Published online Feb 7, 2021. doi: 10.3748/wjg.v27.i5.377
Peer-review started: October 20, 2020
First decision: December 18, 2020
Revised: December 25, 2020
Accepted: January 8, 2021
Article in press: January 8, 2021
Published online: February 7, 2021
Processing time: 100 Days and 16.1 Hours
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has undoubtedly revolutionized the whole globe and given a new point of view on respiratory tract infections. Nevertheless, coronavirus disease 2019 (COVID-19) cannot be perceived as a disease limited only to pneumonia with diverse severity. More and more reports have demonstrated a wide range of possible systemic symptoms, including hepatic complications. Liver injury has been observed in a significant proportion of patients, especially in those with a severe or critical illness. COVID-19 might provoke a deterioration of liver function in patients with already diagnosed chronic liver diseases and without pre-existing liver disorders. The deterioration of liver function worsens the prognosis, increases the risk of a severe course of SARS-CoV-2 infection and prolongs the hospital stay. In general, patients who develop liver dysfunction in COVID-19 are mainly males, elderly people, and those with higher body mass index. The underlying mechanisms for hepatic failure in patients infected with SARS-CoV-2 are still unclear, nevertheless liver damage appears to be directly connected with virus-induced cytopathic effects. A liver injury observed during hospitalization might be simultaneously caused by the use of potentially hepatotoxic drugs, mainly antiviral agents. This minireview focuses on a possible relationship between COVID-19 and the liver, potential molecular mechanisms of liver damage, the characteristics of liver injury and suggested factors predisposing to hepatic manifestations in COVID-19 patients.
Core Tip: The coronavirus disease 2019 (COVID-19) pandemic has revolutionized the priorities of the medical society worldwide. In the natural history of severe acute respiratory syndrome coronavirus-2 infection, liver injury is relatively frequent but quite mild and it is described as any liver damage occurring during disease progression and treatment of infection in patients with or without pre-existing liver disorders. Direct viral cytopathic injury, secondary liver impairment due to systemic inflammatory response or hypoxia, drug-induced liver failure and finally the exacerbation of chronic liver diseases are enumerated as potential etiologic factors for liver injury in COVID-19.
- Citation: Cichoż-Lach H, Michalak A. Liver injury in the era of COVID-19. World J Gastroenterol 2021; 27(5): 377-390
- URL: https://www.wjgnet.com/1007-9327/full/v27/i5/377.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i5.377
December 2019 was the crucial time, when the first cases of severe pneumonia and other infections of the respiratory tract were diagnosed in the city of Wuhan, located in Hubei province, central China. Information regarding the causative factor - severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) emerged and was the beginning of the diagnosis of coronavirus disease 2019 (COVID-19) according to World Health Organization nomenclature[1-3]. Since the start of 2020, COVID-19 has become a pandemic, spreading worldwide, and leading to the disruption of among human societies and significant economic destabilization. Up to October 10th, more than 39.4 million SARS-CoV-2 cases were identified in the world and more than 1.11 million patients died due to its severe course. The situation remains dynamic and new epidemiological data are actualized each day. Current medical knowledge associates SARS-CoV-2 with a spectrum of disorders involving the respiratory tract. COVID-19 mainly presents with dyspnea, pneumonia, dry cough and fever. Nevertheless, a possible manifestation of the disease may be closely related to pathologies of the gastrointestinal tract. Diarrhea, vomiting, abdominal pain or anorexia might precede respiratory symptoms or even behave as isolated signs of the illness[4-6].
In a multicenter study performed in Hubei province on 204 patients with COVID-19, researchers showed that the percentage of persons manifesting gastrointestinal symptoms was 50.5%. With the exception of the group with loss of appetite as the dominant complaint (a relatively low specificity for this symptom), gastrointestinal disorders were present in 18.6% of those enrolled in the study. Interestingly, in patients with digestive tract disorders, the period of time from the beginning of COVID-19 manifestations to hospitalization was significantly longer compared to those with typical respiratory infections (9 d and 7.3 d, respectively; P = 0.013). Another observation in this study was that a more severe course of COVID-19 was associated with more marked gastrointestinal symptoms[7]. Recent observations proved that gastrointestinal manifestations of SARS-CoV-2 infection may involve 39.6% to 50% of patients and usually include nausea, diarrhea, anorexia, abdominal pain, belching and emesis[8-10]. A complex presentation of SARS-CoV-2 infection involving skin disorders, impaired taste and smell, kidney dysfunction, acute heart failure and even multiple organ dysfunction syndrome, proves that COVID-19 is a systemic diseases. Early reports from Wuhan showed that only approximately 2%-10% of patients complained of diarrhea as the first symptom of the infection and in such cases, the virus genome could be isolated from stool and blood samples. However, a recent analysis of the clinical manifestations of SARS-CoV-2 infection in 992 patients revealed that 53% had at least one gastrointestinal symptom at any time during their illness, usually diarrhea (34%), nausea (27%), vomiting (16%), and abdominal pain (11%)[11-14]. Similarly, a broad spectrum of abnormalities can be found in laboratory tests, e.g., lymphopenia, monocytopenia, prolonged prothrombin time, hypo-proteinemia and even hypertransaminasemia. Nowadays, accumulated data suggest a harmful effect of SARS-CoV-2 on the liver; however, the number of available reports is still limited[15,16].
