference Citation Analysis (0)] |
| 12. | Greuter T, Safroneeva E, Bussmann C, Biedermann L, Vavricka SR, Katzka DA, Schoepfer AM, Straumann A. Maintenance Treatment Of Eosinophilic Esophagitis With Swallowed Topical Steroids Alters Disease Course Over A 5-Year Follow-up Period In Adult Patients. Clin Gastroenterol Hepatol. 2019;17:419-428.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Rouphael C, Gordon IO, Thota PN. Lymphocytic esophagitis: Still an enigma a decade later. World J Gastroenterol. 2017;23:949-956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 14. | Hart PA, Romano RC, Moreira RK, Ravi K, Sweetser S. Esophagitis Dissecans Superficialis: Clinical, Endoscopic, and Histologic Features. Dig Dis Sci. 2015;60:2049-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Chan LS. Mucous membrane pemphigoid. Clin Dermatol. 2001;19:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Quispel R, van Boxel OS, Schipper ME, Sigurdsson V, Canninga-van Dijk MR, Kerckhoffs A, Smout AJ, Samsom M, Schwartz MP. High prevalence of esophageal involvement in lichen planus: a study using magnification chromoendoscopy. Endoscopy. 2009;41:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Katzka DA, Smyrk TC, Bruce AJ, Romero Y, Alexander JA, Murray JA. Variations in presentations of esophageal involvement in lichen planus. Clin Gastroenterol Hepatol. 2010;8:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Kern JS, Technau-Hafsi K, Schwacha H, Kuhlmann J, Hirsch G, Brass V, Deibert P, Schmitt-Graeff A, Kreisel W. Esophageal involvement is frequent in lichen planus: study in 32 patients with suggestion of clinicopathologic diagnostic criteria and therapeutic implications. Eur J Gastroenterol Hepatol. 2016;28:1374-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Podboy A, Sunjaya D, Smyrk TC, Murray JA, Binder M, Katzka DA, Alexander JA, Halland M. Oesophageal lichen planus: the efficacy of topical steroid-based therapies. Aliment Pharmacol Ther. 2017;45:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Schauer F, Monasterio C, Technau-Hafsi K, Kern JS, Lazaro A, Deibert P, Hasselblatt P, Schwacha H, Heeg S, Brass V, Küllmer A, Schmidt AR, Schmitt-Graeff A, Kreisel W. Esophageal lichen planus: towards diagnosis of an underdiagnosed disease. Scand J Gastroenterol. 2019;54:1189-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 21. | Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 22. | Payette MJ, Weston G, Humphrey S, Yu J, Holland KE. Lichen planus and other lichenoid dermatoses: Kids are not just little people. Clinics in Dermatology. 2015;33:631-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 24. | Solimani F, Forchhammer S, Schloegl A, Ghoreschi K, Meier K. Lichen planus - a clinical guide. J Dtsch Dermatol Ges. 2021;19:864-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (2)] |
| 25. | Hübner F, Langan EA, Recke A. Lichen Planus Pemphigoides: From Lichenoid Inflammation to Autoantibody-Mediated Blistering. Front Immunol. 2019;10:1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Ma C, Limketkai BN, Montgomery EA. Recently highlighted non-neoplastic pathologic entities of the upper GI tract and their clinical significance. Gastrointest Endosc. 2014;80:960-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Kuten-Shorrer M, Menon RS, Lerman MA. Mucocutaneous Diseases. Dent Clin North Am. 2020;64:139-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Gru AA, Salavaggione AL. Lichenoid and interface dermatoses. Semin Diagn Pathol. 2017;34:237-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 253] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Ioannides D, Vakirlis E, Kemeny L, Marinovic B, Massone C, Murphy R, Nast A, Ronnevig J, Ruzicka T, Cooper SM, Trüeb RM, Pujol Vallverdú RM, Wolf R, Neumann M. European S1 guidelines on the management of lichen planus: a cooperation of the European Dermatology Forum with the European Academy of Dermatology and Venereology. J Eur Acad Dermatol Venereol. 2020;34:1403-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 31. | Lodi G, Manfredi M, Mercadante V, Murphy R, Carrozzo M. Interventions for treating oral lichen planus: corticosteroid therapies. Cochrane Database Syst Rev. 2020;2:CD001168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 32. | DeAngelis LM, Cirillo N, McCullough MJ. The immunopathogenesis of oral lichen planus-Is there a role for mucosal associated invariant T cells? J Oral Pathol Med. 2019;48:552-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Mansouri P, Nikkhah N, Esmaeili B, Khosravi A, Chalangari R, Martits-Chalangari K. The Immunogenetics of Lichen Planus. Adv Exp Med Biol. 2022;1367:119-135. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Blauvelt A, Chiricozzi A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin Rev Allergy Immunol. 2018;55:379-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 489] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 35. | Boutet MA, Nerviani A, Gallo Afflitto G, Pitzalis C. Role of the IL-23/IL-17 Axis in Psoriasis and Psoriatic Arthritis: The Clinical Importance of Its Divergence in Skin and Joints. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 36. | Holstein J, Solimani F, Baum C, Meier K, Pollmann R, Didona D, Tekath T, Dugas M, Casadei N, Hudemann C, Polakova A, Matthes J, Schäfer I, Yazdi AS, Eming R, Hertl M, Pfützner W, Ghoreschi K, Möbs C. Immunophenotyping in pemphigus reveals a TH17/TFH17 cell-dominated immune response promoting desmoglein1/3-specific autoantibody production. J Allergy Clin Immunol. 2021;147:2358-2369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 37. | Gueiros LA, Arão T, Souza T, Vieira CL, Gomez RS, Almeida OP, Lodi G, Leão JC. IL17A polymorphism and elevated IL17A serum levels are associated with oral lichen planus. Oral Dis. 2018;24:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Abdul N, Shenoy M. Role of Salivary and Serum IL17 and IL23 in the Pathogenesis of Oral Lichen Planus: A Systematic Review. Jundishapur J Microb. 2022;15: 858-867. [DOI] [Full Text] |
| 39. | Nagao Y, Nishida N, Toyo-Oka L, Kawaguchi A, Amoroso A, Carrozzo M, Sata M, Mizokami M, Tokunaga K, Tanaka Y. Genome-Wide Association Study Identifies Risk Variants for Lichen Planus in Patients With Hepatitis C Virus Infection. Clin Gastroenterol Hepatol. 2017;15:937-944.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Georgescu SR, Tampa M, Mitran MI, Mitran CI, Sarbu MI, Nicolae I, Matei C, Caruntu C, Neagu M, Popa MI. Potential pathogenic mechanisms involved in the association between lichen planus and hepatitis C virus infection. Exp Ther Med. 2019;17:1045-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Podboy AJ, Alexander JA, Smyrk TC, Halland M, Ravi K, Geno DM, Murray JA, Katzka DA. Occurrence of IgG4 in Esophageal Lichen Planus. Clin Gastroenterol Hepatol. 2017;15:1975-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Cheng YS, Gould A, Kurago Z, Fantasia J, Muller S. Diagnosis of oral lichen planus: a position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:332-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 327] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 43. | McParland H, Warnakulasuriya S. Oral lichenoid contact lesions to mercury and dental amalgam--a review. J Biomed Biotechnol. 2012;2012:589569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Torrente-Castells E, Figueiredo R, Berini-Aytés L, Gay-Escoda C. Clinical features of oral lichen planus. A retrospective study of 65 cases. Med Oral Patol Oral Cir Bucal. 2010;15:e685-e690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Cassol-Spanemberg J, Rodríguez-de Rivera-Campillo ME, Otero-Rey EM, Estrugo-Devesa A, Jané-Salas E, López-López J. Oral lichen planus and its relationship with systemic diseases. A review of evidence. J Clin Exp Dent. 2018;10:e938-e944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Hasan S, Ahmed S, Kiran R, Panigrahi R, Thachil JM, Saeed S. Oral lichen planus and associated comorbidities: An approach to holistic health. J Family Med Prim Care. 2019;8:3504-3517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 47. | Fromme M, Schneider CV, Schlapbach C, Cazzaniga S, Trautwein C, Rader DJ, Borradori L, Strnad P. Comorbidities in lichen planus by phenome-wide association study in two biobank population cohorts. Br J Dermatol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Cerqueira JDM, Moura JR, Arsati F, Lima-Arsati YBO, Bittencourt RA, Freitas VS. Psychological disorders and oral lichen planus: A systematic review. J Investig Clin Dent. 2018;9:e12363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Adamo D, Calabria E, Coppola N, Lo Muzio L, Giuliani M, Bizzoca ME, Azzi L, Croveri F, Colella G, Boschetti CE, Montebugnoli L, Gissi D, Gabriele M, Nisi M, Sardella A, Lodi G, Varoni EM, Giudice A, Antonelli A, Cabras M, Gambino A, Vescovi P, Majorana A, Bardellini E, Campisi G, Panzarella V, Francesco S, Marino S, Pentenero M, Ardore M, Biasotto M, Gobbo M, Guarda Nardini L, Romeo U, Tenore G, Serpico R, Lajolo C, Gioco G, Aria M, Mignogna MD; SIPMO (Italian Society of Oral Pathology, Medicine). Psychological profile and unexpected pain in oral lichen planus: A case-control multicenter SIPMO studya. Oral Dis. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | De Porras-Carrique T, González-Moles MÁ, Warnakulasuriya S, Ramos-García P. Depression, anxiety, and stress in oral lichen planus: a systematic review and meta-analysis. Clin Oral Investig. 2022;26:1391-1408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 51. | Lefer LG. Lichen planus of the esophagus. Am J Dermatopathol. 1982;4:267-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Al-Shihabi BM, Jackson JM. Dysphagia due to pharyngeal and oesophageal lichen planus. J Laryngol Otol. 1982;96:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Keate RF, Williams JW, Connolly SM. Lichen planus esophagitis: report of three patients treated with oral tacrolimus or intraesophageal corticosteroid injections or both. Dis Esophagus. 2003;16:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Reissmann A, Hahn EG, Faller G, Herold C, Schwab D. Sole treatment of lichen planus-associated esophageal stenosis with injection of corticosteroids. Gastrointest Endosc. 2006;63:168-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Bombeccari GP, Pallotti F, Guzzi G, Spadari F. Oral-esophageal lichen planus associated with oral squamous cell carcinoma. Indian J Dermatol Venereol Leprol. 2008;74:509-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 56. | Bombeccari GP, Guzzi GP, Pallotti F, Marino R, Spadari F. Lichen planus esophagitis: diagnostic implications. Am J Dermatopathol. 2009;31:509-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Bombeccari GP, Guzzi G, Tettamanti M, Giannì AB, Baj A, Pallotti F, Spadari F. Oral lichen planus and malignant transformation: a longitudinal cohort study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 58. | Hou JK, Qureshi W. Swallowed fluticasone for the treatment of esophageal lichen planus. Gastrointest Endosc. 2011;74:708-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Miehlke S, Reinhold B, Schröder S. [Successful treatment of Lichen planus esophagitis with topical budesonide]. Z Gastroenterol. 2012;50:1104-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 60. | Ynson ML, Forouhar F, Vaziri H. Case report and review of esophageal lichen planus treated with fluticasone. World J Gastroenterol. 2013;19:1652-1656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Nielsen JA, Law RM, Fiman KH, Roberts CA. Esophageal lichen planus: a case report and review of the literature. World J Gastroenterol. 2013;19:2278-2281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Kolios AG, Marques Maggio E, Gubler C, Cozzio A, Dummer R, French LE, Navarini AA. Oral, esophageal and cutaneous lichen ruber planus controlled with alitretinoin: case report and review of the literature. Dermatology. 2013;226:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Goñi Esarte S, Arín Letamendía A, Vila Costas JJ, Jiménez Pérez FJ, Ruiz-Clavijo García D, Carrascosa Gil J, Almendral López ML. [Rituximab as rescue therapy in refractory esophageal lichen planus]. Gastroenterol Hepatol. 2013;36:264-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | Eustace K, Clowry J, Kiely C, Murphy GM, Harewood G. The challenges of managing refractory oesphageal lichen planus. Ir J Med Sci. 2015;184:75-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Harewood GC, Murray JA, Cameron AJ. Esophageal lichen planus: the Mayo Clinic experience. Dis Esophagus. 1999;12:309-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Linton MS, Zhao L, Gui X, Storr M, Andrews CN. Lichen planus is an uncommon cause of nonspecific proximal esophageal inflammation. Gut Liver. 2013;7:401-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 67. | Bobadilla J, van der Hulst RW, ten Kate FJ, Tytgat GN. Esophageal lichen planus. Gastrointest Endosc. 1999;50:268-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Westbrook R, Riley S. Esophageal lichen planus: case report and literature review. Dysphagia. 2008;23:331-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 69. | Franco DL, Islam SR, Lam-Himlin DM, Fleischer DE, Pasha SF. Presentation, Diagnosis, and Management of Esophageal Lichen Planus: A Series of Six Cases. Case Rep Gastroenterol. 2015;9:253-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 70. | Monasterio C, Decker A, Schauer F, Büttner N, Schmidt A, Schmitt-Graeff A, Kreisel W. [Esophageal Lichen Planus - an Underdiagnosed Disease]. Z Gastroenterol. 2021;59:460-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 71. | Jacobs JW, Kukreja K, Camisa C, Richter JE. Demystifying Esophageal Lichen Planus: A Comprehensive Review of a Rare Disease You Will See in Practice. Am J Gastroenterol. 2022;117:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Li C, Tang X, Zheng X, Ge S, Wen H, Lin X, Chen Z, Lu L. Global Prevalence and Incidence Estimates of Oral Lichen Planus: A Systematic Review and Meta-analysis. JAMA Dermatol. 2020;156:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 73. | González-Moles MÁ, Warnakulasuriya S, González-Ruiz I, González-Ruiz L, Ayén Á, Lenouvel D, Ruiz-Ávila I, Ramos-García P. Worldwide prevalence of oral lichen planus: A systematic review and meta-analysis. Oral Dis. 2021;27:813-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 220] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 74. | Dickens CM, Heseltine D, Walton S, Bennett JR. The oesophagus in lichen planus: an endoscopic study. BMJ. 1990;300:84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 75. | Chandan VS, Murray JA, Abraham SC. Esophageal lichen planus. Arch Pathol Lab Med. 2008;132:1026-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 76. | Straumann A, Katzka DA. Diagnosis and Treatment of Eosinophilic Esophagitis. Gastroenterology. 2018;154:346-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (1)] |
| 77. | Fox LP, Lightdale CJ, Grossman ME. Lichen planus of the esophagus: what dermatologists need to know. J Am Acad Dermatol. 2011;65:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 78. | Rubio CA, Ichiya T, Schmidt PT. Lymphocytic oesophagitis, eosinophilic oesophagitis and compound lymphocytic-eosinophilic oesophagitis I: histological and immunohistochemical findings. J Clin Pathol. 2017;70:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 79. | Pittman ME, Hissong E, Katz PO, Yantiss RK. Lymphocyte-predominant Esophagitis: A Distinct and Likely Immune-mediated Disorder Encompassing Lymphocytic and Lichenoid Esophagitis. Am J Surg Pathol. 2020;44:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 80. | Akhondi H. Sloughing esophagitis: a not so common entity. Int J Biomed Sci. 2014;10:282-286. [PubMed] |
| 81. | Hokama A, Yamamoto Y, Taira K, Nakamura M, Kobashigawa C, Nakamoto M, Hirata T, Kinjo N, Kinjo F, Takahashi K, Fujita J. Esophagitis dissecans superficialis and autoimmune bullous dermatoses: A review. World J Gastrointest Endosc. 2010;2:252-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 82. | Albert DM, Ally MR, Moawad FJ. The sloughing esophagus: a report of five cases. Am J Gastroenterol. 2013;108:1816-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Yamanaka Y, Yamashita M, Innocentini LMA, Macedo LD, Chahud F, Ribeiro-Silva A, Roselino AM, Rocha MJA, Motta AC. Direct Immunofluorescence as a Helpful Tool for the Differential Diagnosis of Oral Lichen Planus and Oral Lichenoid Lesions. Am J Dermatopathol. 2018;40:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 84. | Jobard-Drobacheff C, Blanc D, Quencez E, Zultak M, Paris B, Ottignon Y, Agache P. Lichen planus of the oesophagus. Clin Exp Dermatol. 1988;13:38-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Van Maercke P, Günther M, Groth W, Gheorghiu T, Habermann U. Lichen ruber mucosae with esophageal involvement. Endoscopy. 1988;20:158-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 86. | Wedgeworth EK, Vlavianos P, Groves CJ, Neill S, Westaby D. Management of symptomatic esophageal involvement with lichen planus. J Clin Gastroenterol. 2009;43:915-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 87. | Hafner J, Gubler C, Kaufmann K, Nobbe S, Navarini AA, French LE. Apremilast Is Effective in Lichen Planus Mucosae-Associated Stenotic Esophagitis. Case Rep Dermatol. 2016;8:224-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 88. | Parmentier L, Bron BA, Prins C, Samson J, Masouyé I, Borradori L. Mucocutaneous lichen planus with esophageal involvement: successful treatment with an anti-CD20 monoclonal antibody. Arch Dermatol. 2008;144:1427-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 89. | Sengupta N, Tapper EB, Corban C, Sommers T, Leffler DA, Lembo AJ. The clinical predictors of aetiology and complications among 173 patients presenting to the Emergency Department with oesophageal food bolus impaction from 2004-2014. Aliment Pharmacol Ther. 2015;42:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Poincloux L, Rouquette O, Abergel A. Endoscopic treatment of benign esophageal strictures: a literature review. Expert Rev Gastroenterol Hepatol. 2017;11:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 91. | Fugazza A, Repici A. Endoscopic Management of Refractory Benign Esophageal Strictures. Dysphagia. 2021;36:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 92. | Hoffman RS, Burns MM, Gosselin S. Ingestion of Caustic Substances. N Engl J Med. 2020;382:1739-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (1)] |
| 93. | Stein P, Brun A, Zaidi H, Sejpal DV, Trindade AJ. Successful Treatment of a Persistent Esophageal Lichen Planus Stricture With a Fully Covered Metal Stent. ACG Case Rep J. 2016;3:98-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 94. | Halonen P, Jakobsson M, Heikinheimo O, Riska A, Gissler M, Pukkala E. Cancer risk of Lichen planus: A cohort study of 13,100 women in Finland. Int J Cancer. 2018;142:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 95. | Halonen P, Jakobsson M, Heikinheimo O, Gissler M, Pukkala E. Incidence of Lichen Planus and Subsequent Mortality in Finnish Women. Acta Derm Venereol. 2020;100:adv00303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | González-Moles MÁ, Ruiz-Ávila I, González-Ruiz L, Ayén Á, Gil-Montoya JA, Ramos-García P. Malignant transformation risk of oral lichen planus: A systematic review and comprehensive meta-analysis. Oral Oncol. 2019;96:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 97. | Idrees M, Kujan O, Shearston K, Farah CS. Oral lichen planus has a very low malignant transformation rate: A systematic review and meta-analysis using strict diagnostic and inclusion criteria. J Oral Pathol Med. 2021;50:287-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 98. | Warnakulasuriya S, Kovacevic T, Madden P, Coupland VH, Sperandio M, Odell E, Møller H. Factors predicting malignant transformation in oral potentially malignant disorders among patients accrued over a 10-year period in South East England. J Oral Pathol Med. 2011;40:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 99. | Arduino PG, Magliano A, Gambino A, Macciotta A, Carbone M, Conrotto D, Karimi D, Carrozzo M, Broccoletti R. Risk of Malignant Transformation in 3173 Subjects with Histopathologically Confirmed Oral Lichen Planus: A 33-Year Cohort Study in Northern Italy. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 100. | Chryssostalis A, Gaudric M, Terris B, Coriat R, Prat F, Chaussade S. Esophageal lichen planus: a series of eight cases including a patient with esophageal verrucous carcinoma. A case series. Endoscopy. 2008;40:764-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 101. | Ravi K, Codipilly DC, Sunjaya D, Fang H, Arora AS, Katzka DA. Esophageal Lichen Planus Is Associated With a Significant Increase in Risk of Squamous Cell Carcinoma. Clin Gastroenterol Hepatol. 2019;17:1902-1903.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 102. | Singhi AD, Arnold CA, Crowder CD, Lam-Himlin DM, Voltaggio L, Montgomery EA. Esophageal leukoplakia or epidermoid metaplasia: a clinicopathological study of 18 patients. Mod Pathol. 2014;27:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 103. | Singhi AD, Arnold CA, Lam-Himlin DM, Nikiforova MN, Voltaggio L, Canto MI, McGrath KM, Montgomery EA. Targeted next-generation sequencing supports epidermoid metaplasia of the esophagus as a precursor to esophageal squamous neoplasia. Mod Pathol. 2017;30:1613-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 104. | Kamboj AK, Graham RP, Murray JA. Epidermoid Metaplasia of the Esophagus. Mayo Clin Proc. 2020;95:1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 105. | Dellon ES, Katzka DA, Collins MH, Gupta SK, Lan L, Williams J, Hirano I. Safety and Efficacy of Budesonide Oral Suspension Maintenance Therapy in Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2019;17:666-673.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 106. | Dellon ES, Liacouras CA. Advances in clinical management of eosinophilic esophagitis. Gastroenterology. 2014;147:1238-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 107. | Dellon ES, Gonsalves N, Abonia JP, Alexander JA, Arva NC, Atkins D, Attwood SE, Auth MKH, Bailey DD, Biederman L, Blanchard C, Bonis PA, Bose P, Bredenoord AJ, Chang JW, Chehade M, Collins MH, Di Lorenzo C, Dias JA, Dohil R, Dupont C, Falk GW, Ferreira CT, Fox AT, Genta RM, Greuter T, Gupta SK, Hirano I, Hiremath GS, Horsley-Silva JL, Ishihara S, Ishimura N, Jensen ET, Gutiérrez-Junquera C, Katzka DA, Khoury P, Kinoshita Y, Kliewer KL, Koletzko S, Leung J, Liacouras CA, Lucendo AJ, Martin LJ, McGowan EC, Menard-Katcher C, Metz DC, Miller TL, Moawad FJ, Muir AB, Mukkada VA, Murch S, Nhu QM, Nomura I, Nurko S, Ohtsuka Y, Oliva S, Orel R, Papadopoulou A, Patel DA, Pesek RD, Peterson KA, Philpott H, Putnam PE, Richter JE, Rosen R, Ruffner MA, Safroneeva E, Schreiner P, Schoepfer A, Schroeder SR, Shah N, Souza RF, Spechler SJ, Spergel JM, Straumann A, Talley NJ, Thapar N, Vandenplas Y, Venkatesh RD, Vieira MC, von Arnim U, Walker MM, Wechsler JB, Wershil BK, Wright BL, Yamada Y, Yang GY, Zevit N, Rothenberg ME, Furuta GT, Aceves SS. International Consensus Recommendations for Eosinophilic Gastrointestinal Disease Nomenclature. Clin Gastroenterol Hepatol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 108. | Grossberg LB, Papamichael K, Cheifetz AS. Review article: emerging drug therapies in inflammatory bowel disease. Aliment Pharmacol Ther. 2022;55:789-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 109. | Sandborn WJ, Feagan BG, D'Haens G, Wolf DC, Jovanovic I, Hanauer SB, Ghosh S, Petersen A, Hua SY, Lee JH, Charles L, Chitkara D, Usiskin K, Colombel JF, Laine L, Danese S; True North Study Group. Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2021;385:1280-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 329] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 110. | Wang J, Goren I, Yang B, Lin S, Li J, Elias M, Fiocchi C, Rieder F. Review article: the sphingosine 1 phosphate/sphingosine 1 phosphate receptor axis - a unique therapeutic target in inflammatory bowel disease. Aliment Pharmacol Ther. 2022;55:277-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 111. | Gonzalez Lopez de Turiso F, Guckian K. Selective TYK2 inhibitors as potential therapeutic agents: a patent review (2019-2021). Expert Opin Ther Pat. 2022;32:365-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 112. | Bellinato F, Gisondi P, Girolomoni G. Latest Advances for the Treatment of Chronic Plaque Psoriasis with Biologics and Oral Small Molecules. Biologics. 2021;15:247-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 113. | Gonciarz M, Pawlak-Buś K, Leszczyński P, Owczarek W. TYK2 as a therapeutic target in the treatment of autoimmune and inflammatory diseases. Immunotherapy. 2021;13:1135-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 114. | Danese S, Peyrin-Biroulet L. Selective Tyrosine Kinase 2 Inhibition for Treatment of Inflammatory Bowel Disease: New Hope on the Rise. Inflamm Bowel Dis. 2021;27:2023-2030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 115. | Jo CE, Gooderham M, Beecker J. TYK 2 inhibitors for the treatment of dermatologic conditions: the evolution of JAK inhibitors. Int J Dermatol. 2022;61:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 116. | Donnellan F, Swan MP, May GR, Kandel G, Marcon NE, Kortan PP. Fluticasone propionate for treatment of esophageal lichen planus. A case series. Dis Esophagus. 2011;24:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |