INTRODUCTION
The incidence of esophageal adenocarcinoma (EAC) has been rapidly increasing in the last few decades and is expected to rise further in upcoming years[1,2]. Barrett’s esophagus (BE) is a well-established risk factor for EAC[3], progressing from non-dysplastic BE (NDBE) to low-grade dysplasia, high-grade dysplasia (HGD), and EAC. Thus, it is recommended that patients with BE have regular endoscopic surveillance[4,5]. The ultimate goal of endoscopy in BE patients is to detect subtle early-stage neoplastic lesions, before they can progress to invasive adenocarcinoma. Detection of both dysplasia or early EAC typically permits curative endoscopic therapies[6-9], and an accurate endoscopic assessment is crucial, considering the average 5-year survival rate of only 18% associated with invasive EAC[10].
Unfortunately, despite the widespread use of upper gastrointestinal (GI) endoscopy, standard quality indicators are lacking for BE surveillance. Elsewhere in endoscopy, quality benchmarks, such as adenoma detection rate (ADR) for colonoscopy, have already been shown to improve patient outcomes[11]. As ADR improvement reduces interval colorectal cancer[12], the rates of dysplasia and EAC at index upper GI endoscopy, namely the neoplasia detection rate, might have important clinical implications serving as a quality measure in BE[13]. A recent meta-analysis by Visrodia et al[14] showed that about 20% of HGD/EAC on BE were detected within 1 year from initial diagnosis, likely being missed at that time, highlighting the paramount importance of performing a high-quality endoscopy. Thus, timely endoscopic detection is key to prevent invasive EAC and has been shown to improve outcomes[15].
Over the last two decades, advanced imaging techniques, moving from traditional dye-spray chromoendoscopy to more practical virtual chromoendoscopy (VCE) technologies, have been introduced with the aim of enhancing neoplasia detection in BE. As witnessed in other fields of advanced endoscopy imaging[16-18], thanks to its ability to automatically recognize specific visual patterns, artificial intelligence (AI) has revolutionized the field of diagnostic endoscopy. The aim of this commentary is to summarize both present evidence and future perspectives in order to provide a useful step-by-step approach for performing high-quality endoscopy in BE to increase the diagnostic yield of endoscopy in BE.
HOW TO OPTIMIZE THE ENDOSCOPIC EXAMINATION OF BE?
International guidelines recommend high-definition/high-resolution white light endoscopy for examination[4,5]. The use of magnification may also permit closer evaluation of the microstructures of the mucosa, the glandular pattern and its vasculature[19]; thus, use of a scope with optical zoom capabilities is suggested. Furthermore, use of a distal attachment (i.e. transparent or black cap) could facilitate visualization. In Figure 1 we provide a step-by-step approach for the high-quality endoscopic evaluation of BE.
Figure 1 Eight-step flowchart to perform high-quality endoscopic evaluation of Barrett’s esophagus.
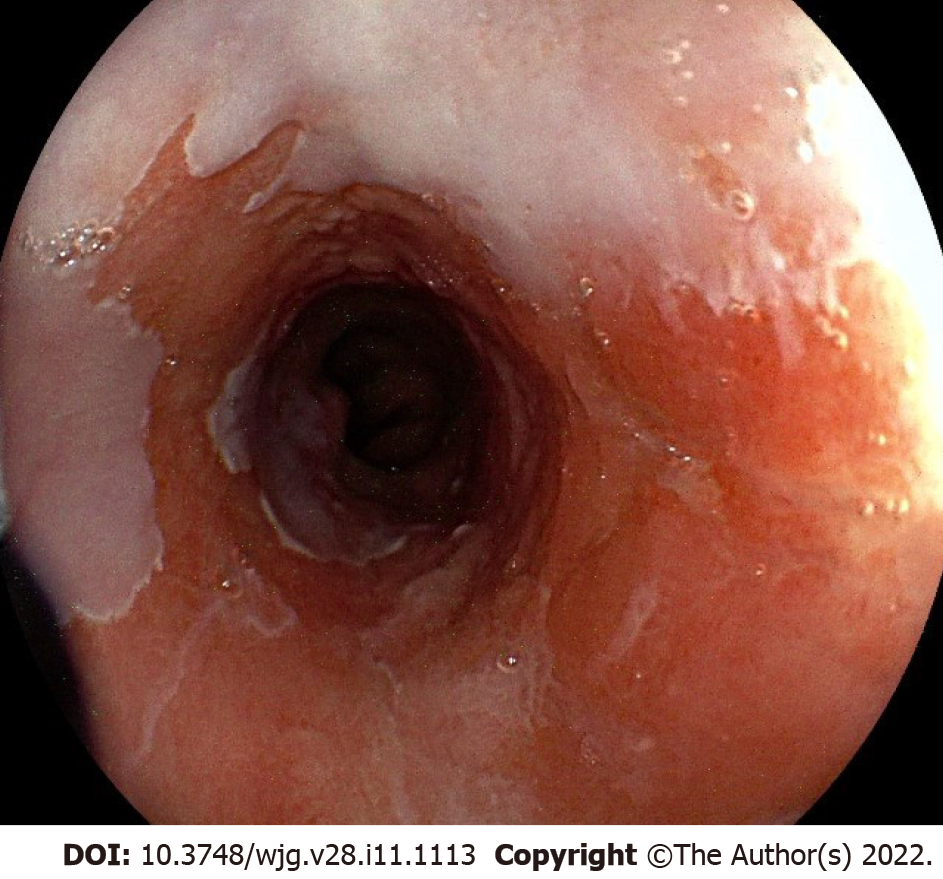
During upper GI endoscopy for BE, the distal esophagus should be carefully cleaned with the water jet to remove any debris or mucus. Esophageal landmarks should be documented, including diaphragmatic hiatus, gastro-esophageal junction, and squamo-columnar junction, using the Prague classification system[20]. Then a detailed examination of BE mucosa is recommended to identify (and biopsy) any visible mucosal irregularity (Figure 2). The endoscopist should spend about 1 min per cm of BE segment carefully inspecting the metaplastic mucosa[21]. The Paris classification[22] should be used to describe any visible lesion. Once the mucosal irregularities (i.e. nodule, erythema, erosion) have been biopsied, four-quadrant random biopsies for every 1-2 cm of the BE segment length should be performed, following the Seattle protocol[4,5], with sensitivity and specificity ranging from 28% to 85% and 56% to 100%, respectively[23].
Figure 2 White light appearance of non-dysplastic Barrett’s mucosa with a slightly elevated lesion (Paris 0-IIa).
Histology: Intramucosal adenocarcinoma.
However, this protocol is far from perfection, as it is prone to several drawbacks. (1) Less than 5% of the BE epithelium is sampled[24], and sampling error is indeed common, increasing by 31% for every cm of BE[25]; (2) Performing such a number of biopsies is time-consuming, which may hamper the widespread adoption of such protocols in clinical practice, with nearly 50% of endoscopists not adhering to it in common practice, significantly decreasing the rate of dysplasia detection[26]; (3) Cost-effectiveness may be also of concern, as highlighted in a recent feasibility study where it was compared to targeted biopsies[27]; and (4) Excessive reliance on random biopsies may undervalue targeted evaluation of subtle lesions, which may therefore be overlooked.
These limitations inherent to the use of white light endoscopy (WLE) with random biopsies may explain why earlier studies underestimated the mortality benefit of endoscopic surveillance. Great effort has been made to implement new technologies that may improve neoplasia detection in BE, and potentially avoid random biopsies; in this regard, the American Society of Gastrointestinal Endoscopy (ASGE) released a Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) document, delineating the performance thresholds required by imaging technologies using targeted biopsies to eliminate the need for random biopsies during endoscopic surveillance of BE[23]. The performance thresholds are: (1) Imaging technology with targeted biopsies should have a per-patient sensitivity of ≥ 90% and a negative predictive value (NPV) of ≥ 98% for detecting HGD or early EAC, compared with the current standard protocol; and (2) The imaging technology should have a specificity that is sufficiently high (80%) to allow a reduction in the number of biopsies (compared with random biopsies).
Different approaches have been proposed to efficiently target esophageal biopsies, with some of them being difficult to implement in a standard community setting [i.e. confocal laser endomicroscopy (CLE), volumetric laser endoscopy (VLE) Autofluorescence imaging (AFI)]. In this commentary we summarize the current knowledge on these strategies, which may be easily integrated in common practice such as both dye-based and electronic chromoendoscopy.
Dye-based chromoendoscopy
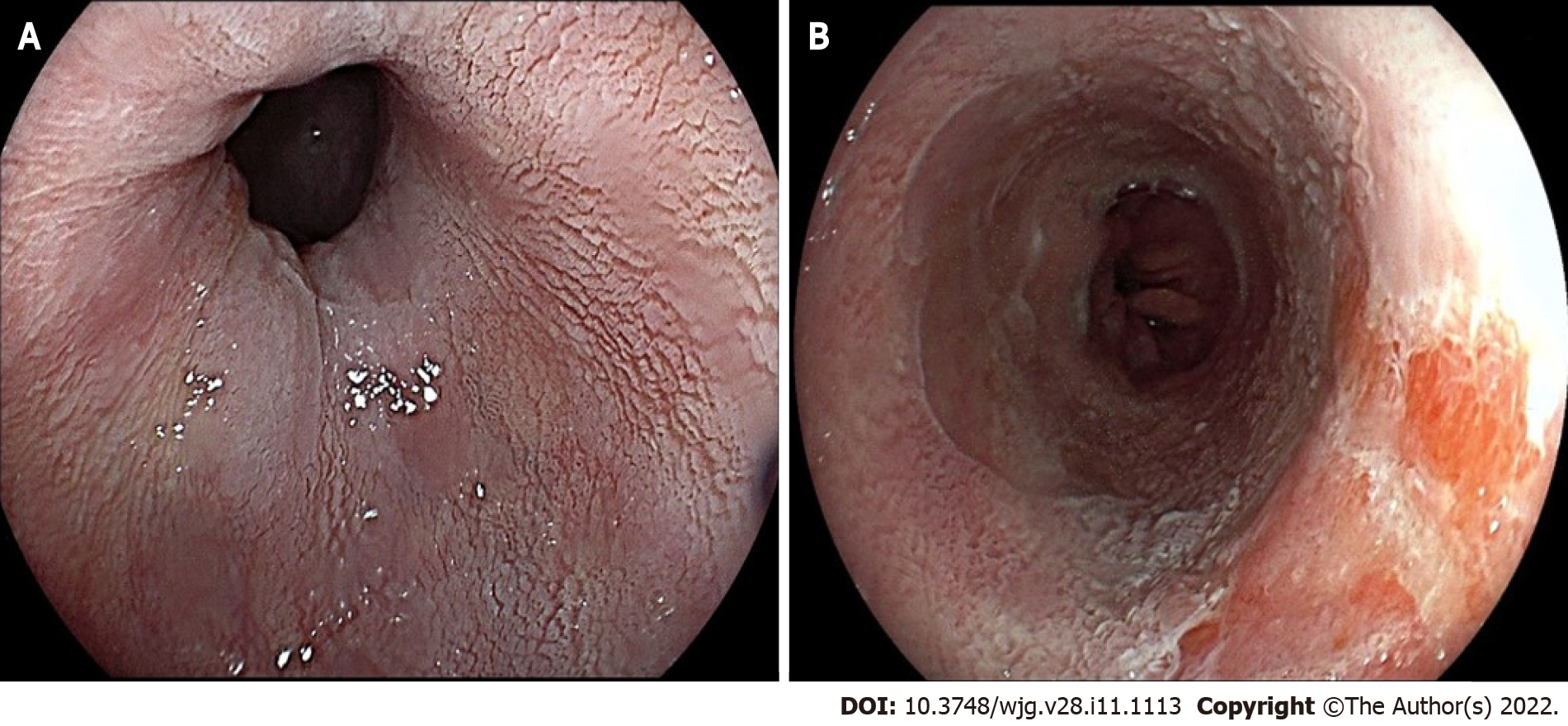
Chromoendoscopy is the most extensively used advanced imaging technique for improving neoplasia detection in BE. The first methodology introduced was dye-chromoendoscopy (DCE). Through a catheter, the dye is sprayed over BE mucosal surface, in an easy but relatively time-consuming process; dedicated equipment and dye must be readily available, as well as expertise in endoscopic mucosal surface evaluation after its application. The most commonly used dyes for BE surveillance are acetic acid (AA), methylene blue, and indigo carmine; however, the only DCE technology that met the PIVI standards in a meta-analysis performed by the ad hoc ASGE committee[23] is AA (sensitivity 96.6%, NPV 98.3%, specificity 84.6%), which performed well enough to be compared to current random biopsies protocols. It works by transiently denaturizing cells in the surface epithelium, leading to a whitening effect. A localized area that loses the whitening reaction earlier than the surrounding mucosa should be considered suspect for neoplasia (Figure 3).
Figure 3 Acetic acid chromoendoscopy in Barrett’s esophagus.
A: Appearance of non-dysplastic mucosa (note the white, regular, and homogeneous glandular pattern); B: Early aceto-whitening loss area consistent with an intramucosal adenocarcinoma.
Recent insights regarding AA chromoendoscopy come from a randomized, cross-over, feasibility trial, where AA-guided targeted biopsies (Portsmouth protocol) were compared to the standard Seattle’s protocol[27]. Patients under BE surveillance with no history of neoplasia were included, undergoing two endoscopies, one with each protocol, 8 wk apart. A total of 200 patients were randomized across six centers, and 174 underwent both procedures. A total of 2139 biopsies were taken in the nontargeted arm and 226 in the AA arm. HGD and cancer were detected with both protocols (AA and nontargeted biopsies). Five low-grade dysplasia were detected (two with AA and four with nontargeted biopsies; one lesion was detected with both techniques). A paired analysis demonstrated a 6.50-fold decrease in the number of biopsies per pathology found by the Portsmouth protocol compared with the Seattle protocol, and a 9.65-fold decrease when restricted to high-risk neoplasia (HGD and cancer), raising an important cost-saving issue. Although the results were promising, the authors concluded that a noninferiority, tandem, crossover trial would require an estimated 2828 patients in order to prove a significant lack of missed pathology, postponing a conclusive indication.
VCE
VCE has rapidly spread in BE surveillance. Over the last decade, several pre-processing chromoendoscopy technologies have been developed and introduced in clinical practice, including narrow band imaging (NBI; Olympus, Tokyo, Japan), blue light imaging (BLI; Fujifilm, Tokyo, Japan), and i-Scan (PENTAX Medical, Montvale, NJ, United States). Such techniques are practical in first instance (no need to apply any dye, rapid button transition to white light to VCE), allowing immediate enhanced visualization of mucosal and vascular patterns, a critical step to detect early neoplastic lesions. Furthermore, they add no cost, being part of the scope, with no additional risk to the patient.
Several studies have investigated the role of VCE imaging in BE. Most evidence has been accumulated on NBI (Olympus), which is based on the optical phenomenon that the depth of light penetration into tissue depends on its wavelength; NBI applies shorter wavelengths (400-540 nm) to maximally highlight the surface mucosa and vascular pattern. Moreover, the narrow spectrum is matched to the maximum absorption of hemoglobin, in order to make structures rich in hemoglobin look darker (surface capillaries appear brown, submucosal vessels appear cyan) than the surrounding mucosa. Such optical enhancement allows for better visualization and characterization of suspected dysplastic areas. In this regard, a specific NBI-based classification has been developed, based on the so-called BING criteria: Mucosal surface pattern, which can be distinguished in regular (circular, ridged/villous, or tubular) or irregular (absent or irregular); and vascular pattern, distinguished in regular (blood vessels regularly situated along or between mucosal ridges with normal branching patterns) or irregular (focally or diffusely distributed vessels not following the normal architecture of the mucosa). The BING criteria identified patients with dysplasia with 85% overall accuracy, 80% sensitivity, 88% specificity, 81% positive predictive value, and 88% NPV, with a good overall inter-observer agreement (k = 0.681)[28]. Several studies and subsequent meta-analyses have investigated the role of NBI as advanced imaging technology in BE surveillance, showing that NBI-targeted biopsies may improve dysplasia detection, at least when performed by experienced endoscopists in advanced imaging, matching the PIVI thresholds[29-31]. In particular, the most recent meta-analysis performed a methodologically robust comparison between NBI and standard WLE in a per-patient analysis, including six studies that reported the diagnostic utility of NBI for detecting dysplasia of all grades[31]. The pooled sensitivity of NBI to detect all-grades of dysplasia was 0.76 [95% confidence interval (CI): 0.61-0.91], the pooled specificity was 0.99 (95%CI: 0.99-1.00).
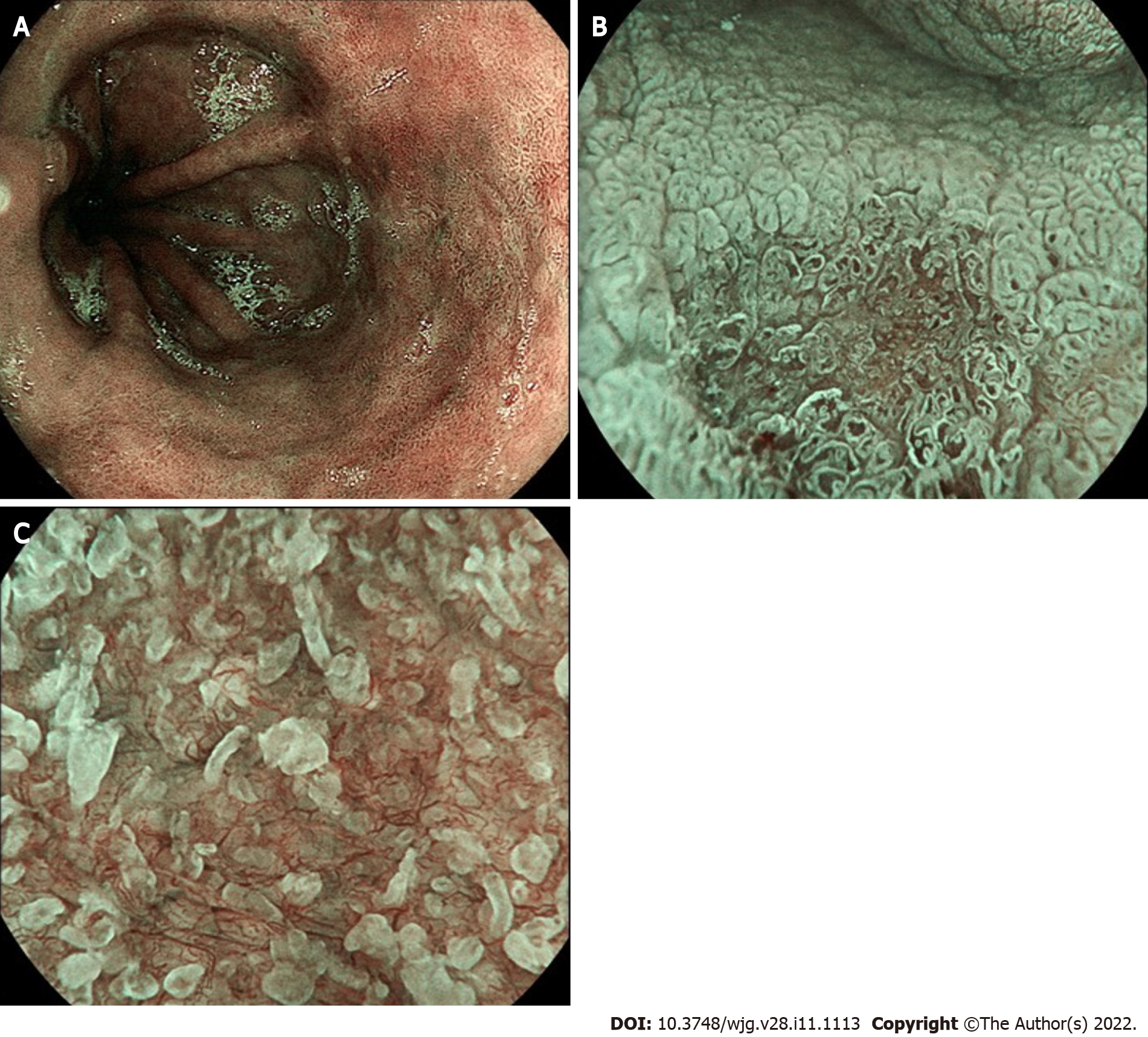
BLI (Fujifilm) is a novel technology that enhances the contrast between mucosal and surface vascular patterns, similarly to NBI technology (Figure 4). A classification (BLINC) that helps to characterize BE lesions has been recently developed and validated, similarly as seen with NBI, showing good interobserver reliability and improvement of neoplasia detection[32]. BLI has been recently tested in a tandem retrospective comparison with high definition WLE (HD-WLE) in a recent Dutch analysis of 40 still images of BE lesions. The authors found that experts appreciated BLI significantly better than HD-WLE for visualization of macroscopic appearance in a visual assisted scale (median 8.0 vs 7.0; P < 0.001), for surface relief (8.0 vs 6.0; P < 0.001), and for lesion demarcation (8.0 vs 6.0; P < 0.001 and 8.0 vs 5.0; P < 0.001)[33]. Similar results, in a similar setting, were reported for nonexpert endoscopists after an appropriate training phase[34].
Figure 4 Blue light imaging appearance of Barrett’s mucosa.
A: Non-dysplastic mucosa; B: Irregular vascular pattern of a slightly depressed (Paris 0-IIc) high-grade dysplastic lesion; C: Detail of irregular mucosal and vascular pattern after zoom magnification.
Finally, the I-SCAN (PENTAX) improves neoplasia detection on endoscopic imaging of BE as well as the accuracy of histology prediction. In a recent study, accuracy of neoplasia detection was significantly higher in all trainees (76% vs 63%) and in six experts (84% vs 77%) who used OE vs HD-WLE. OE improved the sensitivity of dysplasia detection compared with HD-WLE in six trainees (81% vs 71%) and five experts (77% vs 67%). Specificity improved in six trainees who used OE vs HD-WLE (70% vs 55%) and in five experts (92% vs 86%)[19].
In conclusion, it is still debatable if a chromoendoscopy directed targeted-biopsies strategy can replace the four-quadrant random biopsies in the near future. Currently, only AA chromoendoscopy, and VCE with NBI, meet the PIVI thresholds when performed by expert endoscopists[23]. International guidelines continue to support a careful HD-WLE examination of BE, followed by Seattle protocol biopsies[4,5]; however, use of adjunct chromoendoscopy can potentially increase diagnostic yield of the standard guideline supported approach by identifying suspicious areas for targeted biopsies.
HOW TO GET PREPARED FOR AN IMMINENT TOMORROW IN BE EXAMINATION
As already mentioned, different approaches have been proposed to help in dysplasia/neoplasia detection during BE surveillance. Even though it has been years since we started investigating advanced modalities such as CLE, VLE and AFI, what yesterday was considered the “future,” is today considered the “present” of BE endoscopy. AI in endoscopy has been booming in the last few years, and BE examination itself will likely be a part of this revolution.
AI
AI is a branch of computer science that takes advantage of the development of autonomous machines that are able to mimic human behavior. Over the last decade, AI has been applied in several fields; in medicine, one of the most ambitious aims is to aid interpretation of subtle differences in medical imaging. Computer-aided diagnosis (CAD) systems have thus been developed and trained to autonomously learn to distinguish features that a human being cannot easily distinguish. For instance, AI may overcome the physician-dependent limitations related to poor intra- and inter-observer agreement, a burden that affects many aspects of medical imaging and endoscopy. If the system were to be trained to distinguish between neoplastic and non-neoplastic BE mucosa with almost-perfection, it seems reasonable to imagine that in the near future the burden of pathological examination may be considerably relieved.
Especially via deep learning techniques with artificial neural networks, these CAD systems offer many potential advantages for the endoscopic practice; in the last few years, several CAD systems using deep learning machine techniques have been developed in other areas of endoscopy, such as colonic adenomas, with extremely promising results[35]. The Netherlands-based ARGOS consortium is under way in developing a real-time CAD system for BE neoplasia[36]. The development of this deep learning CAD system required the analysis of a total of 494364 normal white light endoscopy images and 1704 unique BE still images. The aim was automatically to detect early neoplastic BE lesions, indicating areas with the highest chance of containing abnormality with a crosshair, The CAD system was in a complex multistep asset: Starting from WLE images collected from all gastro-intestinal segments (Set 1), refinement of CAD system was obtained with a subsequent analysis of 1247 WLE images of BE (Dataset 2:557 NDBE and 690 early neoplasia images) and other 197 WLE images (Dataset 3:168 NDBE and 129 early neoplasia): To ensure maximal accuracy, NDBE images were further reviewed by BE experts endoscopists for confirming the absence of visible lesions and the neoplastic images were re-delineated by other experts; moreover, all suspected neoplastic lesions were histologically confirmed as HGD or adenocarcinoma. It is worth noting that images were acquired with different endoscopic optical systems (both Fujifilm and Olympus). A double external validation was then carried out with 2 other datasets (4 and 5) of 160 different BE images (80 NDBE, 80 neoplastic) and eventually benchmarked with a large group of international endoscopists, including novices and seniors. As a final result, in dataset 4 the CAD system classified images as containing neoplasms or nondysplastic BE with 89% accuracy, 90% sensitivity, and 88% specificity. In dataset 5 values for the CAD system vs those of the general endoscopists were 88% vs 73% accuracy, 93% vs 72% sensitivity, and 83% vs 74% specificity. The CAD system achieved higher accuracy than any of the individual 53 non-expert endoscopists. CAD delineations of the area of neoplasm overlapped with those from the BE experts in all detected neoplasia in data sets 4 and 5. The CAD system also identified the optimal site for biopsy (crosshair) of detected neoplasia in 97% and 92% of cases[37]. The first real-time performance (with video) of this CAD system has been recently reported by the same group in 10 patients[38]: Accuracy, sensitivity, and specificity of the CAD system in per-level analyses were 90%, 91%, and 89%, respectively. In total, 9 out of 10 neoplastic patients were correctly diagnosed. The single lesion not detected by CAD, showed NDBE in the endoscopic resection specimen. In only 1 NDBE patient, the CAD system produced false positive predictions. In 75% of all levels, the CAD system produced 3 concordant predictions. In another study by Ebigbo et al[39], a different CAD system was trained using a total of 129 endoscopic images[39]; real-time validation was performed with 62 additional images (36 of early EAC and 26 of normal BE) in a concurrent assessment made by the CAD system and a BE-expert endoscopist. All images and lesions were then validated by pathological examination of resection specimens (EAC), as well as forceps biopsies (normal BE). The AI system had excellent performance scores in the classification task with a sensitivity and specificity of 83.7% and 100.0%, respectively, and an overall accuracy of 89.9%.
The number of available CAD systems may be rapidly increasing, with several other reports appearing in the last few years[40-42]: A meta-analysis has pooled data from the all the studies available so far (9 in total)[43]. Overall, CAD showed pooled sensitivity of 89% (CI 83%-93%), specificity 88% (CI 84%-91%), PPV 88% (CI 84%-91%), NPV 89% (CI 83%-93%), LR+ 7.5 (CI 5.2-10.4), and LR− 0.120 (CI 0.074-0.198). Heterogeneity was present both for sensitivity and specificity (0.60 and 0.29 between study SD in the logit scale, respectively). Comprehensively, at 50% of early-stage neoplasia prevalence, a positive result of AI would increase the disease probability to 88%, whereas a negative result would decrease the disease probability to 11%. In addition, in subgroup analysis, it was shown that the use of advanced imaging techniques was associated with an increase of specificity, at least in BE-related neoplasia detection, suggesting a potential synergic effect between advanced imaging and AI.
The field of AI will continue to evolve in next few years providing models that would enhance easy detection and mucosal detailing for endoscopists. This will undergo rigorous prospective validation and testing before being available. Ideal AI based model should be easily taught, reproducible with accuracy and such that can be easily incorporated in community GI endoscopy centers.