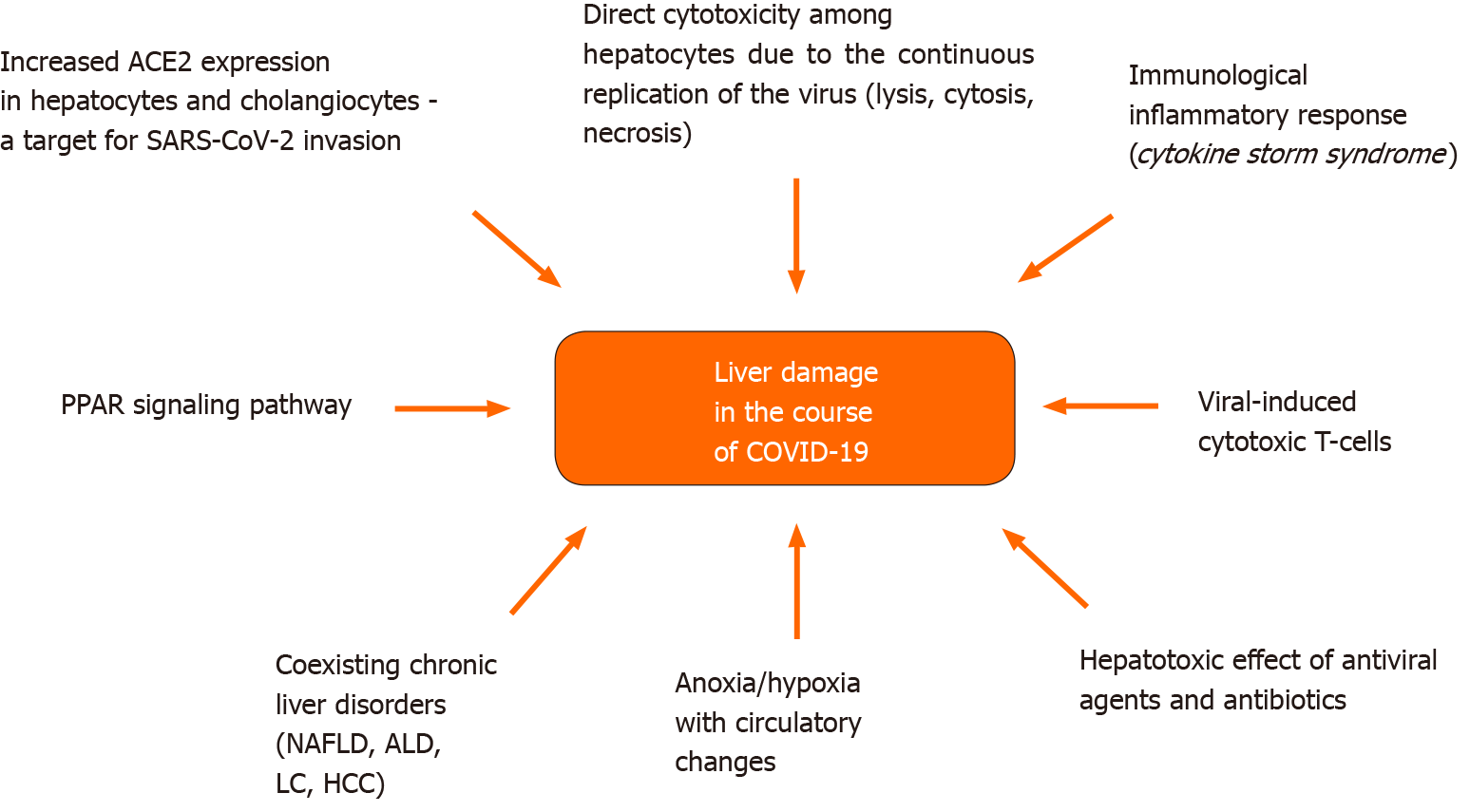
Angiotensin converting enzyme 2 (ACE2) appears to be a key agent in liver injury due to COVID-19. This metallopeptidase, which serves as a functional receptor for SARS-CoV-2, is not only localized on the surface of respiratory tract epithelium (in the lung-specific pulmonary alveolar type II cells), but also on epithelial cells of the upper esophagus, enterocytes of the ileum and colon, in the heart, testicles, cells of smooth muscles and the endothelium of pancreatic, brain and kidney blood vessels[17-20]. Furthermore, increased expression of ACE2 is also observed in cholangiocytes (59.7% of cells) and to a lesser extent in hepatocytes (2.6% of cells). This proves that SARS-CoV-2 infection impairs liver function by direct cytotoxicity due to the continuous replication of the virus in the above-mentioned cell populations[21,22]. Additionally, gene expression of ACE2 transmembrane serine protease 2 together with paired basic amino acid cleaving enzyme have also been proved in cholangiocytes and hepatocytes. Thus, SARS-CoV-2 may exert a cytopathic effect, either by lysis and/or by inducing necrosis and apoptosis[23-27]. ACE2 plays a fundamental role in host cells as a receptor of Spike-I Glycoprotein of COVID-19 which finally leads to infection. A recent finding revealed that the renin-angiotensin system and peroxisome proliferator-activated receptor signaling pathway can even enhance the infection at this stage. Therefore, both angiotensin and peroxisome proliferator-activated receptor family proteins may potentially be perceived as possible therapeutic targets[28,29]. Another survey indicated the potential role of viral-induced cytotoxic T-cells in the severity of the disease[30]. A subsequent pathological pathway responsible for liver dysfunction in COVID-19 is an immunological inflammatory response. This phenomenon has already been confirmed by high levels of inflammatory markers [e.g., C-reactive protein (CRP), ferritin, lactate dehydrogenase (LDH), D-dimers, interleukin-6 (IL-6), IL-2], suggesting a direct link between the presence of the cytokine storm syndrome and disease severity[31]. Furthermore, hepatic anoxia during the course of SARS-CoV-2-related respiratory failure cannot be omitted. On the other hand, antiviral agents in the treatment of COVID-19 (e.g., lopinavir, ritonavir, ramdevpir, umifenovir) or antibiotics used in the case of coexisting bacterial infections, antipyretics or steroids might also cause deterioration of liver function[32,33]. A group of patients with known chronic liver failure is more prone to developing hepatic complications in the presence of SARS-CoV-2 infection. Furthermore, chronic liver disorders are well-known independent causative factors of severe COVID-19. In a recently published survey of 2780 persons with COVID-19, 250 patients with known chronic liver disease (CLD) were at a higher risk of prolonged hospitalization and death[34]. Unfortunately, according to available data, it is difficult to predict which liver diseases are mostly dangerous. Of note, biologics such as tocilizumab and baricitinib administered in severe COVID-19 may lead to a reactivation of HBV infection. Thus, these patients require special supervision. On the other hand, a potential link between SARS-CoV-2 infection and cholestatic liver pathologies still remains an unexplored field[35]. Figure 1 presents the spectrum of mechanisms involved in liver damage accompanying COVID-19.
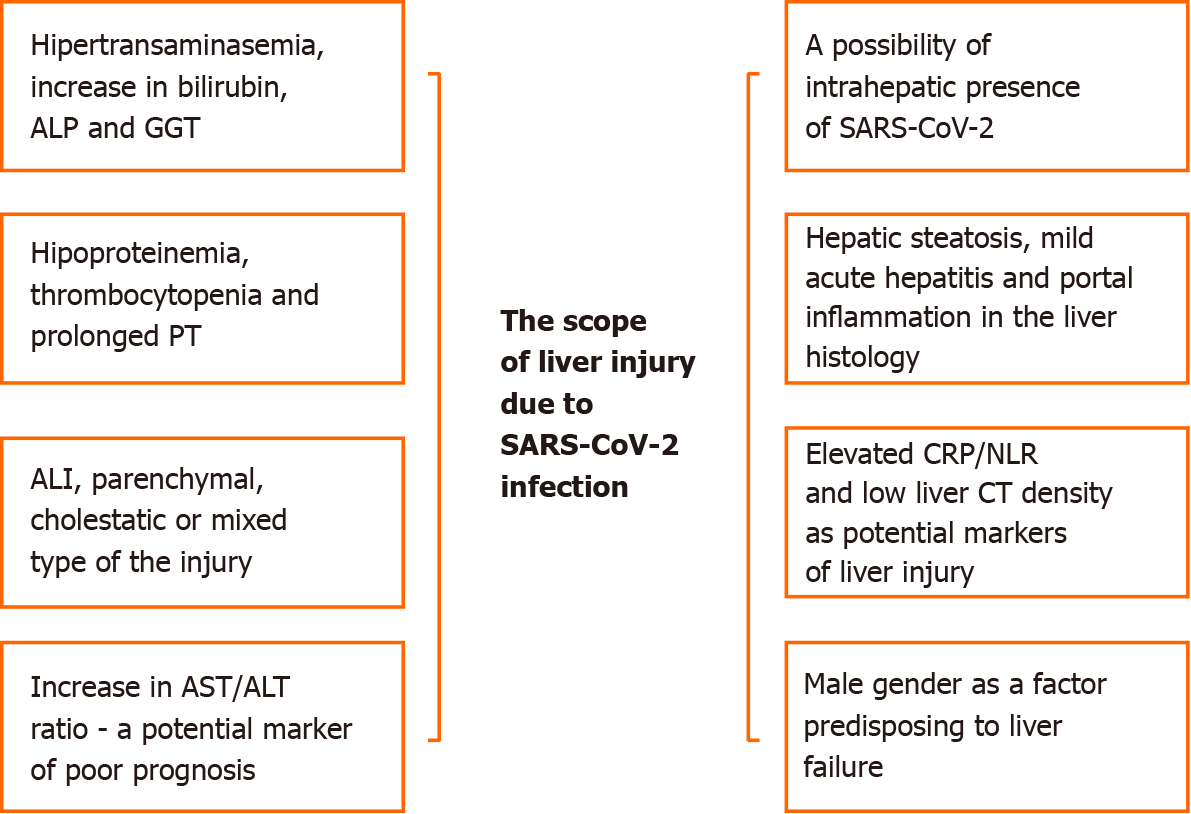
In a great majority of clinical studies, regardless of existing CLD, COVID-19 was associated with mild to moderate liver failure, reflected mainly by hyper-transaminasemia, elevation of gamma-glutamyl transferase (GGT) and alkaline phosphatase (ALP) levels (less frequently), hypoproteinemia and prolonged prothrombin time[36-40]. Accumulated data suggest that more than one-third of patients hospitalized due to SARS-CoV-2 infection might have impaired liver function. An increase in aspartate transaminase (AST) and alanine transaminase (ALT) activity, especially in men, results in a severe course of COVID-19. In general, a higher level of ALT, thrombocytopenia and hypoalbuminemia are indices of increased mortality in COVID-19 patients. Moreover, hypoalbuminemia is recognized as an independent marker of severe SARS-CoV-2 infection, poor prognosis and higher mortality[41-43]. The possible manifestations of liver injury during the course of COVID-19 are shown in Figure 2.
Some reports have even proved the presence of a correlation between abnormal liver tests and coagulation dysfunction in SARS-CoV-2 pneumonia, highlighting the significant role of the liver in this disease[44]. However, the data are too scanty to differentiate an exact background of hypertransaminasemia in COVID-19 patients – a pre-existing chronic liver failure or a certain hepatotoxic impact of SARS-CoV-2 infection.
In large cohort studies of hospitalized patients, an elevation in AST and ALT activity varied from 14% to 53% with reference to the normal range. A study of the clinical profile of COVID-19 patients with abnormal liver test results was performed by Cai et al[45]. This study included 417 Chinese patients hospitalized in Shenzhen. Deviations of liver enzymes were classified as parenchymal (a 3-fold increase in the values of ALT and/or AST above the upper limit of normal (ULN), cholestatic (ALP or GGT values 2-fold higher according to the ULN) and mixed (coexisting above-mentioned pathologies). Additionally, researchers identified a group of patients with liver injury defined as a 3-fold increase in AST and/or ALT above the ULN and a 2-fold increase in ALP, GGT and/or bilirubin above the ULN. Parameters of liver function were assessed on admission to the hospital and during hospitalization. 41% of the examined patients had impaired liver function tests on admission; 5% of them fulfilled the criteria mentioned in the identified subgroup. Deterioration of liver function was most common in men, elderly patients and those with a higher body mass index (BMI). Patients with chronic liver disorders [non-alcoholic fatty liver disease (NAFLD), alcohol-related liver disease (ALD), chronic hepatitis-B] also showed abnormal liver test results; however, the percentage of these conditions was not significant. The examined patients manifested cough as the first symptom of COVID-19. A moderate impairment of liver function was noted in a majority of study participants; the level of evaluated markers did not exceed the ULN by more than 2-fold (the results were higher in only 4% of patients). The most marked elevation was seen in GGT; its activity was 2-fold higher than the ULN in 12.71% of patients and 3-fold higher in 2.4%. An analysis of 417 people with COVID-19 revealed abnormal liver test results in 76.3% patients; 21.5% presented with features of liver injury. The presence of these impairments became more visible during the first two weeks of hospitalization with a higher than 3-fold increase in ALT, AST, bilirubin and GGT levels in 23.4%, 14.8%, 11.5%, and 24.4% of patients, respectively. 26.7% of patients with impaired liver function tests developed severe pneumonia. A mixed type of liver injury was the dominant type (43.4% of patients); parenchymal and cholestatic patterns were less common (20.75% and 29.25% of patients, respectively). An elevation in ALT, AST, bilirubin and GGT levels, exceeding a 3-fold increase according to the ULN, was noted in 10.38%, 2.83% and 11.64% of the study participants, respectively. A significant increase in ALP activity was not observed. Furthermore, a mixed type of liver injury dominated in patients with severe pneumonia due to SARS-CoV-2 infection. There was no significant difference in ALP level in this group compared to patients with a less severe disease course. 11.76% of patients were diagnosed with multiorgan insufficiency; 2 patients had liver insufficiency. Interestingly, nearly half of the patients with liver function impairment belonged to a subgroup with severe COVID-19. Activities of ALT and GGT 3-fold higher than the ULN were also more common in severe cases (41% and 37%, respectively). Increases in AST and bilirubin levels were less frequent and were observed in 20% and 10% of patients, respectively. Abnormal ALP levels were uncommon. 23.26% of severe COVID-19 patients (10 cases) developed multiorgan failure; 2 had liver insufficiency. Three patients died, one with liver failure. All systemic complications were directly related to secondary infections in patients hospitalized in intensive care units. Patients with impaired liver function tests (as previously defined) had a significantly higher risk of a severe course of COVID-19 – the group with a parenchymal subtype – 2-fold higher and mixed type – 4.44-fold [Odds ratio (OR): 2.73; confidence interval (CI): 1.19–6.30; P = 0.02; OR: 4.44; CI: 1.93–10.23; P < 0.001, respectively]. Liver failure (according to the above-mentioned definition) was a factor responsible for a 9-fold higher risk of severe COVID-19 in comparison to patients without hepatic complications (OR: 9.04; 95%CI: 3.19–25.6; P < 0.001). Parenchymal and mixed types of liver insufficiency were associated with a higher probability of the development of severe SARS-CoV-2 infection (3.19-fold and 11.22-fold, respectively). The severity of COVID-19 did not depend on the treatment with ACE inhibitors (ACEI) and angiotensin II receptor blockers. Furthermore, the patients treated with other antihypertensives did not have significant differences in SARS-CoV-2 infection presentation compared to those treated with ACEI/angiotensin II receptor blockers. The authors of this report did not find that antibiotics, nonsteroidal anti-inflammatory drugs, ribavirin or interferon provoked hepatic complications. Lopinavir and ritonavir were the only agents proved to deteriorate liver function (4.44-fold to 5.03-fold, respectively) in a dose-dependent manner, according to an analysis. The antivirals mainly caused an increase in bilirubin and GGT values. It is worth emphasizing that the percentage of patients with COVID-19 presenting with liver failure in the study was relatively high compared with previous reports and only a small subgroup of patients had CLD, suggesting the presence of a direct link between SARS-CoV-2 infection and viral infection of hepatocytes.
To date, the pathway of liver injury during the course of SARS-CoV-2 infection has not been fully explained, and this may be due to the pathogenetic mechanism of the virus or as a result of the use of hepatotoxic drugs. The majority of antipyretics used in COVID-19 patients contain acetaminophen, which is can lead to liver failure. The list of potential agents used in COVID-19 treatment is becoming longer and longer. Currently chloroquine phosphate or hydroxychloroquine sulfate, tocilizumab (IL-6 blocker), ribavirin, remdesivir (nucleotide analogue inhibiting viral ribonucleic acid polymerase), lopinavir/ritonavir and oseltamivir have been tested. There are still single reports on their potential hepatotoxic action, nevertheless this harmful effect is observed more frequently in severe COVID-19, with liver injury at baseline[46-49].
Remdesivir used in the treatment of COVID-19 pneumonia has recently been suspected to induce acute liver injury in two patients, who presented with hyper-transaminasemia, features of coagulopathy and encephalopathy between day 3 and day 10 of therapy. The introduction of acetylcysteine infusions restored the normal level of liver enzymes; however, one of the patients recovered and the other died, probably due to septic shock.
Data on a potential relationship between remdesivir and acute liver injury in the COVID-19 population are limited, but worth further exploration[50].
There are first reports in the literature regarding the use of ursodeoxycholic acid for COVID-19. Abdulrab et al[51] proposed adding this agent to the standard treatment due to its anti-inflammatory and immunomodulating activity. This strategy may be helpful in the increase in cytokines due to possible regulation of the immune response by ursodeoxycholic acid. The suggested dose is 13-15 mg/kg/day. Nevertheless, further detailed analyses are required[52].
Recently, EASL recommendations concerning hepatic care in the COVID-19 pandemic have been published. They present rules concerning the use of diagnostic procedures, supervision of patients with CLDs and those after liver transplantation[53].
Fan et al[54] tried to determine the potential relationship between liver dysfunction and concomitant treatment, by retrospectively enrolling 148 patients with COVID-19 hospitalized in Shanghai. The authors analyzed liver parameter values, treatment and the length of hospital stay. Impaired liver function (characterized by increased activity of aminotransferases, GGT, ALP and total bilirubin level) was diagnosed in 37.2% of patients on the day of hospital admission. Abnormal AST, ALT, GGT and bilirubin results were found in 21.6%, 18.2%, 17.6%, 6.1%, and 4.1% of patients, respectively. The presence of a high fever was more common in this group of patients. Increased levels of hepatic parameters were characteristic in the male population and were often accompanied by elevated levels of inflammatory markers (CRP and procalcitonin). This may have been related to the systemic inflammatory response due to SARS-CoV-2 infection. Similar to other analyses, increased ALP activity was least often observed[55]. Interestingly, the use of antibiotics (levofloxacin, azithromycin, cephalosporin), antiviral agents (umifenovir, oseltamivir, acyclovir) and antipyretics (ibuprofen) prior to hospitalization, was not related to liver function. 57.8% of patients treated with lopinavir or ritonavir presented with abnormal liver function. The duration of hospital stay in patients with deterioration of liver function was significantly prolonged (approximately 15.1 ± 4.8 d) compared to those without liver dysfunction (approximately 12.8 ± 4.4 d; P = 0.021). 48.5% of patients with normal liver function at baseline developed insufficiency approximately 7 d (from 4 to 11 d) after hospitalization. The peak of liver enzymes elevation was noted around day 10 (from 7 to 12 d) after hospital discharge. A less frequently observed peak of increased bilirubin concentration was present on day 5 after hospital discharge (from 4 to 12 d). The authors of the aforementioned publication concluded that abnormal liver function in SARS-CoV-2 infection predisposes to prolonged hospitalization. The observed results of ALT between 41 and 115 U/L and AST between 37 and 107 U/L, suggested mild liver function impairment related to COVID-19.
Comparable conclusions were drawn by Wang et al[56]. AST was evaluated in patients with a severe presentation of COVID-19 as a potential diagnostic marker and its level was significantly higher in non-survivors compared with survivors. Receiver operating characteristic (ROC) curve analysis of AST revealed an area under the curve (AUC) of 0.854, indicating the prognostic value of this marker in infected patients[57].
Furthermore, Medetalibeyoglu et al[58] stated that an AST/ALT ratio > 1 in 554 COVID-19 patients was associated with more frequent admission to the intensive care unit and with the severity of pneumonia, compared to those with an AST/ALT ratio < 1 (P = 0.033 and P = 0.016, respectively). An analysis of the AUC value under the ROC curve for the AST/ALT ratio revealed its high diagnostic accuracy in the prediction of mortality, severity of pneumonia and the risk of intensive care unit admission (AUC = 0.713/P = 0.001, AUC = 0.577/P = 0.002 and AUC = 0.636/P = 0.001, respectively)[58].
Accumulated data indicate that patients with severe pneumonia tend to develop liver failure more frequently. It can be assumed that the strong activity of proin-flammatory cytokines followed by a strong immune response are the main causative factors in this association[59,60].
Nevertheless, resources concerning the activity of less frequently assessed enzymes potentially involved in liver function impairment in COVID-19 patients are very sparse. LDH activity has been proved to increase especially in patients with abnormal liver tests. Fan et al[54] found an association between high LDH activity and an unfavorable course of COVID-19 in a patient who died due to respiratory in-sufficiency. Corresponding observations related to hyperactivity of LDH have been reported in patients with acute respiratory failure diagnosed with SARS-CoV and Middle-east respiratory syndrome infections, which resemble SARS-CoV-2 infection. Thus, LDH may be an independent prognostic marker of severe respiratory insufficiency. However, further analyses are required to determine its role among other alarm markers in the course of COVID-19[61,62].
The clinical presentation of acute liver injury (ALI) during the course of SARS-CoV-2 infection has been rarely presented in the literature. One of the surveys performed on 187 patients with COVID-19 revealed that 15.4% of patients were affected by ALI[63]. Another analysis by Fu et al[64] on the clinical characteristics of ALI due to COVID-19 in 355 patients showed a differential type of this complication. Its course was mild in 211 persons, was severe in 88 and was critical in the remaining 51 patients. Hypo-albuminemia was noted in 62.8% of patients, cholestasis in 42.5% and parenchymal liver failure was found in 28.5% of patients on hospital admission. ALI was frequently present in patients with critical COVID-19, suggesting that liver failure is an unfavorable marker of COVID-19. On the other hand, markers of systemic oxidation and the function of the respiratory tract correlated positively with the serological concentration of proteins, especially albumin. Therefore, a deterioration of respiratory tract function can at least partly induce ALI in COVID-19 patients. Severe cases of COVID-19 followed by death were more often related to hyper-transaminasemia and elevated level of bilirubin, compared to other types of the disease (mild and moderate). Hyperbilirubinemia and increased ALT, ALP and GGT levels were characteristic in the male population - in contrast to hypoalbuminemia. The listed parameters were also higher in elderly patients. Of note, coexisting arterial hypertension was a factor predisposing to greater activity of ALP and GGT. Male gender, older age and lymphopenia were three independent predictors of ALI in SARS-CoV-2 infection. Fu et al[64] observed that hypoproteinemia increased the risk of the death by 9-fold and cholestasis by 2-fold in comparison to patients without these pathologies (RR = 9.471, P < 0.001, RR = 2.182, P < 0.05). Abnormal liver function tests were long-lasting. Appropriate liver function was not restored within 14 d after hospital discharge; a value of at least one of the markers was elevated in two-thirds of patients during this time period. According to the above-listed observations, ALI during the course of COVID-19 is more common in men. It is worth emphasizing that males represent 58% of people infected with SARS-CoV-2[65].
A low concentration of testosterone was proposed by German scientists as one of the risk factors in patients with COVID-19, who were hospitalized in an emergency unit in Hamburg. This hormonal imbalance was noted in two-thirds of male patients on admission[66,67]. This theory is supported by a retrospective study conducted by Naaraayan et al[68] of 370 patients with COVID-19. Younger men (< 65 years) were found to be more likely to develop ALI (defined as elevation of AST and ALT levels greater than 3 x the ULN) compared to women (P = 0.02). Interestingly, in older patients, sex difference did not influence liver function.
Wang et al[69] concluded after studying 657 patients infected with SARS-CoV-2 in Wuhan that not only male sex, but also serum concentration of high sensitivity CRP ≥ 10 mg/L and a neutrophil-to-lymphocyte ratio ≥ 5 predispose to liver injury during the course of COVID-19, defined in this survey as a serum level of ALT or total bilirubin greater than the ULN. Thus, an inflammatory background may be closely related to liver function impairment as a complication of the disease[70,71]. Metabolic syndrome and isolated hepatic steatosis were also evaluated as potential risk factors for liver dysfunction during the course of SARS-CoV-2 infection. Ji et al[72] retro-spectively assessed 202 patients diagnosed with COVID-19; in 76 of these patients (37.6%) NAFLD was an accompanying condition. One half of the study participants manifested biochemical features of liver failure on the day of hospital admission and 75.2% of all patients during hospitalization. The majority of cases with liver function impairment had a parenchymal type; only 2.6% had the cholestatic or mixed type. In 33.2% of patients, liver dysfunction was observed during the hospital stay (one month at maximum). In a great majority of patients (80.7%) the course of COVID-19 was stable; only 19.3% of patients had severe disease progression. The group with severe SARS-CoV-2 infection was represented by elderly patients, those with higher BMI and the presence of other comorbidities (together with NAFLD). The risk of disease progression was 3-fold higher in men and close to 5-fold higher in patients aged sixty years and over (OR: 4.8; 95%CI: 1.5–16.2). Moreover, elevated BMI was responsible for a 1.3-fold higher risk of the development of complications (OR: 1.3; 95%CI: 1.0–1.8) and a 6-fold higher risk of comorbidities with NAFLD (OR: 6.3; 95%CI: 2.3–18.8 and OR 6.4; 95%CI: 1.5-31.2, respectively). COVID-19 patients with accompanying NAFLD were shown to have a significantly greater risk of complications during the course of the infection (44.7% vs 6.6%, P < 0.0001). A similar observation was seen with reference to the probability of prolonged liver function impairment from hospital admission to discharge (70% vs 11.1%, P < 0.0001). It is worth highlighting that the presence of NAFLD was directly related to the prolonged elimination of SARS-CoV-2 (17.5 ± 5.2 d vs 12.1 ± 4.4 d; P < 0.0001, respectively)[72]. Elements of the metabolic syndrome such as arterial hypertension, obesity and type 2 diabetes mellitus have been found to be associated with a severe presentation of COVID-19 and as independent markers of poor prognosis[73,74]. Another cohort study of 342 patients with COVID-19 revealed a relationship between hepatic steatosis and both transaminitis and increased disease severity. However, steatosis did not predispose to the development of clinically relevant liver insufficiency during the course of infection[75].
In addition, patients with metabolic syndrome and liver steatosis were found to be more prone to developing drug-induced liver injury after SARS-CoV-2 infection[76].
A novel observational cohort study by Kim et al[77] of 867 patients with CLDs and coexisting COVID-19 revealed that ALD, decompensated cirrhosis and hepatocellular carcinoma may be predictors of higher overall mortality during the course of infection. This observation was recently confirmed in a subsequent large cohort study of 745 patients with CLDs and concomitant SARS-CoV-2 infection (including 386 patients with cirrhosis and 359 without cirrhosis). Mortality among cirrhotic patients was significantly higher (32% vs 8%, P < 0.001) and increased according to liver function decompensation (Child-Turcotte-Pugh class). Respiratory failure was stated as the key cause of death (71%). Age, liver disease severity and ALD were found to be factors associated with death in all examined patients[78]. Another retrospective analysis of COVID-19 patients in Shanghai revealed that male gender, COVID-19 severity, together with a low liver CT density were causative factors strongly related to liver injury (ORs: 2.936, 6.543, and 3.387, respectively)[79]. In general, CLDs are known to be risk factors for severe COVID-19 infection (57.33%), and higher mortality (17.65%), as shown by Oyelade et al[80] among others in a recent meta-analysis. This phenomenon could be related to low platelets and lymphocytes in these patients, indicating that cirrhosis-associated immune dysfunction is a potential factor predisposing to a greater susceptibility of developing liver damage caused by SARS-CoV-2. Another meta-analysis of 11 observational studies involving 2043 COVID-19 positive patients revealed that the prevalence of CLDs ranged between 3% and 11%[81-83].
Data concerning the histological appearance of the liver in patients infected with SARS-CoV-2 are becoming more and more detailed, as the number of studies is increasing and the quality of our insight into the relationship between the infection and histopathological findings in the liver is improving. The first autopsy examinations were casuistic and concerned individual cases. A liver biopsy from a 69-year-old deceased man revealed mild steatosis of a small number of hepatocytes and their degeneration was probably caused by ischemia and hypoxia. Liver sinusoids were mildly infiltrated by neutrophils, plasmocytes and Kupffer cells[84]. Another autopsy performed in a 50-year-old male patient showed moderate vesicular steatosis and water degeneration in the liver together with a mild inflammatory process within the lobules and portal areas, suggesting that both SARS-CoV-2 and treatment could be the factors leading to this type of liver injury[85]. A study in Milan of 48 liver biopsies from post-mortem COVID-19 patients showed vascular changes in the portal vein, with a coexisting increased number of portal branches, terminal vessel dilations, and thrombi observed in portal and sinusoidal vessels. The features of inflammation were discrete, with mild portal and lobular infiltrates. The authors concluded that histopathological findings in COVID-19 are suggestive of impairments in the intrahepatic blood vessel network secondary to systemic alterations due to SARS-CoV-2. In addition, liver injury in COVID-19 patients might be induced through viral replication itself within hepatocytes, as SARS-CoV-2 binds cells through the ACE2 enzyme, especially in biliary epithelial cells[86]. However, relatively low serum aminotransferase concentrations present in COVID-19 patients do not suggest an exacerbated inflammatory response or direct viral injury to hepatocytes to be of crucial importance. The scheme of the aminotransferase curves in SARS-CoV-2 infection differs from those seen in hepatitis associated with other epidemic viruses that involve dynamic liver function test elevations due to intense parenchymal necrosis (e.g., dengue or yellow fever)[84,87-89]. On the other hand, the pattern of liver injury during the course of COVID-19 resembles that found in patients infected with other viruses, such as SARS, Middle-east respiratory syndrome and influenza[90-92]. Liver histology in other COVID-19 cases presented a mixed inflammatory infiltration with marked bile duct damage, features of endotheliitis and many apoptotic bodies. The intrahepatic presence of SARS-CoV-2 was even suggested in electron microscopy and in-situ hybridization, indicating the possibility of direct cell injury[93]. The ultrastructural assessment of postpartum liver tissue biopsies derived from two deceased COVID-19 patients by Wang et al[94] revealed typical coronavirus particles with their spikes in the cytoplasm of hepatocytes. Virus-related hepatocyte injury was described as mitochondrial swelling, endoplasmic reticulum dilatation, and cell membrane dysfunction. Of note, these authors documented viral ability to replicate in hepatocytes. This seems to be the first study showing the SARS-CoV-2 cytopathic liver cell effect as a background of liver function derangement. Viral ribonucleic acid was also identified in hepatocytes by Lagana et al[95] in the liver sections of 44 COVID-19 autopsies (in 11 of 20 examined patients). Polymerase chain reaction positivity correlated with peak creatinine and ferritin; however, there were no relationships with histological results or liver enzymes. The main findings in this study corresponded with other observations, as hepatic steatosis (75%), mild acute hepatitis (50%) and portal inflammation (50%) were the most common abnormalities.
Impaired liver diagnostic test results constitute common findings in COVID-19 patients and may affect over one-third of inpatients. Deterioration of liver function worsens the prognosis, increases the risk of severe SARS-CoV-2 infection and prolongs the duration of hospitalization. Abnormal liver function test results may be predictors of COVID-19 severity. COVID-19 patients affected by liver dysfunction are mainly male, elderly, and have a higher BMI. Liver injury observed during hospitalization might be simultaneously caused by the use of potentially hepatotoxic drugs, mainly antiviral agents such as lopinavir and ritonavir. Patients with accompanying chronic liver diseases are predisposed to developing a more severe course of COVID-19, but on the other hand, a more complicated presentation of SARS-CoV-2 infection increases the risk of liver failure.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fan X S-Editor: Zhang L L-Editor: Webster JR P-Editor: Liu JH
| 1. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17645] [Article Influence: 3529.0] [Reference Citation Analysis (0)] |
| 2. | Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5202] [Cited by in RCA: 4632] [Article Influence: 926.4] [Reference Citation Analysis (0)] |
| 3. | Asselah T, Durantel D, Pasmant E, Lau G, Schinazi RF. COVID-19: Discovery, diagnostics and drug development. J Hepatol. 2021;74:168-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 263] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 4. | Gao QY, Chen YX, Fang JY. 2019 Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21:125-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 5. | Puli S, Baig M, Walayat S. Gastrointestinal Symptoms and Elevation in Liver Enzymes in COVID-19 Infection: A Systematic Review and Meta-Analysis. Cureus. 2020;12:e9999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Noor FM, Islam MM. Prevalence and Associated Risk Factors of Mortality Among COVID-19 Patients: A Meta-Analysis. J Community Health. 2020;45:1270-1282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 7. | Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1160] [Cited by in RCA: 1205] [Article Influence: 241.0] [Reference Citation Analysis (0)] |
| 8. | Dahiya DS, Kichloo A, Albosta M, Pagad S, Wani F. Gastrointestinal implications in COVID-19. J Investig Med. 2020;68:1397-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Kopel J, Perisetti A, Gajendran M, Boregowda U, Goyal H. Clinical Insights into the Gastrointestinal Manifestations of COVID-19. Dig Dis Sci. 2020;65:1932-1939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 10. | Galanopoulos M, Gkeros F, Doukatas A, Karianakis G, Pontas C, Tsoukalas N, Viazis N, Liatsos C, Mantzaris GJ. COVID-19 pandemic: Pathophysiology and manifestations from the gastrointestinal tract. World J Gastroenterol. 2020;26:4579-4588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 98] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (5)] |
| 11. | Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 554] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 12. | Elmunzer BJ, Spitzer RL, Foster LD, Merchant AA, Howard EF, Patel VA, West MK, Qayed E, Nustas R, Zakaria A, Piper MS, Taylor JR, Jaza L, Forbes N, Chau M, Lara LF, Papachristou GI, Volk ML, Hilson LG, Zhou S, Kushnir VM, Lenyo AM, McLeod CG, Amin S, Kuftinec GN, Yadav D, Fox C, Kolb JM, Pawa S, Pawa R, Canakis A, Huang C, Jamil LH, Aneese AM, Glamour BK, Smith ZL, Hanley KA, Wood J, Patel HK, Shah JN, Agarunov E, Sethi A, Fogel EL, McNulty G, Haseeb A, Trieu JA, Dixon RE, Yang JY, Mendelsohn RB, Calo D, Aroniadis OC, LaComb JF, Scheiman JM, Sauer BG, Dang DT, Piraka CR, Shah ED, Pohl H, Tierney WM, Mitchell S, Condon A, Lenhart A, Dua KS, Kanagala VS, Kamal A, Singh VK, Pinto-Sanchez MI, Hutchinson JM, Kwon RS, Korsnes SJ, Singh H, Solati Z, Willingham FF, Yamchimski PS, Conwell DL, Mosier E, Azab M, Patel A, Buxbaum J, Wani S, Chak A, Hosmer AE, Keswani RN, DiMaio CJ, Bronze MS, Muthusamy R, Canto MI, Gjeorgjievski VM, Imam Z, Odish F, Edhi AI, Orosey M, Tiwari A, Patwardhan S, Brown NG, Patel AA, Ordiah CO, Sloan IP, Cruz L, Koza CL, Okafor U, Hollander T, Furey N, Reykhart O, Zbib NH, Damianos JA, Esteban J, Hajidiacos N, Saul M, Mays M, Anderson G, Wood K, Mathews L, Diakova G, Caisse M, Wakefield L, Nitchie H, Waljee AK, Tang W, Zhang Y, Zhu J, Deshpande AR, Rockey DC, Alford TB, Durkalski V; North American Alliance for the Study of Digestive Manifestations of COVID-19. Digestive Manifestations in Patients Hospitalized with COVID-19. Clin Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 13. | Kotfis K, Skonieczna-Żydecka K. COVID-19: gastrointestinal symptoms and potential sources of SARS-CoV-2 transmission. Anaesthesiol Intensive Ther. 2020;52:171-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12976] [Article Influence: 2595.2] [Reference Citation Analysis (1)] |
| 15. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration; UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6751] [Article Influence: 1350.2] [Reference Citation Analysis (0)] |
| 16. | Shanmugam C, Mohammed AR, Ravuri S, Luthra V, Rajagopal N, Karre S. COVID-2019 - A comprehensive pathology insight. Pathol Res Pract. 2020;216:153222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4149] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 18. | Fierro NA. COVID-19 and the liver: What do we know after six months of the pandemic? Ann Hepatol. 2020;19:590-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Metawea MI, Yousif WI, Moheb I. COVID 19 and liver: An A-Z literature review. Dig Liver Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (2)] |
| 20. | Wang F, Wang H, Fan J, Zhang Y, Wang H, Zhao Q. Pancreatic Injury Patterns in Patients With Coronavirus Disease 19 Pneumonia. Gastroenterology. 2020;159:367-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 325] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 21. | Loganathan S, Kuppusamy M, Wankhar W, Gurugubelli KR, Mahadevappa VH, Lepcha L, Choudhary AK. Angiotensin-converting enzyme 2 (ACE2): COVID 19 gate way to multiple organ failure syndromes. Respir Physiol Neurobiol. 2021;283:103548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 22. | Lizardo-Thiebaud MJ, Cervantes-Alvarez E, Limon-de la Rosa N, Tejeda-Dominguez F, Palacios-Jimenez M, Méndez-Guerrero O, Delaye-Martinez M, Rodriguez-Alvarez F, Romero-Morales B, Liu WH, Huang CA, Kershenobich D, Navarro-Alvarez N. Direct or Collateral Liver Damage in SARS-CoV-2-Infected Patients. Semin Liver Dis. 2020;40:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors AA, Osterhaus AD, van der Voort PH, Mulder DJ, van Goor H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251:228-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 739] [Article Influence: 147.8] [Reference Citation Analysis (0)] |
| 24. | Pirola CJ, Sookoian S. COVID-19 and ACE2 in the Liver and Gastrointestinal Tract: Putative Biological Explanations of Sexual Dimorphism. Gastroenterology. 2020;159:1620-1621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 445] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 26. | Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (2)] |
| 27. | Machhi J, Herskovitz J, Senan AM, Dutta D, Nath B, Oleynikov MD, Blomberg WR, Meigs DD, Hasan M, Patel M, Kline P, Chang RC, Chang L, Gendelman HE, Kevadiya BD. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J Neuroimmune Pharmacol. 2020;15:359-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 357] [Cited by in RCA: 355] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 28. | Chai X, Hu L, Zhang Y, Han W, LuZ, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Fan J, Lan F. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019 nCoV infection. bioRxiv. 2020;. [DOI] [Full Text] |
| 29. | Dey A, Sen S, Maulik U. Unveiling COVID-19-associated organ-specific cell types and cell-specific pathway cascade. Brief Bioinform. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Shi H, Wang W, Yin J, Ouyang Y, Pang L, Feng Y, Qiao L, Guo X, Shi H, Jin R, Chen D. The inhibition of IL-2/IL-2R gives rise to CD8+ T cell and lymphocyte decrease through JAK1-STAT5 in critical patients with COVID-19 pneumonia. Cell Death Dis. 2020;11:429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 31. | Fara A, Mitrev Z, Rosalia RA, Assas BM. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol. 2020;10:200160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 229] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 32. | Gupta R, Misra A. Contentious issues and evolving concepts in the clinical presentation and management of patients with COVID-19 infectionwith reference to use of therapeutic and other drugs used in Co-morbid diseases (Hypertension, diabetes etc). Diabetes Metab Syndr. 2020;14:251-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 33. | Lei P, Zhang L, Han P, Zheng C, Tong Q, Shang H, Yang F, Hu Y, Li X, Song Y. Liver injury in patients with COVID-19: clinical profiles, CT findings, the correlation of the severity with liver injury. Hepatol Int. 2020;14:733-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 34. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology 2020; 159: 768-771. e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 35. | Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020;40:1316-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 36. | Kullar R, Patel AP, Saab S. Hepatic Injury in Patients With COVID-19. J Clin Gastroenterol. 2020;54:841-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Deidda S, Tora L, Firinu D, Del Giacco S, Campagna M, Meloni F, Orrù G, Chessa L, Carta MG, Melis A, Spolverato G, Littera R, Perra A, Onali S, Zorcolo L, Restivo A. Gastrointestinal coronavirus disease 2019: epidemiology, clinical features, pathogenesis, prevention, and management. Expert Rev Gastroenterol Hepatol. 2021;15:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Napodano C, Pocino K, Stefanile A, Marino M, Miele L, Gulli F, Basile V, Pandolfi F, Gasbarrini A, Rapaccini GL, Basile U. COVID-19 and hepatic involvement: The liver as a main actor of the pandemic novel. Scand J Immunol. 2020;e12977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Wong YJ, Tan M, Zheng Q, Li JW, Kumar R, Fock KM, Teo EK, Ang TL. A systematic review and meta-analysis of the COVID-19 associated liver injury. Ann Hepatol. 2020;19:627-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 40. | Ghoda A, Ghoda M. Liver Injury in COVID-19 Infection: A Systematic Review. Cureus. 2020;12:e9487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Liu W, Tao ZW, Wang L, Yuan ML, Liu K, Zhou L, Wei S, Deng Y, Liu J, Liu HG, Yang M, Hu Y. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). 2020;133:1032-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 636] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 42. | Trevenzoli M, Guarnaccia A, Alberici I, Fassan M, Di Meco E, Farinati F, Cattelan AM. SARS-CoV-2 and hepatitis. J Gastrointestin Liver Dis. 2020;29:473-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Gholizadeh P, Safari R, Marofi P, Zeinalzadeh E, Pagliano P, Ganbarov K, Esposito S, Khodadadi E, Yousefi M, Samadi Kafil H. Alteration of Liver Biomarkers in Patients with SARS-CoV-2 (COVID-19). J Inflamm Res. 2020;13:285-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Chen S, Liu H, Li T, Huang R, Gui R, Zhang J. Correlation analysis of coagulation dysfunction and liver damage in patients with novel coronavirus pneumonia: a single-center, retrospective, observational study. Ups J Med Sci. 2020;125:293-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 46. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 47. | Bertolini A, van de Peppel IP, Bodewes FAJA, Moshage H, Fantin A, Farinati F, Fiorotto R, Jonker JW, Strazzabosco M, Verkade HJ, Peserico G. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology. 2020;72:1864-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 193] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 48. | Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1228] [Cited by in RCA: 1219] [Article Influence: 243.8] [Reference Citation Analysis (0)] |
| 49. | Li Y, Hu Y, Yu J, Ma T. Retrospective analysis of laboratory testing in 54 patients with severe- or critical-type 2019 novel coronavirus pneumonia. Lab Invest. 2020;100:794-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 50. | Carothers C, Birrer K, Vo M. Acetylcysteine for the Treatment of Suspected Remdesivir-Associated Acute Liver Failure in COVID-19: A Case Series. Pharmacotherapy. 2020;40:1166-1171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 51. | Abdulrab S, Al-Maweri S, Halboub E. Ursodeoxycholic acid as a candidate therapeutic to alleviate and/or prevent COVID-19-associated cytokine storm. Med Hypotheses. 2020;143:109897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 52. | Işık S, Karaman M, Çilaker Micili S, Çağlayan-Sözmen Ş, Bağrıyanık HA, Arıkan-Ayyıldız Z, Uzuner N, Karaman Ö. Beneficial effects of ursodeoxycholic acid via inhibition of airway remodelling, apoptosis of airway epithelial cells, and Th2 immune response in murine model of chronic asthma. Allergol Immunopathol (Madr). 2017;45:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (1)] |
| 54. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 55. | Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK; Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4155] [Cited by in RCA: 3821] [Article Influence: 764.2] [Reference Citation Analysis (1)] |
| 56. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 57. | Zhu Y, Du Z, Zhu Y, Li W, Miao H, Li Z. Evaluation of organ function in patients with severe COVID-19 infections. Med Clin (Engl Ed). 2020;155:191-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Medetalibeyoglu A, Catma Y, Senkal N, Ormeci A, Cavus B, Kose M, Bayramlar OF, Yildiz G, Akyuz F, Kaymakoglu S, Tukek T. The effect of liver test abnormalities on the prognosis of COVID-19. Ann Hepatol. 2020;19:614-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 59. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30115] [Article Influence: 6023.0] [Reference Citation Analysis (3)] |
| 60. | Yang RX, Zheng RD, Fan JG. Etiology and management of liver injury in patients with COVID-19. World J Gastroenterol. 2020;26:4753-4762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (2)] |
| 61. | Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, Lui SF, Szeto CC, Chung S, Sung JJ. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986-1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1563] [Cited by in RCA: 1602] [Article Influence: 72.8] [Reference Citation Analysis (1)] |
| 62. | Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, Flemban H, Al-Nassir WN, Balkhy HH, Al-Hakeem RF, Makhdoom HQ, Zumla AI, Memish ZA. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752-761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 969] [Cited by in RCA: 1030] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 63. | Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2516] [Cited by in RCA: 2842] [Article Influence: 568.4] [Reference Citation Analysis (0)] |
| 64. | Fu L, Fei J, Xu S, Xiang H, Xiang Y, Tan Z, Li M, Liu F, Li Y, Han M, Li X, Zhao H, Xu D. Acute liver injury and its association with death risk of patients with COVID-19: a hospital-based prospective case-cohort study. medRxiv. 2020;. [DOI] [Full Text] |
| 65. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18199] [Article Influence: 3639.8] [Reference Citation Analysis (0)] |
| 66. | Schroeder M, Tuku B, Jarczak D, Nierhaus A, Bai T, Jacobsen H, Zickler M, Mueller Z, Stanelle-Bertram S, Meinhardt A, Aberle J, Kluge S, Gabriel G. The majority of male patients with COVID-19 present low testosterone levels on admission to Intensive Care in Hamburg, Germany: a retrospective cohort study. medRxiv. 2020;. [DOI] [Full Text] |
| 67. | Pozzilli P, Lenzi A. Commentary: Testosterone, a key hormone in the context of COVID-19 pandemic. Metabolism. 2020;108:154252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 68. | Naaraayan A, Nimkar A, Hasan A, Pant S, Durdevic M, Elenius H, Nava Suarez C, Jesmajian S. Analysis of Male Sex as a Risk Factor in Older Adults With Coronavirus Disease 2019: A Retrospective Cohort Study From the New York City Metropolitan Region. Cureus. 2020;12:e9912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 69. | Wang M, Yan W, Qi W, Wu D, Zhu L, Li W, Wang X, Ma K, Ni M, Xu D, Wang H, Chen G, Yu H, Ding H, Xing M, Han M, Luo X, Chen T, Guo W, Xi D, Ning Q. Clinical characteristics and risk factors of liver injury in COVID-19: a retrospective cohort study from Wuhan, China. Hepatol Int. 2020;14:723-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 70. | Zhang H, Liao YS, Gong J, Liu J, Zhang H. Clinical characteristics and risk factors for liver injury in COVID-19 patients in Wuhan. World J Gastroenterol. 2020;26:4694-4702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 71. | Effenberger M, Grander C, Grabherr F, Griesmacher A, Ploner T, Hartig F, Bellmann-Weiler R, Joannidis M, Zoller H, Weiss G, Adolph TE, Tilg H. Systemic inflammation as fuel for acute liver injury in COVID-19. Dig Liver Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (2)] |
| 72. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 73. | Hussain A, Vasas P, El-Hasani S. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Bramante C, Tignanelli CJ, Dutta N, Jones E, Tamariz L, Clark JM, Usher M, Metlon-Meaux G, Ikramuddin S. Non-alcoholic fatty liver disease (NAFLD) and risk of hospitalization for Covid-19. medRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 75. | Chen VL, Hawa F, Berinstein JA, Reddy CA, Kassab I, Platt KD, Hsu CY, Steiner CA, Louissaint J, Gunaratnam NT, Sharma P. Hepatic Steatosis Is Associated with Increased Disease Severity and Liver Injury in Coronavirus Disease-19. Dig Dis Sci. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 76. | Boeckmans J, Rodrigues RM, Demuyser T, Piérard D, Vanhaecke T, Rogiers V. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020;94:1367-1369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 77. | Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen V, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin K, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch A, Viveiros K, Chan W, Chascsa D, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients with Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 78. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 79. | Guo H, Zhang Z, Zhang Y, Liu Y, Wang J, Qian Z, Zou Y, Lu H. Analysis of liver injury factors in 332 patients with COVID-19 in Shanghai, China. Aging (Albany NY). 2020;12:18844-18852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in Patients with Liver and Kidney Diseases: An Early Systematic Review and Meta-Analysis. Trop Med Infect Dis. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 81. | Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, Khare S, Srivastava A. Clinical Features of COVID-19 and Factors Associated with Severe Clinical Course: A Systematic Review and Meta-Analysis. SSRN. 2020;3566166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 82. | Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 1165] [Article Influence: 233.0] [Reference Citation Analysis (0)] |
| 83. | Qi X, Liu Y, Wang J, Fallowfield JA, Wang J, Li X, Shi J, Pan H, Zou S, Zhang H, Chen Z, Li F, Luo Y, Mei M, Liu H, Wang Z, Li J, Yang H, Xiang H, Li X, Liu T, Zheng MH, Liu C, Huang Y, Xu D, Li X, Kang N, He Q, Gu Y, Zhang G, Shao C, Liu D, Zhang L, Li X, Kawada N, Jiang Z, Wang F, Xiong B, Takehara T, Rockey DC; COVID-Cirrhosis-CHESS Group. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 84. | Brito CA, Barros FM, Lopes EP. Mechanisms and consequences of COVID-19 associated liver injury: What can we affirm? World J Hepatol. 2020;12:413-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 85. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5782] [Article Influence: 1156.4] [Reference Citation Analysis (2)] |
| 86. | Sonzogni A, Previtali G, Seghezzi M, Alessio MG, Gianatti A, Licini L, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver and COVID 19 infection: a very preliminary lesson learnt from histological post-mortem findings in 48 patients. Preprints 2020; 2020040438. [DOI] [Full Text] |
| 87. | Souza LJ, Alves JG, Nogueira RM, Gicovate Neto C, Bastos DA, Siqueira EW, Souto Filho JT, Cezário Tde A, Soares CE, Carneiro Rda C. Aminotransferase changes and acute hepatitis in patients with dengue fever: analysis of 1,585 cases. Braz J Infect Dis. 2004;8:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (2)] |
| 88. | Samanta J, Sharma V. Dengue and its effects on liver. World J Clin Cases. 2015;3:125-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 130] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (2)] |
| 89. | Escosteguy CC, Pereira AGL, Marques MRVE, Lima TRA, Galliez RM, Medronho RA. Yellow fever: profile of cases and factors associated with death in a hospital in the State of Rio de Janeiro, 2017-2018. Rev Saude Publica. 2019;53:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Chang HL, Chen KT, Lai SK, Kuo HW, Su IJ, Lin RS, Sung FC. Hematological and biochemical factors predicting SARS fatality in Taiwan. J Formos Med Assoc. 2006;105:439-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, Selim MA, Al Mutairi M, Al Nakhli D, Al Aidaroos AY, Al Sherbeeni N, Al-Khashan HI, Memish ZA, Albarrak AM. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 376] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 92. | Cao B, Li XW, Mao Y, Wang J, Lu HZ, Chen YS, Liang ZA, Liang L, Zhang SJ, Zhang B, Gu L, Lu LH, Wang DY, Wang C; National Influenza A Pandemic (H1N1) 2009 Clinical Investigation Group of China. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med. 2009;361:2507-2517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 423] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 93. | Fiel MI, El Jamal SM, Paniz-Mondolfi A, Gordon RE, Reidy J, Bandovic J, Advani R, Kilaru S, Pourmand K, Ward S, Thung SN, Schiano T. Findings of Severe Hepatic Severe Acute Respiratory Syndrome Coronavirus-2 Infection. Cell Mol Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 94. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 95. | Lagana SM, Kudose S, Iuga AC, Lee MJ, Fazlollahi L, Remotti HE, Del Portillo A, De Michele S, de Gonzalez AK, Saqi A, Khairallah P, Chong AM, Park H, Uhlemann AC, Lefkowitch JH, Verna EC. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147-2155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |