Published online Mar 14, 2022. doi: 10.3748/wjg.v28.i10.1009
Peer-review started: October 17, 2021
First decision: November 17, 2021
Revised: November 26, 2021
Accepted: February 10, 2022
Article in press: February 10, 2022
Published online: March 14, 2022
Processing time: 145 Days and 7.3 Hours
Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) arise from neuroendocrine cells found throughout the gastrointestinal tract and islet cells of the pancreas. The incidence and prevalence of GEP-NENs have been increasing each year due to higher awareness, improved diagnostic modalities, and increased incidental detection on cross-sectional imaging and endoscopy for cancer screening and other conditions and symptoms. GEP-NENs are a heterogeneous group of tumors and have a wide range in clinical presentation, histopathologic features, and molecular biology. Clinical presentation most commonly depends on whether the GEP-NEN secretes an active hormone. The World Health Organization recently updated the classification of GEP-NENs to introduce a distinction between high-grade neuroendocrine tumors and neuroendocrine carcinomas, which can be identified using histology and molecular studies and are more aggressive with a worse prognosis compared to high-grade neuroendocrine tumors. As our understanding of the biology of GEP-NENs has grown, new and improved diagnostic modalities can be developed and optimized. Here, we discuss clinical features and updates in diagnosis, including histopathological analysis, biomarkers, molecular techniques, and radiology of GEP-NENs. We review established diagnostic tests and discuss promising novel diagnostic tests that are currently in development or require further investigation and validation prior to broad utilization in patient care.
Core Tip: Over the past decade, our understanding of the molecular biology underlying gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) has improved our available diagnostic tools. Although there have been many reviews on the diagnosis of GEP-NENs, the majority focus on one aspect of the diagnosis. This review provides a comprehensive discussion of the current and upcoming diagnostic tools available for GEP-NENs, including histopathology, new biomarkers, molecular techniques, and radiology.
- Citation: Fang JM, Li J, Shi J. An update on the diagnosis of gastroenteropancreatic neuroendocrine neoplasms. World J Gastroenterol 2022; 28(10): 1009-1023
- URL: https://www.wjgnet.com/1007-9327/full/v28/i10/1009.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i10.1009
Neuroendocrine neoplasms (NENs) account for 0.5% of all malignancies, and 62%-70% of these are found in the gastroenteropancreatic system (GEP-NENs)[1-3]. They arise from neuroendocrine cells, which are characterized by their ability to synthesize and secrete neuropeptides and hormones as well as the expression of neuroendocrine markers such as synaptophysin and chromogranin[2]. Neuroendocrine cells are most commonly found throughout the gastrointestinal tract, the islets of Langerhans of the pancreas, and in the lungs[2]. GEP-NENs are often slow-growing and indolent, so they can go undetected for years prior to diagnosis[4]. Though GEP-NENs were previously considered rare, the incidence has dramatically increased over the years as awareness of GEP-NENs grew and diagnostic modalities improved[1,4-7]. GEP-NENs are currently the second most prevalent gastrointestinal neoplasm, second only to colorectal adenocarcinoma[4]. Despite their indolent behavior, GEP-NENs can cause significant morbidity. Furthermore, their clinical presentation may mimic other classes of neoplasms, leading to inappropriate treatment and delays in appropriate therapy. Due to delays in diagnosis, metastases are present in 21% to 69% of patients at the time of diagnosis[6,7]. Therefore, it is imperative to come to an accurate diagnosis in a timely manner.
Oberndorfer first described GEP-NENs in 1907 as “Karzinoide” to describe benign-appearing tumors of the small intestine[2,6]. Now, the term “carcinoid” is no longer recommended as these neoplasms have been found to have the malignant potential[6]. The World Health Organization (WHO) classification of GEP-NENs has changed over the years. A major recent update is the division of NENs into neuroendocrine tumors (NETs) and neuroendocrine carcinomas (NECs). Previous editions classified GEP-NENs into grade 1 and grade 2 NETs, while grade 3 tumors were classified as NECs; however, molecular discoveries have aided in distinguishing grade 3 NETs from NECs and this distinction has been implemented as of the WHO 2019[8]. In addition to the changes in WHO classification, there are new developments in diagnosing GEP-NENs, including histopathology, biomarkers, and imaging. Here, we discuss updates on the diagnosis of GEP-NENs across these various modalities.
GEP-NENs can broadly be divided into functional and nonfunctional neoplasms. Though nonfunctional NENs can secrete calcitonin, chromogranins, ghrelin, neuron-specific enolase, or pancreatic polypeptide, they do not present with a hormone-related clinical syndrome[9]. By contrast, functional GEP-NENs secrete a hormone with an associated clinical syndrome caused by an excess of that hormone.
Nonfunctional GEP-NENs present with symptoms as primary tumor growth or metastases progress. For example, esophageal NENs are rare but present with dysphagia and vomiting due to physical obstruction[10]. Nonfunctional pancreatic NENs can present with symptoms of abdominal pain, early satiety, and obstructive jaundice[10,11]. Colorectal NENs present with hematochezia, change in bowel habits, abdominal pain, and anorectal symptoms[12-14].
With the increased usage of cross-sectional imaging and endoscopies to screen for cancer, many nonfunctional GEP-NENs are detected incidentally[15]. For example, gastric and colorectal NENs can be detected on upper and lower endoscopy, respectively[12,13,16,17]. Similarly, appendiceal NENs have been found incidentally in less than 1% of appendectomy specimens[14,18].
Functional GEP-NENs present with a clinical syndrome consistent with the hormone that they secrete. Due to the associated clinical syndrome, these often present earlier than nonfunctional GEP-NENs. Insulinomas are insulin-secreting tumors that present with symptoms of hypoglycemia such as palpitations, diaphoresis, and altered mental status[19]. Gastrinomas cause Zollinger-Ellison syndrome, in which excess gastrin leads to hypersecretion of gastric acid, resulting in severe peptic ulcer disease, gastroesophageal reflux disease, and chronic diarrhea[20]. Glucagonomas present with necrolytic migratory erythema, diabetes mellitus, weight loss, and diarrhea[21-23]. VIPomas are characterized by autonomous secretion of VIP leading to watery diarrhea, hypokalemia, and achlorhydria syndrome[24,25]. Secretion of serotonin and other active amines and peptides leads to carcinoid syndrome, which presents with episodic flushing, wheezing, and diarrhea[26,27].
The WHO classification of GEP-NENs has undergone numerous changes over the past couple of decades. The first WHO in 2000 described three categories: (1) Well-differentiated endocrine tumors (carcinoids) to describe neoplasms with low malignant potential and well-differentiated endocrine carcinomas for those with aggressive behavior and metastases; (2) Poorly-differentiated endocrine carcinomas; and (3) Mixed exocrine-endocrine tumors[28]. It was not until 2010 that the WHO updated the classification to “neuroendocrine” to describe the cell origin better and discouraged the use of “carcinoid”[28]. A critical change in 2010 was an introduction of a broader classification system for GEP-NENs that was not based on anatomic sites[8]. GEP-NENs were then categorized as grade 1 and grade 2 NETs, with grade 3 tumors being classified as NECs. This division was based on the Ki-67 proliferation index and mitotic count of the tumor (Table 1)[8,29].
| Grade 1 NET | Grade 2 NET | Grade 3 NET | Grade 3 NEC | |||||
| Mitoses/10 hpf | Ki-67 | Mitoses/10 hpf | Ki-67 | Mitoses/10 hpf | Ki-67 | Mitoses/10 hpf | Ki-67 | |
| 2010 | < 2 | < 3% | 2-20 | 3%-20% | - | - | > 20 | > 20% |
| 20171 | < 2 | < 3% | 2-20 | 3%-20% | > 20 | > 20% | > 20 | > 20% |
| 2019 | < 2 | < 3% | 2-20 | 3%-20% | > 20 | > 20% | > 20 | > 20% |
Following the WHO 2010, it became apparent that there were two groups of grade 3 GEP-NENs with drastically different prognoses. One group was made up of neoplasms with a better prognosis that were proliferative but rarely displayed high-grade features (e.g., nuclear pleomorphism and necrosis), while the other group included poorly differentiated NECs that were more aggressive with a poorer prognosis[29]. This distinction was best established in pancreatic NENs, and in 2017, the WHO introduced an additional classification distinguishing between well-differentiated grade 3 NETs and poorly-differentiated NECs in the pancreas[30]. The most recent WHO 2019 applied this distinction to all GEP-NENs (Table 1)[8].
Molecular discoveries have recently shown that NETs and NECs are distinct entities with different molecular profiles[31-33]. This has been best described in pancreatic NENs. Whole exome sequencing of pancreatic NETs led to the discovery that most pancreatic NETs are associated with multiple endocrine neoplasia type 1 (MEN1) inactivation (44%) and death domain-associated protein (DAXX)/alpha-thalassemia/mental retardation X-linked (ATRX) gene mutations (43%)[31]. Less commonly, alterations in the mTOR pathway (15%) have also been identified including in PTEN, TSC2, and PIK3CA[30,31].
These genetic alterations have been essential in distinguishing high-grade NETs from NECs. Up to 43% of high-grade pancreatic NETs demonstrate mutated DAXX/ATRX, whereas pancreatic NECs are not known to have this mutation[30-32]. Instead, up to 92% of pancreatic NECs have TP53 or RB1 mutations[30,32,34]. Other less commonly seen mutations in NECs include mutations in KRAS, SMAD4, CDKN2A/p16, and BCL2[8,30,32].
For non-pancreatic gastrointestinal NENs, genomic studies are still emerging but suggest that NECs similarly harbor TP53 and RB1 mutations[8]. On the other hand, genomic sequencing identified a very low mutation rate in extrapancreatic NETs, and no recurrent mutation has been identified[8,35]. Although no specific mutation has been validated in small intestinal NENs, analysis of chromosomal changes showed that approximately 50% of cases had a loss of chromosome 18 and 10%-30% of cases had a gain of chromosomes 4, 5, 7, 14, or 20[33]. Overall, due to the heterogeneity of gastrointestinal NETs, identifying recurrent mutations in extrapancreatic NETs has been challenging[8,33].
In addition to molecular classification, genome sequencing has been utilized to identify risk factors of genetic susceptibility to developing sporadic GEP-NENs. Autosomal dominantly inherited genetic syndromes account for a minority of GEP-NENs and include multiple endocrine neoplasia types 1 and 2, Von Hippel-Lindau syndrome, tuberous sclerosis, and neurofibromatosis type 1[36]. A 2011 study by Ter-Minassian et al[37] evaluated single-nucleotide polymorphisms (SNPs) in patients with sporadic NETs, including small bowel and pancreas primaries. They identified 2 SNPs that were associated with increased overall risk of NET, IL12A rs2243123, and DAD1 rs8005354, suggesting that inflammatory and apoptosis pathways play a role in tumorigenesis of NENs[37]. However, a larger follow-up genomic study in 2016 was not able to confirm these associations. Instead, Du et al[36] found a potential risk locus on 12q23 that may be associated with developing small bowel NENs. This locus is in proximity to ELK3, which is implicated in angiogenesis. A 2018 study by Obazee et al[38] analyzed susceptibility loci associated with pancreatic ductal adenocarcinoma for possible overlap association with pancreatic NENs and found that rs9543325, rs10919791, and rs1561927 may increase the risk of developing pancreatic NENs. Genome-wide association studies for GEP-NENs have been limited by sample size, but further studies along these lines may yield a greater understanding of the molecular pathways underlying the pathogenesis of GEP-NENs and potentially facilitate the identification of therapeutic targets.
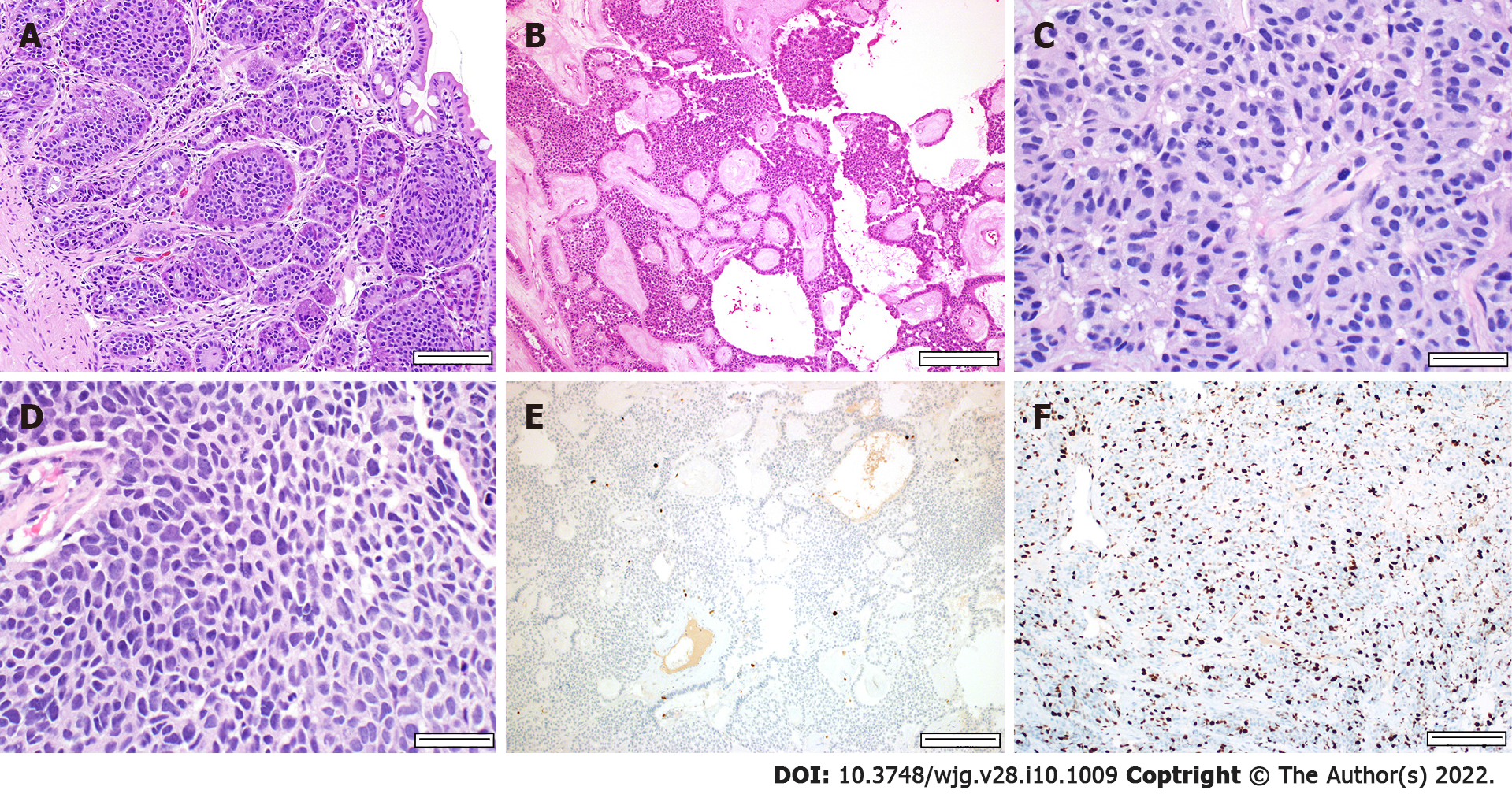
Histologic analysis of the tumor is necessary to establish the diagnosis of a GEP-NEN. The WHO 2019 classification divided GEP-NENs into NETs, grades 1 through 3, and NECs (Table 1). Low-grade NETs (grades 1 and 2) classically show an organoid architecture, but various patterns may be appreciated, including trabecular, glandular, tubuloacinar, and solid (Figure 1). Tumor cells are monotonous with round nuclei and finely granular cytoplasm. The chromatin is finely stippled and classically referred to as “salt and pepper.” High-grade NETs (grade 3) have many overlapping morphologic features with low-grade NETs, with the key difference being that they show higher mitotic activity and higher proliferation indices (Ki-67). Rarely, high-grade NETs may show marked nuclear pleomorphism, diffuse infiltrative patterns, and necrosis. Such features can make it difficult to distinguish from NECs, which is why ancillary studies may be needed in NENs with high-grade features.
By contrast, NECs are poorly differentiated with significant atypia and frequently have geographic necrosis. They can be further subclassified into small cell NEC and large cell NEC. The small cell variant typically grows in a solid, diffuse, sheet-like pattern, and the tumor cells have scant cytoplasm and show nuclear molding with hyperchromatic nuclei and inconspicuous nucleoli. The large cell variant has moderate to abundant cytoplasm, vesicular nuclei, and prominent nucleoli. The classic “salt and pepper” chromatin is not appreciated.
Immunohistochemical stains for neuroendocrine differentiation are used to confirm the diagnosis. The most common markers are chromogranin and synaptophysin, with the former being more specific for neuroendocrine differentiation and the latter more sensitive. Other neuroendocrine markers include neuron-specific enolase and CD56. Insulinoma-associated protein 1 (INSM1) is a recently identified marker proposed to have high specificity for neuroendocrine differentiation[34,39]. In more poorly differentiated NENs, multiple markers may be needed to confirm the neuroendocrine etiology. Approximately 25% of NECs may lack chromogranin and synaptophysin expression, attributed to the decreased number of dense-core granules[30,34].
Tumor grading is dependent on mitotic activity and Ki-67. In situations where the Ki-67 index and mitotic index are discrepant, the higher grade is assigned, as studies have shown tumors tend to behave more like those of the higher grade[29,40]. Current recommendations include counting Ki-67 in at least 500 cells in “hot spots,” areas with increased activity, and counting 50 high power fields for mitoses. “Eyeballing” the mitotic count and Ki-67 is discouraged due to the lack of reproducibility, and it is preferred to count on printed images of the “hot spots” manually. Automated systems for counting have been explored but are currently limited by high costs.
Distinguishing between high-grade NETs from NECs by morphology can be challenging. The molecular differences between NETs and NECs can assist in these situations, especially in pancreatic NENs[7,8,29,30,40]. As previously described, DAXX/ATRX mutations can be detected in approximately 43% of pancreatic NETs, including high-grade NETs. These mutations can be detected by loss of protein expression by DAXX and ATRX immunohistochemistry[32]. As for NECs, immunohistochemical stains for p53 and RB1 may be used, either showing aberrant p53 expression (diffuse positivity or null) or absent RB1 staining[32]. A subset of NECs also shows loss of p16 expression, which is not appreciated in NETs[33]. Overexpression of BCL2 has also been reported, especially in the small cell variant (up to 100%); however, approximately 18% of NETs may also demonstrate this[32]. Although most cases of NETs can be morphologically differentiated from NECs; immunohistochemistry is available when the morphology is not definitive.
Despite the advancements made in histopathology, it still has limitations[41]. Biopsies are invasive, prone to sampling error, and can only provide a snapshot of a single time point in the course of the disease. It is not capable of providing a real-time evaluation of disease progression, recurrence, or therapy response. Other diagnostic modalities such as anatomical and functional imaging and clinical symptoms and biomarkers are needed in the surveillance of disease[42].
Due to the invasiveness of biopsy and limitations of histopathology, there is demand for non-invasive, reproducible biomarkers that can provide not only a diagnosis but also longitudinal data on prognosis, disease evolution, therapy response, and disease recurrence[41]. This has been challenging, and currently, there are no widely available biomarkers that can act as a standalone indicator[43]. However, with the emergence of multianalyte analysis, the field of biomarkers for GEP-NENs is expanding.
Monoanalytes are measured in plasma by enzyme-linked immunosorbent assays (ELISA). The primary targets were identified based on secretory products and include chromogranin A (CgA), pancreastatin, neuron-specific enolase, and neurokinin A[7,41,44]. While these biomarkers were initially regarded with much praise and are currently the only widely utilized biomarkers, they have limited sensitivity, specificity, and reproducibility[41,42,44,45]. Furthermore, they cannot identify early disease progression[41]. Overall, the greatest challenge is that a single analyte is incapable of providing information on the tumor molecular biology, such as cell proliferation and growth factor signaling[41,42,44].
Chromogranin A is a glycoprotein found in neuroendocrine cells and was first introduced as a biomarker over 3 decades ago[46,47]. It is currently the most commonly used biomarker for GEP-NENs[7]. It has a 10%-35% specificity, and its sensitivity ranges from 32% to 92%[44,48]. False elevations are common, especially in patients on proton pump inhibitors and those with chronic atrophic gastritis, renal insufficiency, arterial hypertension, and adenocarcinoma[6,7,42,48]. It is not recommended to use CgA as a screening tool, and it has greater utility in monitoring response to therapy and surveillance after a diagnosis has been made[7,44,49]. Studies originally showed that CgA correlated with tumor size and prognosis, though this is now considered controversial[41].
Another traditional biomarker for GEP-NENs, especially in patients with carcinoid syndrome, is 5-hydroxyindoleacetic acid (5-HIAA), a product of serotonin metabolism[50,51]. Although it may be measured in the plasma, it is more commonly measured in the urine. It is helpful in serotonin-secreting tumors, which account for only 15%-20% of GEP-NENs[41]. Elevation of urinary 5-HIAA has a sensitivity of 70% and a specificity of 90%-100% for NENs in the presence of carcinoid syndrome[41,52]. However, the sensitivity of this biomarker is as low as 35% in the absence of carcinoid syndrome[41,53,54]. Similar to other monoanalyte biomarkers, urinary 5-HIAA levels can be falsely elevated in many scenarios, including recent consumption of foods rich in serotonin, tryptophan, and dopamine, as well as malabsorptive diseases like celiac sprue and Whipple disease[51,53]. A study of 371 patients with NENs showed that urine 5-HIAA level was not a useful prognosticator for overall survival[55]. Other specific hormone markers such as insulin, gastrin, glucagon, VIP, somatostatin, ACTH, and calcitonin are also available; however, these collectively only apply to < 2% of GEP-NENs[7,41].
In 2008, Leja et al[56] analyzed serotonin-producing metastatic small intestinal NENs and identified six possible novel marker genes, including paraneoplastic antigen Ma2. Cui et al[57] used ELISA to detect Ma2 autoantibodies in 124 patients with small intestinal NENs at different stages of the disease and showed a sensitivity that ranged from 46%-50% and a specificity of 98% compared to the healthy patients. Their findings suggested that Ma2 may be a better biomarker than CgA.
Angiogenic factors have also been suggested as potential biomarkers as GEP-NENs are highly vascularized tumors[58]. Angiopoietin-2 (Ang-2) and the receptor tyrosine kinase Tie-2 have gained the most attention as potential biomarkers out of the angiogenic factors[59]. Ang-2 binds to its receptor, Tie-2, promoting endothelial cell survival and influencing vascular remodeling[60]. In 2009, Srirajaskanthan et al[61] and Detjen et al[59] found that Ang-2 serum levels were increased in patients with NENs compared to healthy patients. In contrast, Melen-Mucha et al[58] compared multiple angiogenic factors, including Ang-2 and Tie-2, and CgA serum levels in patients with NENs to those without and found that only Tie-2 and CgA were elevated in patients with NENs compared to controls. However, they found that Ang-2 was increased in the subgroup of patients with metastatic disease compared to those with localized disease. Figueroa-Vega et al[60] also found that Ang-2 and Tie-2 Levels were elevated in patients with metastatic disease. Another key angiogenic factor is the vascular endothelial growth factor, which is largely studied for its prognostic role as a possible therapeutic target instead of diagnosing GEP-NENs[62-65]. Overall, unlike the current monoanalytes that rely on secretory products, angiogenic factors reflect the tumorigenesis of NENs and represent a potential future category of biomarkers. Isidori et al[66] have an ongoing clinical trial (NCT04464122) to evaluate how Tie-2 and other angiogenic factors change in GEP-NENs after treatment.
Due to the limitations of monoanalyte analyses, multianalyte approaches have been studied over the last decade to improve the accuracy of biomarkers and correlate with tissue expression[41,44]. A panel of analytes, instead of a single biomarker as described above, is measured and interpreted to provide a more comprehensive picture of a tumor’s biology[41,42,44]. For example, disease-specific analytes can be evaluated alongside markers associated with cell proliferation to provide a diagnosis and predict tumor behavior[44].
Neuroendocrine gene transcript assay (NETest) is the first neuroendocrine tumor liquid biopsy, using polymerase chain reaction (PCR) to detect 51 transcriptomic signatures of NENs[35,44,67-69]. These genes were identified to have significant differences in expression in patients with GEP-NENs and bronchopulmonary NENs vs those without NENs[41,70]. After measuring RNA expression in whole blood, an algorithm calculates a risk score that ranges from 0% to 100%[45]. Current cutoffs are < 20% normal, 21% to 40% stable/low risk disease, and 41%-100% progressive/high risk disease[41,45]. These cutoffs will likely be refined as more studies are performed. However, even with the current parameters, NETest has shown promising results with high sensitivity and specificity (> 95% and > 90%, respectively)[69].
In 2014, Modlin et al[44] compared NETest with the monoanlytes CgA, pancreastatin, and neurokinin A in 40 patients with grade 1 and grade 2 GEP-NETs and found that NETest was superior to the monoanalytes in sensitivity and specificity. The authors concluded that NETest could facilitate early detection of disease recurrence and predict therapy response[44]. In 2017, Pavel et al[42] followed patients with GEP-NENs for a median of 4 years to compare NETest and CgA and found that NETest more accurately correlated with the clinical status and was able to identify those with progressive disease approximately one year before being detectable by imaging. In 2016, Modlin et al[69,71] also found that the NETest risk score fell after tumor debulking, suggesting it can be used to identify residual disease after surgery[41]. A current clinical trial (NCT03012789) by Wren Laboratories investigates whether NETest accurately correlates with surgical excision, identifies residual tumor, and predicts early disease relapse[72].
Although NETest is highly sensitive and has shown value in disease monitoring, studies on its use as a screening tool are less promising. Van Treijen et al[70] compared patients with diagnosed GEP-NENs to healthy patients and found that NETest was less specific than CgA (56%-72% vs 83%, respectively). Al-Toubah et al[45] compared patients with metastatic GEP-NENs and bronchopulmonary NENs with a mixed group of healthy patients and patients with metastatic non-NEN gastrointestinal malignancies. Unlike the previous study, they found that NETest successfully ruled out 100% of healthy patients, but specificity was only 67% when compared to patients with non-NEN gastrointestinal malignancies[45]. This is likely because NETest includes genes whose expression is associated with proliferation and metabolism which may be upregulated in non-NEN malignancies as well as nonspecific environments of stress and inflammation[70]. Additionally, studies have shown that NETest does not correlate with tumor grade[70].
Overall, the sensitivity of NETest far exceeds that of other currently used biomarkers for GEP-NENs, while specificity has varied depending on the cohort[41,70]. Importantly, unlike current monoanalyte biomarkers, NETest is not affected by proton pump inhibitor use and diet[41]. Although it shows promise as a valuable biomarker for GEP-NENs, further studies are still warranted in non-gastrointestinal NENs such as paragangliomas and malignancies with mixed epithelial or neuroendocrine phenotype, such as prostate cancer[43]. Wren Laboratories is conducting a clinical trial (NCT02270567) on patients with confirmed diagnoses of NENs to have a better overall understanding of NETest in clinical practice[73]. Another clinical trial (NCT02948946) by H. Lee Moffitt Cancer Center and Research Institute to evaluate the NETest sensitivity and specificity in GEP-NENs and lung NENs recently concluded[74]. Currently, studies show that NETest has great promise in identifying early disease progression, assessing therapy response, and evaluating if the surgical tumor resection is complete[43]. NETest is currently available at select accredited laboratories in the United States and Europe[75].
In addition to NETest, other multianalyte biomarkers are also being explored. MicroRNAs (miRNAs) are small non-coding RNAs that regulate gene expression at the post-transcriptional level and act as oncogenes or tumor suppressors[76]. They can be detected by PCR and have been investigated as a potential serum target. More than 100 miRNAs are differentially expressed in NENs[48,76]. About 10% of these are nonspecific in terms of tumor location, such as miRNA-375 and miRNA-7, while the remaining 90% appear to be specific to the anatomic site[76]. For GEP-NENs, potential biomarker targets have been identified in the small intestine (miRNA-7-75-p, miRNA-182, miRNA-183, and miRNA-96-5p), stomach (miRNA-375, miRNA-7, miR-96-5p, and miRNA-222), and pancreas (miRNA-193b, miRNA-144/451, miRNA-21, miR-1290, miRNA-103, miRNA-107, miRNA-155, miRNA-204, miR-328, miRNA-642, miRNA-3653, miRNA-23b, miRNA-137, miRNA-196a, and miRNA449a)[76,77]. Clinical applications of miRNAs have been challenging, and currently, there is no standardization of the process[41,78]. Existing studies have small sample sizes and inconsistent methodologies, making it difficult to draw conclusions[41,69].
Another area of development in multianalyte analyses is next-generation sequencing (NGS) and other genetic analyses. These include studies on samples consisting of primary tumor tissue and circulating tumor DNA (ctDNA), the fraction of cell-free DNA that is released by dying tumor cells and can be detected in plasma for analysis[79,80]. Recent studies have been guided by the current understanding of genes implicated in GEP-NENs, including MEN1, DAXX, ATRX, and mTOR pathway genes[31]. A 2017 study by Gleeson et al[81] used a 15 gene NGS panel to determine if any genes involved in commonly implicated pathways could be used as prognosticators in patients with pancreatic NENs. Only variants in TSC2, KRAS, and TP53 were identified to have prognostic significance, and each of these variants was present in fewer than 10% of samples examined[81]. Further, 40% of tumors assessed were wild-type for all 15 genes assessed, and this set of patients did not demonstrate meaningful differences in tumor or clinical characteristics[81]. A 2018 study analyzed ctDNA in pancreatic NENs in 10 patients and showed a correlation with genetic characteristics of ctDNA and tumor tissue, suggesting a role for less invasive liquid biopsies in pancreatic NEN diagnosis and monitoring[82]. Zakka et al[83] performed a larger study in 2020 that further demonstrated the feasibility of NGS analysis of ctDNA in 320 patients with NENs, including those outside the gastrointestinal system. While their gene panel did not include implicated genes like MEN1, ATRX, or DAXX, and they lacked data for clinical or histopathological correlation in many patients, the study further reinforced the promise and necessity of future studies on liquid biopsies, ctDNA, and NGS[83]. Another 2021 study on NENs of various origins identified actionable mutations in over 50% of patients using NGS on liquid biopsies and formalin-fixed paraffin-embedded tissue[84]. Studies to date on GEP-NEN ctDNA analysis through NGS and other methods are promising and offer proof-of-concept of feasibility, clinical applicability, and potential prognostication of disease progression and survival. However, larger-scale prospective studies correlating genetic, histopathologic, and clinical data are needed before the widespread use of these tests. Additionally, an increased understanding of molecular pathways underlying the development and progression of GEP-NENs will refine genetic tests analyzing ctDNA and tumor tissue.
Multianalyte analysis of neuroendocrine metabolites has also been explored as a diagnostic strategy for GEP-NENs. A 2021 study from Jiménez et al[85] used nuclear magnetic resonance to compare urine samples from patients with GEP-NENs and healthy controls and generated a model that could accurately discriminate between the two groups. The study suggests that nuclear magnetic resonance could be a useful clinical tool for diagnosing GEP-NENs[85]. They identified several metabolites that were either increased or decreased in GEP-NENs, including kynurenine, hippurate, and phenylacetylglutamine[85]. These novel biomarkers represent areas of future study and suggest that a multianalyte test involving multiple metabolites that are altered in GEP-NENs could be more effective than monoanalyte tests.
Conventional cross-sectional imaging such as computed tomography (CT) and magnetic resonance imaging (MRI) are critical diagnostic tools in localizing, characterizing, and staging GEP-NENs[9,86]. MRI is used less commonly than CT due to increased cost, acquisition time, and potential for motion artifact[87]. Multiphasic CT with intravenous contrast is essential to increase diagnostic yield[87]. NENs are generally hypervascular and show enhancement in the late arterial phase, and NEN metastases are also hypervascular and best visualized in the arterial phase[87]. For detection of metastases to the liver, MRI is more sensitive than CT[88,89]. A 2003 study of different MRI techniques suggested that hepatic arterial phase and fast spin-echo T2 weighted images were most sensitive for hepatic metastases of NENs, further emphasizing the importance of multiphase imaging[89].
Recent advances in conventional cross-sectional imaging of GEP-NENs include studies assessing hepatic metastases of GEP-NENs with contrast-enhanced MRI utilizing hepatocellular phase-contrast agents[90]. The most studied agent is gadoxetate disodium, a gadolinium-based contrast with hepatobiliary excretion[91]. A 2018 study from Tirumani et al[92] compared the ability of 6 MRI phases after gadoxetate disodium injection to assess hepatic metastases of GEP-NENs and found that the hepatocellular phase was superior to all other phases examined. Another study demonstrated that combining diffusion-weighted and hepatobiliary phases of gadoxetate disodium-enhanced MRI in patients with suspected neuroendocrine liver metastases had the best diagnostic yield, with a sensitivity of 86% and a specificity of 94%, compared to other combinations of contrast-enhanced phases[93]. These studies highlight an essential role for contrast-enhanced MRI with gadoxetate disodium or other liver-targeted contrast agents in the assessment of GEP-NENs with potential liver metastases and long-term surveillance of disease with known liver involvement.
Somatostatin receptor scintigraphy (SRS) has been used since the 1990s to assess GEP-NENs. Studies from 1995 and 1996 showed that 111In-pentetreotide, a radiolabeled somatostatin analog, could safely and effectively detect GEP-NENs more effectively than conventional imaging[94,95]. A 2001 study examined 68Ga-DOTATOC compared to older SRS techniques and found a higher diagnostic yield by 30%[96]. More tracers for GEP-NENs have been developed over the years, including Ga-DOTANOC[68] and Ga-DOTATATE[97-99]. Two 2016 studies compared 111in-pentetreotide and 68Ga-DOTATATE imaging for identification of primary tumor and metastatic lesions of NENs[98,100]. Both studies showed that 68Ga-DOTATATE had identified more lesions than 111In-pentetreotide, and altered management in 33%-36% of patients with GEP-NENs[98,100]. An established principle of functional imaging for GEP-NENs is the distinction in imaging characteristics between low-grade NENs vs high-grade NENs and NECs. Low-grade NENs express high levels of somatostatin receptors and are less metabolically active, and thus, 68Ga-DOTATATE and other somatostatin analogs are superior to 18F-fluorodeoxyglucose (18F-FDG) as a tracer for functional imaging of grade 1 and 2 NENs[87,101,102]. High-grade NENs and NECs tend to have higher rates of glucose metabolism and lower expression of somatostatin receptors. As a result, 18F-FDG is superior to somatostatin analogs for functional imaging of high-grade NENs[103,104].
Recent studies have made advances in comparing head-to-head imaging modalities and tracers to determine which are optimal for functional imaging of GEP-NENs. A 2017 study compared positron emission tomography (PET)/CT and PET/MRI using 68Ga-DOTATOC and found that both imaging modalities performed comparably in identifying abdominal primary tumors and yield of lymph node metastases[105,106]. Sawicki et al[105] found that PET/CT performed better in identifying bone lesions, but PET/MRI with 68Ga-DOTATOC outperformed PET/CT in identifying hepatic lesions. However, the further identification of metastases did not alter the management of patients in this study, as most already had advanced stages of the disease[105]. Given the prevalence of liver metastases in GEP-NENs, these data suggest that there may be a valuable role for PET/MRI over or in conjunction with PET/CT in the diagnosis and staging of GEP-NENs[107]. A similar 2021 study of 11 patients with GEP-NENs prospectively compared PET/MRI with 68Ga-DOTATOC and PET/CT with 68Ga-DOTATOC[88]. For detection of all lesions, PET/MRI with 68Ga-DOTATOC outperformed PET/CT with 68Ga-DOTATOC[88]. Consistent with the 2017 study, 68Ga-DOTATOC PET/MRI was superior to 68Ga-DOTATOC PET/CT in detecting liver metastases[88]. These studies suggest that PET/MRI with 68Ga-DOTATOC may be superior to PET/CT in guiding the management of GEP-NENs.
Other recent studies have examined specific clinical scenarios when functional imaging is likely to influence the clinical management of patients with GEP-NENs. A 2017 study of 40 patients with metastatic NENs who had undergone CT or MRI but still had an unknown primary tumor location showed that 68Ga-DOTATOC PET/CT could effectively localize the primary tumor to facilitate treatment[108]. A meta-analysis of studies on 68Ga-DOTATOC PET/CT identified that this imaging modality changed management when the patient had a known NEN around half of the time[109]. By contrast, in patients with symptoms consistent with a NEN and elevated biomarkers but no proven NEN, there was only a 13% yield[109]. Notably, four studies included in this meta-analysis showed a 44% yield for detection of primary tumor site in patients with metastatic disease[109].
Finally, advances in the application of automation and artificial intelligence could improve diagnostic consistency and accuracy of functional imaging for GEP-NENs. This area has been more extensively studied for 18F-FDG PET/CT[110,111]. A retrospective 2021 study retrospectively demonstrated that deep learning could facilitate the automation of detection of hepatic metastases, though future studies with larger sample sizes are required for further validation[112]. Continued optimization of imaging techniques and development of more selective tracers will continue to improve diagnostic yield and ability of functional imaging to guide the management of GEP-NENs effectively.
Endoscopic ultrasound (EUS) has been used for decades to assess gastrointestinal tract tumors, including GEP-NENs[113,114]. This technique combines endoscopy with ultrasound to image structures and can diagnose, stage, and sample malignancies[115]. EUS is especially useful for gastric, duodenal, pancreatic, and rectal NENs[116,117]. EUS is more sensitive than other modalities such as CT or MRI for pancreatic NENs and is the most sensitive method for detection of rectal NENs[118-122]. It provides additional information on the depth of invasion and can assess local lymph node involvement. Further advantages of EUS include the ability to perform a fine needle aspiration (FNA) to obtain tissue for cytologic and molecular analysis and to place a radiofrequency ablation probe for poor surgical candidates[114,116,123]. Cytologic analysis of pancreatic NENs facilitated by EUS-FNA including Ki-67 index correlates with tumor grade confirmed after resection, influences management, and predicts survival, especially when sampling is sufficient[124,125]. Disadvantages include operator dependence and limited assessment beyond the local area compared to broader imaging techniques[116,117].
Recent updates on EUS for the diagnosis of GEP-NENs include technical advances in ultrasonography and new histologic and molecular analyses of tissue obtained using EUS. A recent 2021 study compared the diagnostic accuracy of EUS-FNA and EUS with fine needle biopsy (EUS-FNB) and found that EUS-FNB may be superior to EUS-FNA for pancreatic NENs, including a better correlation of Ki-67 proliferation index between EUS-FNB and the surgical specimen[126]. However, there was no difference in the accuracy of grade estimation between EUS-FNB and EUS-FNA[118].
Immunocytochemical analysis can be performed on EUS-FNA samples for confirmation of the diagnosis of pancreatic NEN. A recent study immunocytochemically analyzed INSM1 expression in 14 EUS-FNA samples of pancreatic NENs and 15 pancreatic ductal adenocarcinomas (PDACs)[127]. All pancreatic NENs contained cells expressing INSM1, and INSM1 was expressed at a higher rate in pancreatic NEN samples than PDAC samples[127]. Advances in molecular analysis of EUS-FNA samples can also improve diagnostic accuracy for pancreatic lesions. A 2020 study used digital droplet PCR to detect KRAS mutations in EUS-FNA samples from PDAC, pancreatic NENs, and chronic pancreatitis. Combining molecular and cytologic analyses improved diagnostic accuracy from 74% with cytology alone to 91%[128]. Further technical advancement and refinement of molecular and cytologic analyses will continue to improve the efficacy of EUS and EUS-FNA.
There are many new developments in the pathologic, molecular, and imaging diagnosis of GEP-NENs. The WHO classification of GEP-NENs has changed over the years, with the most recent significant update being the distinction between high-grade NETs and NECs. Due to the heterogeneity of GEP-NENs, a multimodal approach to diagnosis and disease surveillance is necessary. A better understanding of the molecular biology of GEP-NENs has allowed for the distinction between high-grade NETs and NECs, the introduction of exciting new biomarker tests such as the NETest, and continued advances toward eventual validation and implementation of other multianalyte tests assessing biomarkers such as miRNA and ctDNA. Recent advances in imaging include the validation of improved PET tracers and determination of which imaging modalities are optimal for anatomic and functional imaging of primary GEP-NENs and metastases, especially to the liver. Updates to EUS and EUS-FNA include technological advances and improved molecular and cytological analysis of tissue obtained using EUS.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: United States and Canadian Academy of Pathology, 28212; American Association for Cancer Research (AACR), 76093; Pancreatobiliary Pathology Society; Rodger C. Haggitt Gastrointestinal Pathology Society.
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gazouli M, Nakamura M S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Taal BG, Visser O. Epidemiology of neuroendocrine tumours. Neuroendocrinology. 2004;80 Suppl 1:3-7. [PubMed] [DOI] [Full Text] |
| 2. | Fraenkel M, Faggiano A, Valk GD. Epidemiology of Neuroendocrine Tumors. Front Horm Res. 2015;44:1-23. [PubMed] [DOI] [Full Text] |
| 3. | Klöppel G. Neuroendocrine Neoplasms: Dichotomy, Origin and Classifications. Visc Med. 2017;33:324-330. [PubMed] [DOI] [Full Text] |
| 4. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [PubMed] [DOI] [Full Text] |
| 5. | Andreasi V, Partelli S, Muffatti F, Manzoni MF, Capurso G, Falconi M. Update on gastroenteropancreatic neuroendocrine tumors. Dig Liver Dis. 2021;53:171-182. [PubMed] [DOI] [Full Text] |
| 6. | Raphael MJ, Chan DL, Law C, Singh S. Principles of diagnosis and management of neuroendocrine tumours. CMAJ. 2017;189:E398-E404. [PubMed] [DOI] [Full Text] |
| 7. | Janson ET, Knigge U, Dam G, Federspiel B, Grønbaek H, Stålberg P, Langer SW, Kjaer A, Arola J, Schalin-Jäntti C, Sundin A, Welin S, Thiis-Evensen E, Sorbye H. Nordic guidelines 2021 for diagnosis and treatment of gastroenteropancreatic neuroendocrine neoplasms. Acta Oncol. 2021;60:931-941. [PubMed] [DOI] [Full Text] |
| 8. | Assarzadegan N, Montgomery E. What is New in the 2019 World Health Organization (WHO) Classification of Tumors of the Digestive System: Review of Selected Updates on Neuroendocrine Neoplasms, Appendiceal Tumors, and Molecular Testing. Arch Pathol Lab Med. 2021;145:664-677. [PubMed] [DOI] [Full Text] |
| 9. | Dillon JS. Workup of Gastroenteropancreatic Neuroendocrine Tumors. Surg Oncol Clin N Am. 2020;29:165-183. [PubMed] [DOI] [Full Text] |
| 10. | Ma Z, Cai H, Cui Y. Progress in the treatment of esophageal neuroendocrine carcinoma. Tumour Biol. 2017;39:1010428317711313. [PubMed] [DOI] [Full Text] |
| 11. | Terada T. Small cell neuroendocrine carcinoma of the esophagus: report of 6 cases with immunohistochemical and molecular genetic analysis of KIT and PDGFRA. Int J Clin Exp Pathol. 2013;6:485-491. [PubMed] |
| 12. | Eggenberger JC. Carcinoid and other neuroendocrine tumors of the colon and rectum. Clin Colon Rectal Surg. 2011;24:129-134. [PubMed] [DOI] [Full Text] |
| 13. | Yoon SN, Yu CS, Shin US, Kim CW, Lim SB, Kim JC. Clinicopathological characteristics of rectal carcinoids. Int J Colorectal Dis. 2010;25:1087-1092. [PubMed] [DOI] [Full Text] |
| 14. | Ahmed M. Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol. 2020;12:791-807. [PubMed] [DOI] [Full Text] |
| 15. | Gorelik M, Ahmad M, Grossman D, Grossman M, Cooperman AM. Nonfunctioning Incidental Pancreatic Neuroendocrine Tumors: Who, When, and How to Treat? Surg Clin North Am. 2018;98:157-167. [PubMed] [DOI] [Full Text] |
| 16. | Kaltsas G, Grozinsky-Glasberg S, Alexandraki KI, Thomas D, Tsolakis AV, Gross D, Grossman AB. Current concepts in the diagnosis and management of type 1 gastric neuroendocrine neoplasms. Clin Endocrinol (Oxf). 2014;81:157-168. [PubMed] [DOI] [Full Text] |
| 17. | Scherübl H. Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy. 2009;41:162-165. [PubMed] [DOI] [Full Text] |
| 18. | Connor SJ, Hanna GB, Frizelle FA. Appendiceal tumors: retrospective clinicopathologic analysis of appendiceal tumors from 7,970 appendectomies. Dis Colon Rectum. 1998;41:75-80. [PubMed] [DOI] [Full Text] |
| 19. | Sprague JE, Arbeláez AM. Glucose counterregulatory responses to hypoglycemia. Pediatr Endocrinol Rev. 2011;9:463-73; quiz 474. [PubMed] |
| 20. | Epelboym I, Mazeh H. Zollinger-Ellison syndrome: classical considerations and current controversies. Oncologist. 2014;19:44-50. [PubMed] [DOI] [Full Text] |
| 21. | Wermers RA, Fatourechi V, Wynne AG, Kvols LK, Lloyd RV. The glucagonoma syndrome. Clinical and pathologic features in 21 patients. Medicine (Baltimore). 1996;75:53-63. [PubMed] [DOI] [Full Text] |
| 22. | Song X, Zheng S, Yang G, Xiong G, Cao Z, Feng M, Zhang T, Zhao Y. Glucagonoma and the glucagonoma syndrome. Oncol Lett. 2018;15:2749-2755. [PubMed] [DOI] [Full Text] |
| 23. | Stacpoole PW. The glucagonoma syndrome: clinical features, diagnosis, and treatment. Endocr Rev. 1981;2:347-361. [PubMed] [DOI] [Full Text] |
| 24. | Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia. 2017;19:991-1002. [PubMed] [DOI] [Full Text] |
| 25. | Verner JV, MORRISON AB. Islet cell tumor and a syndrome of refractory watery diarrhea and hypokalemia. Am J Med. 1958;25:374-380. [PubMed] [DOI] [Full Text] |
| 26. | Ito T, Lee L, Jensen RT. Carcinoid-syndrome: recent advances, current status and controversies. Curr Opin Endocrinol Diabetes Obes. 2018;25:22-35. [PubMed] [DOI] [Full Text] |
| 27. | Boutzios G, Kaltsas G. Clinical Syndromes Related to Gastrointestinal Neuroendocrine Neoplasms. Front Horm Res. 2015;44:40-57. [PubMed] [DOI] [Full Text] |
| 28. | de Herder WW, Rehfeld JF, Kidd M, Modlin IM. A short history of neuroendocrine tumours and their peptide hormones. Best Pract Res Clin Endocrinol Metab. 2016;30:3-17. [PubMed] [DOI] [Full Text] |
| 29. | Tang LH, Untch BR, Reidy DL, O'Reilly E, Dhall D, Jih L, Basturk O, Allen PJ, Klimstra DS. Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas. Clin Cancer Res. 2016;22:1011-1017. [PubMed] [DOI] [Full Text] |
| 30. | Fang JM, Shi J. A Clinicopathologic and Molecular Update of Pancreatic Neuroendocrine Neoplasms With a Focus on the New World Health Organization Classification. Arch Pathol Lab Med. 2019;143:1317-1326. [PubMed] [DOI] [Full Text] |
| 31. | Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA, Velculescu VE, Diaz LA Jr, Vogelstein B, Kinzler KW, Hruban RH, Papadopoulos N. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199-1203. [PubMed] [DOI] [Full Text] |
| 32. | Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, de Wilde RF, Maitra A, Hicks J, Demarzo AM, Shi C, Sharma R, Laheru D, Edil BH, Wolfgang CL, Schulick RD, Hruban RH, Tang LH, Klimstra DS, Iacobuzio-Donahue CA. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36:173-184. [PubMed] [DOI] [Full Text] |
| 33. | Mafficini A, Scarpa A. Genetics and Epigenetics of Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr Rev. 2019;40:506-536. [PubMed] [DOI] [Full Text] |
| 34. | Bellizzi AM. Immunohistochemistry in the diagnosis and classification of neuroendocrine neoplasms: what can brown do for you? Hum Pathol. 2020;96:8-33. [PubMed] [DOI] [Full Text] |
| 35. | Di Domenico A, Wiedmer T, Marinoni I, Perren A. Genetic and epigenetic drivers of neuroendocrine tumours (NET). Endocr Relat Cancer. 2017;24:R315-R334. [PubMed] [DOI] [Full Text] |
| 36. | Du Y, Ter-Minassian M, Brais L, Brooks N, Waldron A, Chan JA, Lin X, Kraft P, Christiani DC, Kulke MH. Genetic associations with neuroendocrine tumor risk: results from a genome-wide association study. Endocr Relat Cancer. 2016;23:587-594. [PubMed] [DOI] [Full Text] |
| 37. | Ter-Minassian M, Wang Z, Asomaning K, Wu MC, Liu CY, Paulus JK, Liu G, Bradbury PA, Zhai R, Su L, Frauenhoffer CS, Hooshmand SM, De Vivo I, Lin X, Christiani DC, Kulke MH. Genetic associations with sporadic neuroendocrine tumor risk. Carcinogenesis. 2011;32:1216-1222. [PubMed] [DOI] [Full Text] |
| 38. | Obazee O, Capurso G, Tavano F, Archibugi L, De Bonis A, Greenhalf W, Key T, Pasquali C, Milanetto AC, Hackert T, Fogar P, Liço V, Dervenis C, Lawlor RT, Landoni L, Gazouli M, Zambon CF, Funel N, Strobel O, Jamroziak K, Cantù C, Malecka-Panas E, Landi S, Neoptolemos JP, Basso D, Talar-Wojnarowska R, Rinzivillo M, Andriulli A, Canzian F, Campa D. Common genetic variants associated with pancreatic adenocarcinoma may also modify risk of pancreatic neuroendocrine neoplasms. Carcinogenesis. 2018;39:360-367. [PubMed] [DOI] [Full Text] |
| 39. | McHugh KE, Mukhopadhyay S, Doxtader EE, Lanigan C, Allende DS. INSM1 Is a Highly Specific Marker of Neuroendocrine Differentiation in Primary Neoplasms of the Gastrointestinal Tract, Appendix, and Pancreas. Am J Clin Pathol. 2020;153:811-820. [PubMed] [DOI] [Full Text] |
| 40. | Tang LH, Basturk O, Sue JJ, Klimstra DS. A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. Am J Surg Pathol. 2016;40:1192-1202. [PubMed] [DOI] [Full Text] |
| 41. | Öberg K. Molecular Genomic Blood Biomarkers for Neuroendocrine Tumors: The Long and Winding Road from Berzelius and Bence Jones to a Neuroendocrine Destination. Neuroendocrinology. 2021;111:297-303. [PubMed] [DOI] [Full Text] |
| 42. | Pavel M, Jann H, Prasad V, Drozdov I, Modlin IM, Kidd M. NET Blood Transcript Analysis Defines the Crossing of the Clinical Rubicon: When Stable Disease Becomes Progressive. Neuroendocrinology. 2017;104:170-182. [PubMed] [DOI] [Full Text] |
| 43. | Oberg K, Modlin IM, De Herder W, Pavel M, Klimstra D, Frilling A, Metz DC, Heaney A, Kwekkeboom D, Strosberg J, Meyer T, Moss SF, Washington K, Wolin E, Liu E, Goldenring J. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015;16:e435-e446. [PubMed] [DOI] [Full Text] |
| 44. | Modlin IM, Drozdov I, Alaimo D, Callahan S, Teixiera N, Bodei L, Kidd M. A multianalyte PCR blood test outperforms single analyte ELISAs (chromogranin A, pancreastatin, neurokinin A) for neuroendocrine tumor detection. Endocr Relat Cancer. 2014;21:615-628. [PubMed] [DOI] [Full Text] |
| 45. | Al-Toubah T, Cives M, Valone T, Blue K, Strosberg J. Sensitivity and Specificity of the NETest: A Validation Study. Neuroendocrinology. 2021;111:580-585. [PubMed] [DOI] [Full Text] |
| 46. | Eriksson B, Arnberg H, Oberg K, Hellman U, Lundqvist G, Wernstedt C, Wilander E. Chromogranins--new sensitive markers for neuroendocrine tumors. Acta Oncol. 1989;28:325-329. [PubMed] [DOI] [Full Text] |
| 47. | Schürmann G, Betzler M, Buhr HJ. Chromogranin A, neuron-specific enolase and synaptophysin as neuroendocrine cell markers in the diagnosis of tumours of the gastro-entero-pancreatic system. Eur J Surg Oncol. 1990;16:298-303. [PubMed] |
| 48. | Hofland J, Zandee WT, de Herder WW. Role of biomarker tests for diagnosis of neuroendocrine tumours. Nat Rev Endocrinol. 2018;14:656-669. [PubMed] [DOI] [Full Text] |
| 49. | Papantoniou D, Grönberg M, Landerholm K, Welin S, Ziolkowska B, Nordvall D, Janson ET. Assessment of hormonal levels as prognostic markers and of their optimal cut-offs in small intestinal neuroendocrine tumours grade 2. Endocrine. 2021;72:893-904. [PubMed] [DOI] [Full Text] |
| 50. | Haverback BJ, SJOERDSMA A, TERRY LL. Urinary excretion of the serotonin metabolite, 5-hydroxyindoleacetic acid, in various clinical conditions. N Engl J Med. 1956;255:270-272. [PubMed] [DOI] [Full Text] |
| 51. | Corcuff JB, Chardon L, El Hajji Ridah I, Brossaud J. Urinary sampling for 5HIAA and metanephrines determination: revisiting the recommendations. Endocr Connect. 2017;6:R87-R98. [PubMed] [DOI] [Full Text] |
| 52. | Meijer WG, Kema IP, Volmer M, Willemse PH, de Vries EG. Discriminating capacity of indole markers in the diagnosis of carcinoid tumors. Clin Chem. 2000;46:1588-1596. [PubMed] |
| 53. | O'Toole D, Grossman A, Gross D, Delle Fave G, Barkmanova J, O'Connor J, Pape UF, Plöckinger U; Mallorca Consensus Conference participants; European Neuroendocrine Tumor Society. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: biochemical markers. Neuroendocrinology. 2009;90:194-202. [PubMed] [DOI] [Full Text] |
| 54. | Kanakis G, Kaltsas G. Biochemical markers for gastroenteropancreatic neuroendocrine tumours (GEP-NETs). Best Pract Res Clin Gastroenterol. 2012;26:791-802. [PubMed] [DOI] [Full Text] |
| 55. | Zandee WT, Kamp K, van Adrichem RC, Feelders RA, de Herder WW. Limited value for urinary 5-HIAA excretion as prognostic marker in gastrointestinal neuroendocrine tumours. Eur J Endocrinol. 2016;175:361-366. [PubMed] [DOI] [Full Text] |
| 56. | Leja J, Essaghir A, Essand M, Wester K, Oberg K, Tötterman TH, Lloyd R, Vasmatzis G, Demoulin JB, Giandomenico V. Novel markers for enterochromaffin cells and gastrointestinal neuroendocrine carcinomas. Mod Pathol. 2009;22:261-272. [PubMed] [DOI] [Full Text] |
| 57. | Cui T, Hurtig M, Elgue G, Li SC, Veronesi G, Essaghir A, Demoulin JB, Pelosi G, Alimohammadi M, Öberg K, Giandomenico V. Paraneoplastic antigen Ma2 autoantibodies as specific blood biomarkers for detection of early recurrence of small intestine neuroendocrine tumors. PLoS One. 2010;5:e16010. [PubMed] [DOI] [Full Text] |
| 58. | Melen-Mucha G, Niedziela A, Mucha S, Motylewska E, Lawnicka H, Komorowski J, Stepien H. Elevated peripheral blood plasma concentrations of tie-2 and angiopoietin 2 in patients with neuroendocrine tumors. Int J Mol Sci. 2012;13:1444-1460. [PubMed] [DOI] [Full Text] |
| 59. | Detjen KM, Rieke S, Deters A, Schulz P, Rexin A, Vollmer S, Hauff P, Wiedenmann B, Pavel M, Scholz A. Angiopoietin-2 promotes disease progression of neuroendocrine tumors. Clin Cancer Res. 2010;16:420-429. [PubMed] [DOI] [Full Text] |
| 60. | Figueroa-Vega N, Díaz A, Adrados M, Alvarez-Escolá C, Paniagua A, Aragonés J, Martín-Pérez E, Leskela S, Moreno-Otero R, González-Amaro R, Marazuela M. The association of the angiopoietin/Tie-2 system with the development of metastasis and leukocyte migration in neuroendocrine tumors. Endocr Relat Cancer. 2010;17:897-908. [PubMed] [DOI] [Full Text] |
| 61. | Srirajaskanthan R, Dancey G, Hackshaw A, Luong T, Caplin ME, Meyer T. Circulating angiopoietin-2 is elevated in patients with neuroendocrine tumours and correlates with disease burden and prognosis. Endocr Relat Cancer. 2009;16:967-976. [PubMed] [DOI] [Full Text] |
| 62. | Abdel-Rahman O. Vascular endothelial growth factor (VEGF) pathway and neuroendocrine neoplasms (NENs): prognostic and therapeutic considerations. Tumour Biol. 2014;35:10615-10625. [PubMed] [DOI] [Full Text] |
| 63. | Zhang J, Jia Z, Li Q, Wang L, Rashid A, Zhu Z, Evans DB, Vauthey JN, Xie K, Yao JC. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer. 2007;109:1478-1486. [PubMed] [DOI] [Full Text] |
| 64. | Pavel ME, Hassler G, Baum U, Hahn EG, Lohmann T, Schuppan D. Circulating levels of angiogenic cytokines can predict tumour progression and prognosis in neuroendocrine carcinomas. Clin Endocrinol (Oxf). 2005;62:434-443. [PubMed] [DOI] [Full Text] |
| 65. | Berardi R, Torniai M, Partelli S, Rubini C, Pagliaretta S, Savini A, Polenta V, Santoni M, Giampieri R, Onorati S, Barucca F, Murrone A, Bianchi F, Falconi M. Impact of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) single nucleotide polymorphisms on outcome in gastroenteropancreatic neuroendocrine neoplasms. PLoS One. 2018;13:e0197035. [PubMed] [DOI] [Full Text] |
| 66. | Isidori AM. Rediscovering Biomarkers for the Diagnosis and Early Treatment Response in NEN. [accessed 2021 Oct 12]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04464122 ClinicalTrials.gov Identifier: NCT04464122. |
| 67. | Liu E, Paulson S, Gulati A, Freudman J, Grosh W, Kafer S, Wickremesinghe PC, Salem RR, Bodei L. Assessment of NETest Clinical Utility in a U.S. Registry-Based Study. Oncologist. 2019;24:783-790. [PubMed] [DOI] [Full Text] |
| 68. | Modlin IM, Drozdov I, Kidd M. The identification of gut neuroendocrine tumor disease by multiple synchronous transcript analysis in blood. PLoS One. 2013;8:e63364. [PubMed] [DOI] [Full Text] |
| 69. | Modlin IM, Kidd M, Malczewska A, Drozdov I, Bodei L, Matar S, Chung KM. The NETest: The Clinical Utility of Multigene Blood Analysis in the Diagnosis and Management of Neuroendocrine Tumors. Endocrinol Metab Clin North Am. 2018;47:485-504. [PubMed] [DOI] [Full Text] |
| 70. | van Treijen MJC, Korse CM, van Leeuwaarde RS, Saveur LJ, Vriens MR, Verbeek WHM, Tesselaar MET, Valk GD. Blood Transcript Profiling for the Detection of Neuroendocrine Tumors: Results of a Large Independent Validation Study. Front Endocrinol (Lausanne). 2018;9:740. [PubMed] [DOI] [Full Text] |
| 71. | Modlin IM, Frilling A, Salem RR, Alaimo D, Drymousis P, Wasan HS, Callahan S, Faiz O, Weng L, Teixeira N, Bodei L, Drozdov I, Kidd M. Blood measurement of neuroendocrine gene transcripts defines the effectiveness of operative resection and ablation strategies. Surgery. 2016;159:336-347. [PubMed] [DOI] [Full Text] |
| 72. | Wren Laboratories LLC. Diagnosis of Neuroendocrine Neoplasms and Assessment of Response to Surgery by Means of Measurement of Gene Transcripts in Blood. [accessed 2021 Oct 12]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03012789 ClinicalTrials.gov Identifier: NCT03012789. |
| 73. | Wren Laboratories LLC. A Registry for Neuroendocrine Tumors in the USA. [accessed 2021 Oct 12]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02270567 ClinicalTrials.gov Identifier: NCT02270567. |
| 74. | H Lee Moffitt Cancer Center and Research Institute. The Clinical Utility of a Blood-Based Multitranscriptome Assay as a Biomarker for Gastroenteropancreatic and Lung Neuroendocrine Tumors. [accessed 2021 Oct 12]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02948946 ClinicalTrials.gov Identifier: NCT02948946. |
| 75. | Öberg K, Califano A, Strosberg JR, Ma S, Pape U, Bodei L, Kaltsas G, Toumpanakis C, Goldenring JR, Frilling A, Paulson S. A meta-analysis of the accuracy of a neuroendocrine tumor mRNA genomic biomarker (NETest) in blood. Ann Oncol. 2020;31:202-212. [PubMed] [DOI] [Full Text] |
| 76. | Korotaeva A, Mansorunov D, Apanovich N, Kuzevanova A, Karpukhin A. MiRNA Expression in Neuroendocrine Neoplasms of Frequent Localizations. Noncoding RNA. 2021;7. [PubMed] [DOI] [Full Text] |
| 77. | Panarelli N, Tyryshkin K, Wong JJM, Majewski A, Yang X, Scognamiglio T, Kim MK, Bogardus K, Tuschl T, Chen YT, Renwick N. Evaluating gastroenteropancreatic neuroendocrine tumors through microRNA sequencing. Endocr Relat Cancer. 2019;26:47-57. [PubMed] [DOI] [Full Text] |
| 78. | Malczewska A, Kos-Kudła B, Kidd M, Drozdov I, Bodei L, Matar S, Oberg K, Modlin IM. The clinical applications of a multigene liquid biopsy (NETest) in neuroendocrine tumors. Adv Med Sci. 2020;65:18-29. [PubMed] [DOI] [Full Text] |
| 79. | Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426-437. [PubMed] [DOI] [Full Text] |
| 80. | Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, Pacey S, Baird R, Rosenfeld N. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223-238. [PubMed] [DOI] [Full Text] |
| 81. | Gleeson FC, Voss JS, Kipp BR, Kerr SE, Van Arnam JS, Mills JR, Marcou CA, Schneider AR, Tu ZJ, Henry MR, Levy MJ. Assessment of pancreatic neuroendocrine tumor cytologic genotype diversity to guide personalized medicine using a custom gastroenteropancreatic next-generation sequencing panel. Oncotarget. 2017;8:93464-93475. [PubMed] [DOI] [Full Text] |
| 82. | Boons G, Vandamme T, Peeters M, Beyens M, Driessen A, Janssens K, Zwaenepoel K, Roeyen G, Van Camp G, Op de Beeck K. Cell-Free DNA From Metastatic Pancreatic Neuroendocrine Tumor Patients Contains Tumor-Specific Mutations and Copy Number Variations. Front Oncol. 2018;8:467. [PubMed] [DOI] [Full Text] |
| 83. | Zakka K, Nagy R, Drusbosky L, Akce M, Wu C, Alese OB, El-Rayes BF, Kasi PM, Mody K, Starr J, Shaib WL. Blood-based next-generation sequencing analysis of neuroendocrine neoplasms. Oncotarget. 2020;11:1749-1757. [PubMed] [DOI] [Full Text] |
| 84. | Burak GI, Ozge S, Cem M, Gulgun B, Zeynep DY, Atil B. The emerging clinical relevance of genomic profiling in neuroendocrine tumours. BMC Cancer. 2021;21:234. [PubMed] [DOI] [Full Text] |
| 85. | Jiménez B, Abellona U MR, Drymousis P, Kyriakides M, Clift AK, Liu DSK, Rees E, Holmes E, Nicholson JK, Kinross JM, Frilling A. Neuroendocrine Neoplasms: Identification of Novel Metabolic Circuits of Potential Diagnostic Utility. Cancers (Basel). 2021;13. [PubMed] [DOI] [Full Text] |
| 86. | Kos-Kudła B, Blicharz-Dorniak J, Strzelczyk J, Bałdys-Waligórska A, Bednarczuk T, Bolanowski M, Boratyn-Nowicka A, Borowska M, Cichocki A, Ćwikła JB, Falconi M, Foltyn W, Handkiewicz-Junak D, Hubalewska-Dydejczyk A, Jarząb B, Junik R, Kajdaniuk D, Kamiński G, Kolasińska-Ćwikła A, Kowalska A, Król R, Królicki L, Krzakowski M, Kunikowska J, Kuśnierz K, Lampe P, Lange D, Lewczuk-Myślicka A, Lewiński A, Lipiński M, Londzin-Olesik M, Marek B, Nasierowska-Guttmejer A, Nawrocki S, Nowakowska-Duława E, Pilch-Kowalczyk J, Rosiek V, Ruchała M, Siemińska L, Sowa-Staszczak A, Starzyńska T, Steinhof-Radwańska K, Sworczak K, Syrenicz A, Szawłowski A, Szczepkowski M, Wachuła E, Zajęcki W, Zemczak A, Zgliczyński W, Zieniewicz K. Diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol Pol. 2017;68:79-110. [PubMed] [DOI] [Full Text] |
| 87. | Malla S, Kumar P, Madhusudhan KS. Radiology of the neuroendocrine neoplasms of the gastrointestinal tract: a comprehensive review. Abdom Radiol (NY). 2021;46:919-935. [PubMed] [DOI] [Full Text] |
| 88. | Jawlakh H, Velikyan I, Welin S, Sundin A. 68 Ga-DOTATOC-PET/MRI and 11 C-5-HTP-PET/MRI are superior to 68 Ga-DOTATOC-PET/CT for neuroendocrine tumour imaging. J Neuroendocrinol. 2021;33:e12981. [PubMed] [DOI] [Full Text] |
| 89. | Dromain C, de Baere T, Lumbroso J, Caillet H, Laplanche A, Boige V, Ducreux M, Duvillard P, Elias D, Schlumberger M, Sigal R, Baudin E. Detection of liver metastases from endocrine tumors: a prospective comparison of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging. J Clin Oncol. 2005;23:70-78. [PubMed] [DOI] [Full Text] |
| 90. | Feuerlein S, Boll DT, Gupta RT, Ringe KI, Marin D, Merkle EM. Gadoxetate disodium-enhanced hepatic MRI: dose-dependent contrast dynamics of hepatic parenchyma and portal vein. AJR Am J Roentgenol. 2011;196:W18-W24. [PubMed] [DOI] [Full Text] |
| 91. | van Montfoort JE, Stieger B, Meijer DK, Weinmann HJ, Meier PJ, Fattinger KE. Hepatic uptake of the magnetic resonance imaging contrast agent gadoxetate by the organic anion transporting polypeptide Oatp1. J Pharmacol Exp Ther. 1999;290:153-157. [PubMed] |
| 92. | Tirumani SH, Jagannathan JP, Braschi-Amirfarzan M, Qin L, Balthazar P, Ramaiya NH, Shinagare AB. Value of hepatocellular phase imaging after intravenous gadoxetate disodium for assessing hepatic metastases from gastroenteropancreatic neuroendocrine tumors: comparison with other MRI pulse sequences and with extracellular agent. Abdom Radiol (NY). 2018;43:2329-2339. [PubMed] [DOI] [Full Text] |
| 93. | Caton MT Jr, Shinagare AB, Lee B, Tirumani SH. Optimization of timing of hepatocellular phase imaging after gadoxetate disodium injection for evaluation of patients with neuroendocrine tumor. Abdom Radiol (NY). 2020;45:2358-2369. [PubMed] [DOI] [Full Text] |
| 94. | Jamar F, Fiasse R, Leners N, Pauwels S. Somatostatin receptor imaging with indium-111-pentetreotide in gastroenteropancreatic neuroendocrine tumors: safety, efficacy and impact on patient management. J Nucl Med. 1995;36:542-549. [PubMed] |
| 95. | Meko JB, Doherty GM, Siegel BA, Norton JA. Evaluation of somatostatin-receptor scintigraphy for detecting neuroendocrine tumors. Surgery. 1996;120:975-83; discussion 983. [PubMed] [DOI] [Full Text] |
| 96. | Hofmann M, Maecke H, Börner R, Weckesser E, Schöffski P, Oei L, Schumacher J, Henze M, Heppeler A, Meyer J, Knapp H. Biokinetics and imaging with the somatostatin receptor PET radioligand (68)Ga-DOTATOC: preliminary data. Eur J Nucl Med. 2001;28:1751-1757. [PubMed] [DOI] [Full Text] |
| 97. | Wild D, Mäcke HR, Waser B, Reubi JC, Ginj M, Rasch H, Müller-Brand J, Hofmann M. 68Ga-DOTANOC: a first compound for PET imaging with high affinity for somatostatin receptor subtypes 2 and 5. Eur J Nucl Med Mol Imaging. 2005;32:724. [PubMed] [DOI] [Full Text] |
| 98. | Sadowski SM, Neychev V, Millo C, Shih J, Nilubol N, Herscovitch P, Pacak K, Marx SJ, Kebebew E. Prospective Study of 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography for Detecting Gastro-Entero-Pancreatic Neuroendocrine Tumors and Unknown Primary Sites. J Clin Oncol. 2016;34:588-596. [PubMed] [DOI] [Full Text] |
| 99. | Naswa N, Sharma P, Kumar A, Nazar AH, Kumar R, Chumber S, Bal C. Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic neuroendocrine tumors: a prospective single-center study. AJR Am J Roentgenol. 2011;197:1221-1228. [PubMed] [DOI] [Full Text] |
| 100. | Deppen SA, Liu E, Blume JD, Clanton J, Shi C, Jones-Jackson LB, Lakhani V, Baum RP, Berlin J, Smith GT, Graham M, Sandler MP, Delbeke D, Walker RC. Safety and Efficacy of 68Ga-DOTATATE PET/CT for Diagnosis, Staging, and Treatment Management of Neuroendocrine Tumors. J Nucl Med. 2016;57:708-714. [PubMed] [DOI] [Full Text] |
| 101. | van Essen M, Sundin A, Krenning EP, Kwekkeboom DJ. Neuroendocrine tumours: the role of imaging for diagnosis and therapy. Nat Rev Endocrinol. 2014;10:102-114. [PubMed] [DOI] [Full Text] |
| 102. | Kayani I, Bomanji JB, Groves A, Conway G, Gacinovic S, Win T, Dickson J, Caplin M, Ell PJ. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1,Tyr3-octreotate) and 18F-FDG. Cancer. 2008;112:2447-2455. [PubMed] [DOI] [Full Text] |
| 103. | Howe JR. The Supporting Role of (18)FDG-PET in Patients with Neuroendocrine Tumors. Ann Surg Oncol. 2015;22:2107-2109. [PubMed] [DOI] [Full Text] |
| 104. | Squires MH 3rd, Volkan Adsay N, Schuster DM, Russell MC, Cardona K, Delman KA, Winer JH, Altinel D, Sarmiento JM, El-Rayes B, Hawk N, Staley CA 3rd, Maithel SK, Kooby DA. Octreoscan Versus FDG-PET for Neuroendocrine Tumor Staging: A Biological Approach. Ann Surg Oncol. 2015;22:2295-2301. [PubMed] [DOI] [Full Text] |
| 105. | Sawicki LM, Deuschl C, Beiderwellen K, Ruhlmann V, Poeppel TD, Heusch P, Lahner H, Führer D, Bockisch A, Herrmann K, Forsting M, Antoch G, Umutlu L. Evaluation of 68Ga-DOTATOC PET/MRI for whole-body staging of neuroendocrine tumours in comparison with 68Ga-DOTATOC PET/CT. Eur Radiol. 2017;27:4091-4099. [PubMed] [DOI] [Full Text] |
| 106. | Riihimäki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. The epidemiology of metastases in neuroendocrine tumors. Int J Cancer. 2016;139:2679-2686. [PubMed] [DOI] [Full Text] |
| 107. | Hermans BCM, de Vos-Geelen J, Derks JL, Latten L, Liem IH, van der Zwan JM, Speel EM, Dercksen MW, Dingemans AC. Unique Metastatic Patterns in Neuroendocrine Neoplasms of Different Primary Origin. Neuroendocrinology. 2021;111:1111-1120. [PubMed] [DOI] [Full Text] |
| 108. | Menda Y, O'Dorisio TM, Howe JR, Schultz M, Dillon JS, Dick D, Watkins GL, Ginader T, Bushnell DL, Sunderland JJ, Zamba GKD, Graham M, O'Dorisio MS. Localization of Unknown Primary Site with 68Ga-DOTATOC PET/CT in Patients with Metastatic Neuroendocrine Tumor. J Nucl Med. 2017;58:1054-1057. [PubMed] [DOI] [Full Text] |
| 109. | Graham MM, Gu X, Ginader T, Breheny P, Sunderland JJ. 68Ga-DOTATOC Imaging of Neuroendocrine Tumors: A Systematic Review and Metaanalysis. J Nucl Med. 2017;58:1452-1458. [PubMed] [DOI] [Full Text] |
| 110. | Pfaehler E, Mesotten L, Kramer G, Thomeer M, Vanhove K, de Jong J, Adriaensens P, Hoekstra OS, Boellaard R. Repeatability of two semi-automatic artificial intelligence approaches for tumor segmentation in PET. EJNMMI Res. 2021;11:4. [PubMed] [DOI] [Full Text] |
| 111. | Häggström I, Schmidtlein CR, Campanella G, Fuchs TJ. DeepPET: A deep encoder-decoder network for directly solving the PET image reconstruction inverse problem. Med Image Anal. 2019;54:253-262. [PubMed] [DOI] [Full Text] |
| 112. | Wehrend J, Silosky M, Xing F, Chin BB. Automated liver lesion detection in 68Ga DOTATATE PET/CT using a deep fully convolutional neural network. EJNMMI Res. 2021;11:98. [PubMed] [DOI] [Full Text] |
| 113. | Rösch T, Lightdale CJ, Botet JF, Boyce GA, Sivak MV Jr, Yasuda K, Heyder N, Palazzo L, Dancygier H, Schusdziarra V. Localization of pancreatic endocrine tumors by endoscopic ultrasonography. N Engl J Med. 1992;326:1721-1726. [PubMed] [DOI] [Full Text] |
| 114. | Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172-173. [PubMed] [DOI] [Full Text] |
| 115. | Chung A, Kwan V. Endoscopic ultrasound: an overview of its role in current clinical practice. Australas J Ultrasound Med. 2009;12:21-29. [PubMed] [DOI] [Full Text] |
| 116. | Walczyk J, Sowa-Staszczak A. Diagnostic imaging of gastrointestinal neuroendocrine neoplasms with a focus on ultrasound. J Ultrason. 2019;19:228-235. [PubMed] [DOI] [Full Text] |
| 117. | Sahani DV, Bonaffini PA, Fernández-Del Castillo C, Blake MA. Gastroenteropancreatic neuroendocrine tumors: role of imaging in diagnosis and management. Radiology. 2013;266:38-61. [PubMed] [DOI] [Full Text] |
| 118. | Ginès A, Vazquez-Sequeiros E, Soria MT, Clain JE, Wiersema MJ. Usefulness of EUS-guided fine needle aspiration (EUS-FNA) in the diagnosis of functioning neuroendocrine tumors. Gastrointest Endosc. 2002;56:291-296. [PubMed] [DOI] [Full Text] |
| 119. | Ardengh JC, de Paulo GA, Ferrari AP. EUS-guided FNA in the diagnosis of pancreatic neuroendocrine tumors before surgery. Gastrointest Endosc. 2004;60:378-384. [PubMed] [DOI] [Full Text] |
| 120. | Pais SA, Al-Haddad M, Mohamadnejad M, Leblanc JK, Sherman S, McHenry L, DeWitt JM. EUS for pancreatic neuroendocrine tumors: a single-center, 11-year experience. Gastrointest Endosc. 2010;71:1185-1193. [PubMed] [DOI] [Full Text] |
| 121. | Manta R, Nardi E, Pagano N, Ricci C, Sica M, Castellani D, Bertani H, Piccoli M, Mullineris B, Tringali A, Marini F, Germani U, Villanacci V, Casadei R, Mutignani M, Conigliaro R, Bassotti G, Zullo A. Pre-operative Diagnosis of Pancreatic Neuroendocrine Tumors with Endoscopic Ultrasonography and Computed Tomography in a Large Series. J Gastrointestin Liver Dis. 2016;25:317-321. [PubMed] [DOI] [Full Text] |
| 122. | Chen HT, Xu GQ, Teng XD, Chen YP, Chen LH, Li YM. Diagnostic accuracy of endoscopic ultrasonography for rectal neuroendocrine neoplasms. World J Gastroenterol. 2014;20:10470-10477. [PubMed] [DOI] [Full Text] |
| 123. | Hasan MK, Hawes RH. EUS-guided FNA of solid pancreas tumors. Gastrointest Endosc Clin N Am. 2012;22:155-167, vii. [PubMed] [DOI] [Full Text] |
| 124. | Hasegawa T, Yamao K, Hijioka S, Bhatia V, Mizuno N, Hara K, Imaoka H, Niwa Y, Tajika M, Kondo S, Tanaka T, Shimizu Y, Kinoshita T, Kohsaki T, Nishimori I, Iwasaki S, Saibara T, Hosoda W, Yatabe Y. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy. 2014;46:32-38. [PubMed] [DOI] [Full Text] |
| 125. | Sugimoto M, Takagi T, Hikichi T, Suzuki R, Watanabe K, Nakamura J, Kikuchi H, Konno N, Waragai Y, Asama H, Takasumi M, Watanabe H, Obara K, Ohira H. Efficacy of endoscopic ultrasonography-guided fine needle aspiration for pancreatic neuroendocrine tumor grading. World J Gastroenterol. 2015;21:8118-8124. [PubMed] [DOI] [Full Text] |
| 126. | Crinò SF, Ammendola S, Meneghetti A, Bernardoni L, Conti Bellocchi MC, Gabbrielli A, Landoni L, Paiella S, Pin F, Parisi A, Mastrosimini MG, Amodio A, Frulloni L, Facciorusso A, Larghi A, Manfrin E. Comparison between EUS-guided fine-needle aspiration cytology and EUS-guided fine-needle biopsy histology for the evaluation of pancreatic neuroendocrine tumors. Pancreatology. 2021;21:443-450. [PubMed] [DOI] [Full Text] |
| 127. | Takase Y, Naito Y, Okabe Y, Ishida Y, Yamaguchi T, Abe H, Murata K, Ito T, Tanigawa M, Kawahara A, Yano H, Akiba J. Insulinoma-associated protein 1 expression in pancreatic neuroendocrine tumours in endoscopic ultrasound-guided fine-needle aspiration cytology: An analysis of 14 patients. Cytopathology. 2019;30:194-200. [PubMed] [DOI] [Full Text] |
| 128. | Cazacu IM, Semaan A, Stephens B, Swartzlander DB, Guerrero PA, Singh BS, Lungulescu CV, Danciulescu MM, Cherciu Harbiyeli IF, Streata I, Popescu C, Saftoiu A, Roy-Chowdhuri S, Maitra A, Bhutani MS. Diagnostic value of digital droplet polymerase chain reaction and digital multiplexed detection of single-nucleotide variants in pancreatic cytology specimens collected by EUS-guided FNA. Gastrointest Endosc. 2021;93:1142-1151.e2. [PubMed] [DOI] [Full Text] |