Published online Apr 7, 2022. doi: 10.3748/wjg.v28.i13.1338
Peer-review started: November 23, 2021
First decision: January 8, 2022
Revised: January 17, 2022
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: April 7, 2022
Processing time: 126 Days and 16.9 Hours
Post-colonoscopy colorectal cancer (CRC) rates for patients with inflammatory bowel disease (IBD) are unacceptably high. During colonoscopy, an intravenous fluorescent anti-c-MET probe may improve endoscopic detection of lesions. However, c-MET expression in IBD lesions is poorly defined, limiting translational studies.
To comprehensively define c-MET expression in sporadic and IBD-associated colorectal carcinogenesis.
c-MET expression was immunohistochemically assessed in 319 formalin-fixed paraffin-embedded tissue specimens, colonoscopically or surgically retrieved between 1994-2017. Tissue included: 30 normal colorectal biopsies, 30 hyperplastic polyps (HP), 31 sessile serrated lesions (SSL), 55 tubular/tubulovillous adenomas with low (TA-LGD, n = 32) or high grade dysplasia (TA-HGD, n = 23), 26 sporadic (s)-CRCs, 16 quiescent IBD biopsies, 11 active/inflamed IBD biopsies, 18 IBD-associated dysplastic lesions (IBD-dys), and 102 IBD-CRCs. Expression was scored by two independent observers as: 0 = absent, 1 = weak, 2 = moderate or 3 = strong. Mann-Whitney U and Kruskal-Wallis tests were used to assess significan
Positive epithelial cytoplasmic and membranous c-MET expression was observed in all tissues, indicating there is ubiquitous expression in the colorectum. c-MET expression was weak in normal colonic epithelium compared with each of the sporadic colonic lesions, including TA-LGD (P < 0.001), TA-HGD (P = 0.004), HP (P < 0.001), SSL (P < 0.001), and s-CRC (P < 0.001). Specifically, in sporadic (non-IBD) lesions, expression was stronger in TA-LGD compared with normal mucosa (P < 0.001), and stronger in s-CRC compared with TA-HGD (P = 0.004). However, there was no significant difference between TA-LGD and TA-HGD (P = 0.852). Further, there was no difference in c-MET expression between HP and SSL (P = 0.065). In IBD, expression was weaker in quiescent colonic mucosa compared with inflamed colonic mucosa (P < 0.001). There was no difference between inflamed colonic mucosa and IBD-dys (P = 0.512) or IBD-CRC (P = 0.296). However, expression was stronger in IBD-dys (P < 0.001) and IBD-CRC (P < 0.001) compared with quiescent IBD colonic mucosa.
The characterisation of c-MET expression suggest that an intravenous probe may improve the endoscopic detection of lesions in both non-IBD patients and IBD patients with quiescent disease.
Core Tip: During colonoscopy, an intravenous fluorescent anti-c-MET probe may improve endoscopic detection of dysplasia and cancer. However, c-MET expression in inflammatory bowel disease (IBD) lesions is poorly defined, limiting translational studies. We demonstrate that stronger immunohistochemical c-MET expression is associated with dysplasia and cancer in both sporadic and IBD-associated lesions. Therefore, c-MET expression could be exploited clinically to enhance endoscopic detection of pre-malignant lesions and cancer, particularly in IBD where post-colonoscopy colorectal cancer rates are unacceptably high.
- Citation: Halliday G, Porter RJ, Black CJ, Arends MJ, Din S. c-MET immunohistochemical expression in sporadic and inflammatory bowel disease associated lesions. World J Gastroenterol 2022; 28(13): 1338-1346
- URL: https://www.wjgnet.com/1007-9327/full/v28/i13/1338.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i13.1338
Patients with inflammatory bowel disease (IBD) have an increased risk of developing colorectal cancer (CRC), with poorer survival compared with the general population[1,2]. The British Society of Gastroenterology currently recommends high-definition surveillance ileocolonoscopy ± chromoendoscopy, with targeted biopsies starting 8-years after the onset of IBD symptoms[3]. Despite this, IBD post-colonoscopy CRC rates - defined as a diagnosis of cancer or high-grade dysplasia > 6 mo to 3 years following a colonoscopy that was negative for cancer - are unacceptably high, at 28%-45%[4,5]. One challenge in identifying dysplastic and malignant lesions in IBD is the morphological changes associated with flat lesions which are difficult to detect endoscopically. With an ageing population and rising global burden of IBD[6], there is an increasing requirement for endoscopic surveillance indicating an urgent clinical need to improve the endoscopic detection of IBD-associated dysplasia and cancer.
c-MET is a receptor tyrosine kinase (encoded by the MET gene on chromosome 7q21-31) overexpressed at a protein level in a variety of human primary tumours, including in the colorectum where it is associated with the sporadic adenoma-carcinoma pathway[7-11]. In 2015, Burggraaf and colleagues published a first-in-human study demonstrating that an intravenous injection of a fluorescently labelled peptide with a high affinity for c-MET was safe, well tolerated in humans, and could improve the detection of colonic polyps in a high-risk asymptomatic patient cohort[12]. Since then, translational data have emerged in support of this technology for sporadic CRC[13,14].
The original data from Burggraaf and colleagues stated that there were several lesions visible with fluorescence assisted colonoscopy that were not identified during first or second pass conventional white light colonoscopy. These small lesions were mostly < 6 mm and non-polypoid[12]. Given this, we hypothesise that this technology may be especially useful for identifying IBD-associated lesions with similar flat morphology. However, the clinical utility of c-MET is unclear in the setting of chronic inflammation and injury to the colonic mucosa, as c-MET has been reported to be upregulated in tissue repair[15].
While the efficacy of an in vivo c-MET probe has never been investigated in the setting of human IBD, there are some murine data. The azoxymethane (AOM)/dextran sulphate sodium (DSS) mouse model is commonly used to simulate colitis-associated carcinogenesis in the laboratory[16]. Using this model, Tao and colleagues reported that there was an accumulation of fluorescence from their c-MET probe (Crizotinib and MPA, a water-soluble cyanine dye, covalently conjugate via PEG4) in colonic lesions, while there was minimal fluorescence in adjacent tissue[17]. While an acceptable surrogate model, AOM/DSS lesions are not identical to those seen in human disease. Further, Tao et al[17] resected colons for imaging in vitro, rather than assessment by in situ fluorescence colonoscopy. Nonetheless, these data are encouraging and warrant investigation in human IBD: there are a paucity of histopathological studies that define c-MET expression in human IBD and IBD-associated carcinogenesis.
To address this need, this study comprehensively defines the expression of c-MET in a large cohort of 319 paraffin-embedded tissue sections, representing the spectrum of both sporadic and IBD-associated colorectal carcinogenesis. Our results suggest that c-MET could be exploited clinically to enhance detection of potentially malignant lesions in IBD.
217 formalin fixed paraffin embedded (FFPE) human tissue specimens, colonoscopically or surgically retrieved between January 2000 to December 2017, were identified from the Edinburgh Pathology database. Tissue included: 30 normal colorectal biopsies, 30 hyperplastic polyps (HP), 31 sessile serrated lesions (SSL), 55 tubular/tubulovillous adenomas with low (TA-LGD, n = 32) or high (TA-HGD, n = 23) grade dysplasia, 26 sporadic colorectal adenocarcinomas (s-CRC), 16 quiescent IBD biopsies, 11 active/inflamed IBD biopsies, and 18 conventional IBD-associated dysplastic lesions (IBD-dys)-15 were considered low-grade and 3 were considered high-grade. A tissue microarray comprising 102 IBD-associated CRC (IBD-CRC) cores from 43 patient tumours, retrieved from surgical resection specimens between 1994 and 2011, was also used. For all tissue, the original haematoxylin and eosin (HE) diagnostic slide, case history and pathology report were reviewed by two expert gastrointestinal pathologists (MJA, CJB) to ensure consensual agreement and accurate tissue diagnosis. All selected cases were anonymised. Ethical approval was obtained from Lothian NRS Bioresource Research Tissue Bank (15/ES/0094; SR148, SR389, SR400 and SR588).
Immunohistochemistry (IHC) was performed using an optimised protocol. In summary, 3μm tissue sections were cut by microtomy from each selected FFPE block and floated onto positively charged glass slides. Tissue was oven-dried overnight. Sections were deparaffinised and underwent heat-mediated antigen retrieval in pH6 citrate buffer for 15 min (BOND Epitope Retrieval Solution 1, Leica Biosystems, United Kingdom). After 5 min of peroxidase blocking, tissue was stained for 15 min with recombinant monoclonal anti-MET (c-MET) antibody (ab51067, clone EP1454Y, Abcam, United Kingdom) at a 1:200 dilution, on Leica Bond-III and BONDMAX autostainers (Leica Biosystems, United Kingdom). Primary antibody detection used the BOND Polymer Refine Detection Kit (Leica Biosystems, United Kingdom).
Two expert gastrointestinal pathologists (MJA, CJB) independently assessed c-MET expression as: 0=no staining, 1+ = weak intensity staining, 2+ = moderate intensity staining, and 3+ = strong intensity staining. Epithelial cytoplasmic and epithelial membranous immunopositivity was ubiquitously equivalent in this study which meant one representative score was assigned to each biopsy. Discrepant scores were resolved by discussion and consensus reached. Use of a histoscore was not considered appropriate in small dysplastic lesions. Scoring methodology and staining intensity agreement came from comparison with internal control normal tissue adjacent to the lesions, and previously published staining intensities[7,9-12,14,18]. Negative control slides were used to allow observers to account for background staining (which was negligible due to IHC optimisation).
Statistical analysis was performed using IBM®SPSS® V25.0.0.1 and GraphPad Prism software. Associations between ordinal and categorical variables were assessed using exact two-tailed Mann-Whitney U and Kruskal-Wallis tests. Scores of 0, 1+, 2+ and 3+ were thus compared and statistical significance was determined at P ≤ 0.05.
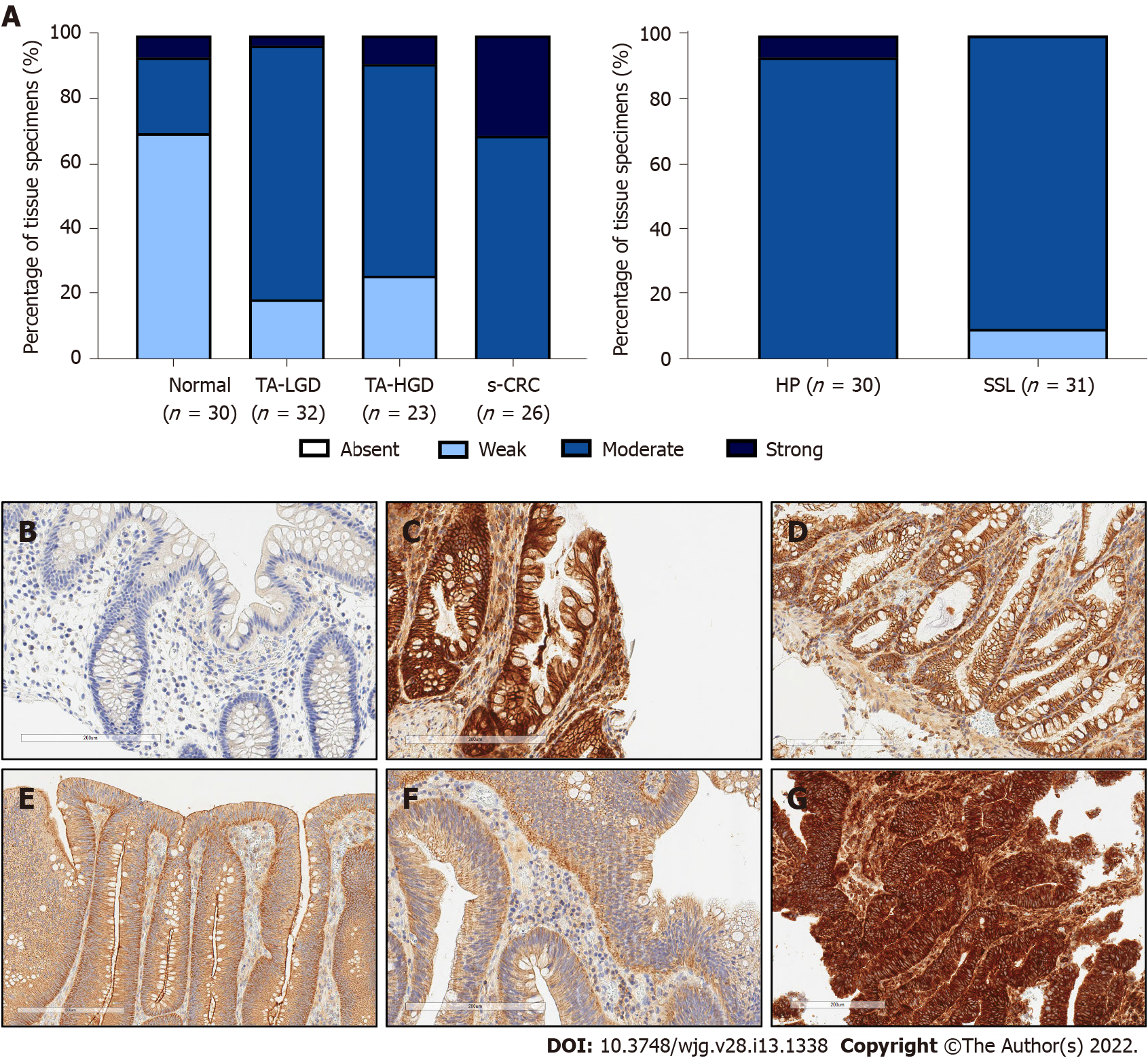
Positive epithelial cytoplasmic and membranous c-MET expression was observed in all tissues, indicating there is ubiquitous expression in the colorectum. As anticipated, c-MET expression was weak in normal colonic epithelium compared with each of the sporadic colonic lesions, including TA-LGD (P < 0.001), TA-HGD (P = 0.004), HP (P < 0.001), SSL (P < 0.001), and s-CRC (P < 0.001) (Figure 1).
c-MET expression was stronger in TA-LGD compared with normal colonic mucosa (P < 0.001), and stronger in s-CRC compared with TA-HGD (P = 0.004). There was no significant difference in c-MET expression between TA-LGD and TA-HGD (P = 0.852).
Given the association between c-MET expression and malignancy, we investigated whether there was a difference between hyperplastic polyps (with low malignant potential) and sessile serrated lesions (with higher malignant potential). There was no statistically significant difference in c-MET expression between these lesions (P = 0.065).
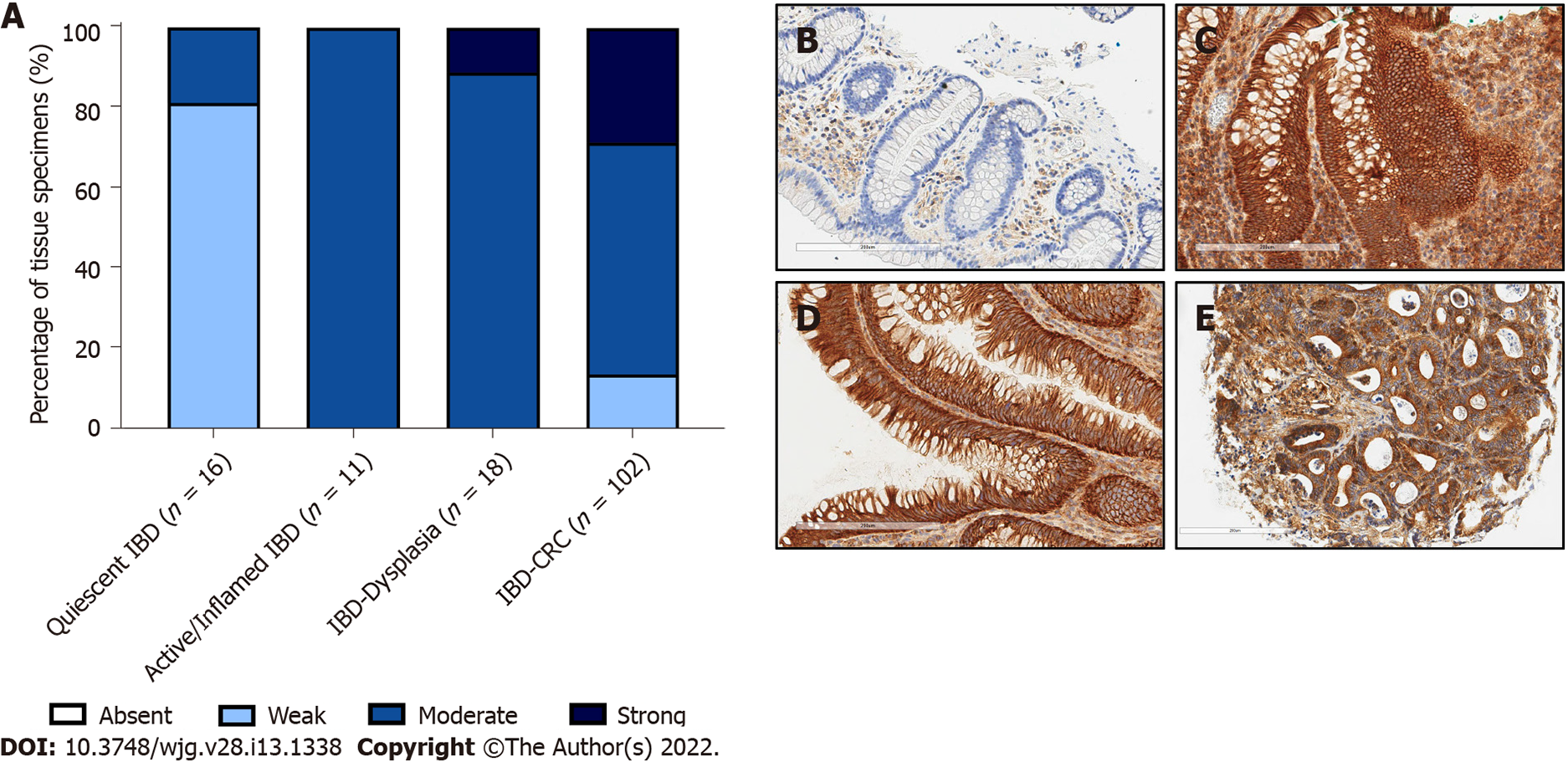
Given the association between c-MET expression and sporadic colorectal carcinogenesis, we assessed whether this was also true for IBD-associated dysplasia and cancer, where detection of dysplasia and cancer is more challenging. c-MET expression was weak in quiescent IBD mucosa compared with active / inflamed IBD (P < 0.001). There was no difference in c-MET expression between inflamed IBD mucosa and IBD-dys (P = 0.512) or IBD-CRC (P = 0.296). However, c-MET expression was stronger in IBD-dys (P < 0.001) and IBD-CRC (P < 0.001) compared with quiescent IBD mucosa. There was no difference between IBD-dys and IBD-CRC (P = 0.673) (Figure 2).
This immunohistochemistry study of 319 human tissue specimens provides a comprehensive overview of c-MET expression in both sporadic and IBD-associated colorectal carcinogenesis (Supplemen
Previous histopathological studies report overexpression of c-MET in sporadic CRC, associated with tumour invasion, metastasis, local recurrence, and poor overall survival[7-10]. Less data exist for pre-cancerous lesions. However, studies suggest that c-MET expression is increased across the adenoma-dysplasia-carcinoma sequence[11]. These data are in keeping with our comprehensive assessment of sporadic lesions; there is an increase in c-MET expression from normal colonic mucosa to dysplasia to colorectal adenocarcinoma.
Gayyed and colleagues reported increased c-MET expression in colonic polyps with HGD compared with LGD[11]. Our study reported no statistically significant difference between these LGD and HGD groups, using a rigorous approach to achieve full diagnostic agreement from two expert gastrointestinal pathologists, based upon review of the HE slide, and all available clinico-pathological data. The proposed use for c-MET is not necessarily to discriminate LGD from HGD; instead, it is as an in vivo probe to improve endoscopic detection of colonic lesions. Both lesions had stronger c-MET expression compared with normal mucosa suggesting that either lesion would be positively identified at colonoscopy allowing targeted biopsy for histopathological assessment.
Identification of SSL from HP is useful as the former have higher malignant potential which is not fully appreciated during endoscopic assessment[19]. Joshi and colleagues report increased c-MET expression in SSL compared with HP, using immunofluorescence on FFPE SSL (n = 17) and HP (n = 10) tissue. This was also observed by Wu et al[13]. In our study, there was no statistically significant difference between SSL (n = 31) and HP (n = 30) (P = 0.065). One reason could be that we used IHC whereas previous studies used immunofluorescence. There has been inconsistent reporting of SSLs; due to previous high inter-observer variability, lack of robust diagnostic criteria and prior misclassification of hyperplastic polyps[19-21]. As previously discussed, our study ensured accurate polyp sub-classification (through consensual agreement by two expert gastrointestinal pathologists) and it is reassuring both lesions had increased expression compared with normal mucosa, as this infers both lesions would be positively identified for histopathological assessment and SSL would thus not be missed.
Our study found no difference in c-MET expression between inflamed IBD mucosa, IBD-dys and IBD-CRC. This is in agreement with the IHC study from Harpaz et al[18]. A key challenge is detecting dysplasia in the context of inflammation-both endoscopically and histopathologically. While this is a major priority, it is based upon the assumption that the post-colonoscopy CRC rate in IBD is related to active inflammation which reduces the ability to detect dysplasia. Therefore, we need to improve detection across the board. While an inability to distinguish between inflamed IBD mucosa, IBD-dys and IBD-CRC could thus be perceived as a barrier to clinical translation, our study reports lower expression of c-MET in quiescent IBD mucosa. Therefore, careful selection of patients could help identify a cohort which would benefit from the use of such an adjunct in detecting subtle lesions during surveillance colonoscopy. Objective biomarkers such as faecal calprotectin ± serum C-reactive protein levels could indicate whether a patient is more likely to have quiescent disease. Indeed, The British Society of Gastroenterology recommend surveillance colonoscopy in patients during quiescent phases of disease where possible[3]. Careful protocol optimisation will minimise any background fluorescence, due to microscopic histological inflammation, which may be present in endoscopically normal and/or quiescent mucosa in patients with IBD.
There are several practical questions that now need to be answered to determine the clinical viability of introducing a c-MET probe into IBD surveillance programs: (1) Is a c-MET probe safe and well tolerated in IBD patients; (2) Does inflammation confound endoscopic assessment of colonic lesions using a fluorescent c-MET probe; (3) Can a c-MET probe be optimised to differentiate between background quiescent/non-inflamed colonic mucosa and IBD-associated lesions, as assessed by fluorescence colonoscopy; (4) Can pre-screening patients using symptom questionnaires, faecal calprotectin ± serum C-reactive protein accurately identify quiescent disease, resulting in low false-positive fluorescence; and (5) Does a c-MET probe offer benefit compared with standard care or other advanced endoscopy techniques for the detection of IBD lesions: for example, can the probe detect endoscopically ‘invisible’ or flat dysplasia (i.e. dysplasia seen only histopathologically on a random biopsy of macroscopically normal mucosa)?
In this comprehensive study, we have robustly defined the expression of c-MET in both sporadic and IBD-associated colorectal carcinogenesis. These data provide a platform for clinical studies to investigate the efficacy of an in vivo c-MET probe to enhance the endoscopic detection of colonic lesions during IBD surveillance colonoscopy. Such an application would be especially important for identifying IBD-associated dysplasia, to reduce the high post-colonoscopy CRC rate within a pre-selected cohort of patients with quiescent disease.
Patients with inflammatory bowel disease (IBD) are more likely to develop colorectal cancer (CRC) compared with the general population, and surveillance colonoscopy is therefore performed at defined intervals to identify pre-malignant lesions. Despite this, IBD post-colonoscopy CRC rates remain unacceptably high. One key challenge is endoscopically identifying the more subtle and flat lesions associated with dysplasia and cancer in IBD.
There is an urgent need to improve endoscopic detection of pre-malignant lesions, especially in patients with IBD. Recent studies have suggested that an intravenously administered fluorescent probe against c-MET protein may improve the detection of sporadic colorectal lesions-specifically small non-polypoid lesions of similar morphology to IBD-associated (pre-) malignant lesions. However, most data come from murine studies or sporadic disease, and there are lack of immunohistochemical data defining c-MET expression in IBD-associated colonic lesions. This is limiting translational studies.
This study was designed to systematically assess the immunohistochemical expression of c-MET in both sporadic and inflammatory bowel disease-associated colonic lesions.
c-MET expression intensity was semi-quantitatively assessed after immunohistochemically staining formalin-fixed paraffin-embedded tissue specimens with an anti-c-MET antibody. Tissue had been colonoscopically or surgically retrieved from patients with and without IBD between 1994-2017, and included normal colonic mucosa, hyperplastic polyps, sessile serrated lesions, tubular/tubulovillous adenomas with low or high grade dysplasia, sporadic-CRC, quiescent IBD mucosa, inflamed IBD mucosa, IBD-associated dysplastic lesions, and IBD-associated CRC.
There was ubiquitous expression of c-MET in normal colonic mucosa, as well as in sporadic and IBD lesions. c-MET expression intensity was similar between low vs high grade dysplasia, and between hyperplastic polyps vs sessile serrated lesions. However, c-MET expression was stronger in sporadic dysplasia and cancer compared with normal colonic mucosa. Similarly, c-MET expression was stronger in IBD-associated dysplastic and malignant lesions compared with quiescent IBD mucosa. There was no difference in c-MET expression between inflamed IBD mucosa and IBD-associated dysplasia or malignant lesions.
c-MET expression intensity is stronger in dysplastic and malignant lesions compared with normal colonic epithelium and quiescent IBD mucosa. These data provide a platform to allow future studies to investigate whether an intravenous anti-c-MET probe could help endoscopically identify dysplasia and malignancy, particularly within surveillance colonoscopy programmes for IBD patients where post-colonoscopy CRC rates are unacceptably high.
Further study is needed to determine whether histopathological expression correlates with mucosal expression at endoscopy in the context of IBD. The ability of such a probe to improve the endoscopic detection of colorectal lesions and reduce the post-colonoscopy CRC rate should then be assessed, in patients with quiescent IBD.
Tissue was provided by Lothian NRS Bioresource. SD acknowledges the support of NHS Research Scotland via NHS Lothian.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Madian A, Egypt; Shahini E, Italy S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Olén O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, Ekbom A, Sørensen HT, Ludvigsson JF. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020;395:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 312] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 2. | Olén O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, Ekbom A, Sørensen HT, Ludvigsson JF. Colorectal cancer in Crohn's disease: a Scandinavian population-based cohort study. Lancet Gastroenterol Hepatol. 2020;5:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 3. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1402] [Cited by in RCA: 1567] [Article Influence: 261.2] [Reference Citation Analysis (0)] |
| 4. | Wintjens DSJ, Bogie RMM, van den Heuvel TRA, le Clercq CMC, Oostenbrug LE, Romberg-Camps MJL, Straathof JW, Stassen LPS, Masclee AAM, Jonkers DMAE, Sanduleanu-Dascalescu S, Pierik MJ. Incidence and Classification of Postcolonoscopy Colorectal Cancers in Inflammatory Bowel Disease: A Dutch Population-Based Cohort Study. J Crohns Colitis. 2018;12:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Stjärngrim J, Ekbom A, Hammar U, Hultcrantz R, Forsberg AM. Rates and characteristics of postcolonoscopy colorectal cancer in the Swedish IBD population: what are the differences from a non-IBD population? Gut. 2019;68:1588-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Jones GR, Lyons M, Plevris N, Jenkinson PW, Bisset C, Burgess C, Din S, Fulforth J, Henderson P, Ho GT, Kirkwood K, Noble C, Shand AG, Wilson DC, Arnott ID, Lees CW. IBD prevalence in Lothian, Scotland, derived by capture-recapture methodology. Gut. 2019;68:1953-1960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 7. | Takeuchi H, Bilchik A, Saha S, Turner R, Wiese D, Tanaka M, Kuo C, Wang HJ, Hoon DS. c-MET expression level in primary colon cancer: a predictor of tumor invasion and lymph node metastases. Clin Cancer Res. 2003;9:1480-1488. [PubMed] |
| 8. | Lee SJ, Lee J, Park SH, Park JO, Lim HY, Kang WK, Park YS, Kim ST. c-MET Overexpression in Colorectal Cancer: A Poor Prognostic Factor for Survival. Clin Colorectal Cancer. 2018;17:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Liu Y, Li Q, Zhu L. Expression of the hepatocyte growth factor and c-Met in colon cancer: correlation with clinicopathological features and overall survival. Tumori. 2012;98:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 10. | Al-Maghrabi J, Emam E, Gomaa W, Saggaf M, Buhmeida A, Al-Qahtani M, Al-Ahwal M. c-MET immunostaining in colorectal carcinoma is associated with local disease recurrence. BMC Cancer. 2015;15:676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Gayyed MF, Abd El-Maqsoud NM, El-Hameed El-Heeny AA, Mohammed MF. c-MET expression in colorectal adenomas and primary carcinomas with its corresponding metastases. J Gastrointest Oncol. 2015;6:618-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 12. | Burggraaf J, Kamerling IM, Gordon PB, Schrier L, de Kam ML, Kales AJ, Bendiksen R, Indrevoll B, Bjerke RM, Moestue SA, Yazdanfar S, Langers AM, Swaerd-Nordmo M, Torheim G, Warren MV, Morreau H, Voorneveld PW, Buckle T, van Leeuwen FW, Ødegårdstuen LI, Dalsgaard GT, Healey A, Hardwick JC. Detection of colorectal polyps in humans using an intravenously administered fluorescent peptide targeted against c-Met. Nat Med. 2015;21:955-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 13. | Wu X, Zhou J, Wang F, Meng X, Chen J, Chang TS, Lee M, Li G, Li X, Appelman HD, Kuick R, Wang TD. Detection of colonic neoplasia in vivo using near-infrared-labeled peptide targeting cMet. Sci Rep. 2019;9:17917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | de Jongh SJ, Vrouwe JPM, Voskuil FJ, Schmidt I, Westerhof J, Koornstra JJ, de Kam ML, Karrenbeld A, Hardwick JCH, Robinson DJ, Burggraaf J, Kamerling IMC, Nagengast WB. The Optimal Imaging Window for Dysplastic Colorectal Polyp Detection Using c-Met-Targeted Fluorescence Molecular Endoscopy. J Nucl Med. 2020;61:1435-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Chmielowiec J, Borowiak M, Morkel M, Stradal T, Munz B, Werner S, Wehland J, Birchmeier C, Birchmeier W. c-Met is essential for wound healing in the skin. J Cell Biol. 2007;177:151-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 254] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 16. | Okayasu I, Ohkusa T, Kajiura K, Kanno J, Sakamoto S. Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut. 1996;39:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 170] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Tao J, Tu Y, Liu P, Tang Y, Wang F, Li Z, Li C, Li Y, Ma Y, Gu Y. Detection of colorectal cancer using a small molecular fluorescent probe targeted against c-Met. Talanta. 2021;226:122128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Harpaz N, Taboada S, Ko HM, Yu J, Yang Q, Xu H, Cao W. Expression of MACC1 and MET in inflammatory bowel disease-associated colonic neoplasia. Inflamm Bowel Dis. 2014;20:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | East JE, Atkin WS, Bateman AC, Clark SK, Dolwani S, Ket SN, Leedham SJ, Phull PS, Rutter MD, Shepherd NA, Tomlinson I, Rees CJ. British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut. 2017;66:1181-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 203] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 20. | Singh H, Bay D, Ip S, Bernstein CN, Nugent Z, Gheorghe R, Wightman R. Pathological reassessment of hyperplastic colon polyps in a city-wide pathology practice: implications for polyp surveillance recommendations. Gastrointest Endosc. 2012;76:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Kim SW, Cha JM, Lee JI, Joo KR, Shin HP, Kim GY, Lim SJ. A significant number of sessile serrated adenomas might not be accurately diagnosed in daily practice. Gut Liver. 2010;4:498-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |