Published online Sep 28, 2021. doi: 10.3748/wjg.v27.i36.6128
Peer-review started: June 19, 2021
First decision: July 14, 2021
Revised: July 26, 2021
Accepted: August 13, 2021
Article in press: August 13, 2021
Published online: September 28, 2021
Processing time: 95 Days and 15 Hours
Multiple gastrointestinal stromal tumors (MGISTs) are specific and rare. Little is known about the impact of MGISTs on the survival of patients with gastrointestinal stromal tumors (GIST). The diagnosis, treatment and follow-up strategies of MGISTs is not specifically described in guidelines.
To compare the clinicopathological characteristics and prognosis of MGISTs and solitary GISTs (SGISTs)
Patients diagnosed with primary GISTs from March 2010 to January 2020 were included. Due to the inhomogeneous distribution of several baseline characteristics and uneven MGIST and SGIST group sizes, propensity score matching was performed according to comorbidities, body mass index, tumor location, mitotic index, sex, age and American Society of Anesthesiologists score. Differences in clinicopathological characteristics and prognosis between patients with MGISTs and patients with SGISTs were compared.
Among the entire cohort of 983 patients, the incidence of MGISTs was 4.17%. Before matching, patients with MGISTs and those with SGISTs had disparities in body mass index, surgical approach, tumor size and mitotic index. After 1:4 ratio matching, the clinical baseline data were comparable. The 5-year progression-free survival rate was 52.17% in the MGIST group and 75.00% in the SGIST group (P = 0.031). On multivariate analysis, tumor location, tumor size, mitotic index, imatinib treatment and MGISTs (hazard ratio = 2.431, 95% confidence interval = 1.097-5.386, P = 0.029) were identified as independent prognostic factors of progression-free survival. However, overall survival was similar between the SGIST and MGIST groups.
Patients with MGISTs had poorer progression-free survival than patients with SGISTs. Risk criteria and diagnostic and treatment strategies should be developed to achieve personalized precision therapy and maximize the survival benefit.
Core Tip: Whether the clinicopathological characteristics and long‑term survival of patients with multiple gastrointestinal stromal tumors are different from those of patients with solitary gastrointestinal stromal tumors is unclear. This is the first study to compare and describe these features. For accuracy and clarity, propensity score matching was used to balance the differences to explore the prognostic factors for patients with multiple gastrointestinal stromal tumors. To date, this study has the most detailed data and the largest number of patients, which may bring new insight to the diagnosis and treatment of multiple gastrointestinal stromal tumors.
- Citation: Wu H, Li C, Li H, Shang L, Jing HY, Liu J, Fang Z, Du FY, Liu Y, Fu MD, Jiang KW, Li LP. Clinicopathological characteristics and longterm survival of patients with synchronous multiple primary gastrointestinal stromal tumors: A propensity score matching analysis. World J Gastroenterol 2021; 27(36): 6128-6141
- URL: https://www.wjgnet.com/1007-9327/full/v27/i36/6128.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i36.6128
As one of the most common mesenchymal tumors with an incidence of approximately 10 per million population, gastrointestinal stromal tumors (GISTs) are receiving increasing attention[1-3]. GISTs are commonly located in the stomach and small intestine and rarely found in the esophagus, colon and rectum[4].
Multiple GISTs (MGISTs) refer to GISTs with two or more synchronous tumors in the gastrointestinal tract[5]. With the rapid advancement of precise diagnostic techniques and detailed pathological examinations, the detection and reporting of MGISTs have increased gradually. MGISTs accounted for nearly 2% of all GISTs in a multicenter study in China from 2001 to 2014[6]. However, due to the low incidence, there is currently no large-scale demographic survey showing the incidence of MGISTs.
Whether the clinical and pathological features of MGISTs are different from those of solitary GISTs (SGISTs) also remains unclear. Additionally, little is known about the impact of MGISTs on the survival of patients with GISTs. The diagnosis, treatment and follow-up strategies for MGISTs are not specifically described in the guidelines from the National Comprehensive Cancer Network, European Society for Medical Oncology and other academic institutions. Thus, we analyzed the clinicopathological characteristics and long-term survival of a large cohort of patients with MGISTs. It is urgent to gain insight into these questions to achieve personalized precision therapy in the future.
This retrospective cohort study was performed based on a prospectively collected database of GISTs at our hospital. All relevant procedures were approved by the Institutional Review Board. This study was designed in compliance with the Declaration of Helsinki and approved by the Ethics Committee of our hospital. The Reporting and Guidelines in propensity score analysis were also followed[7].
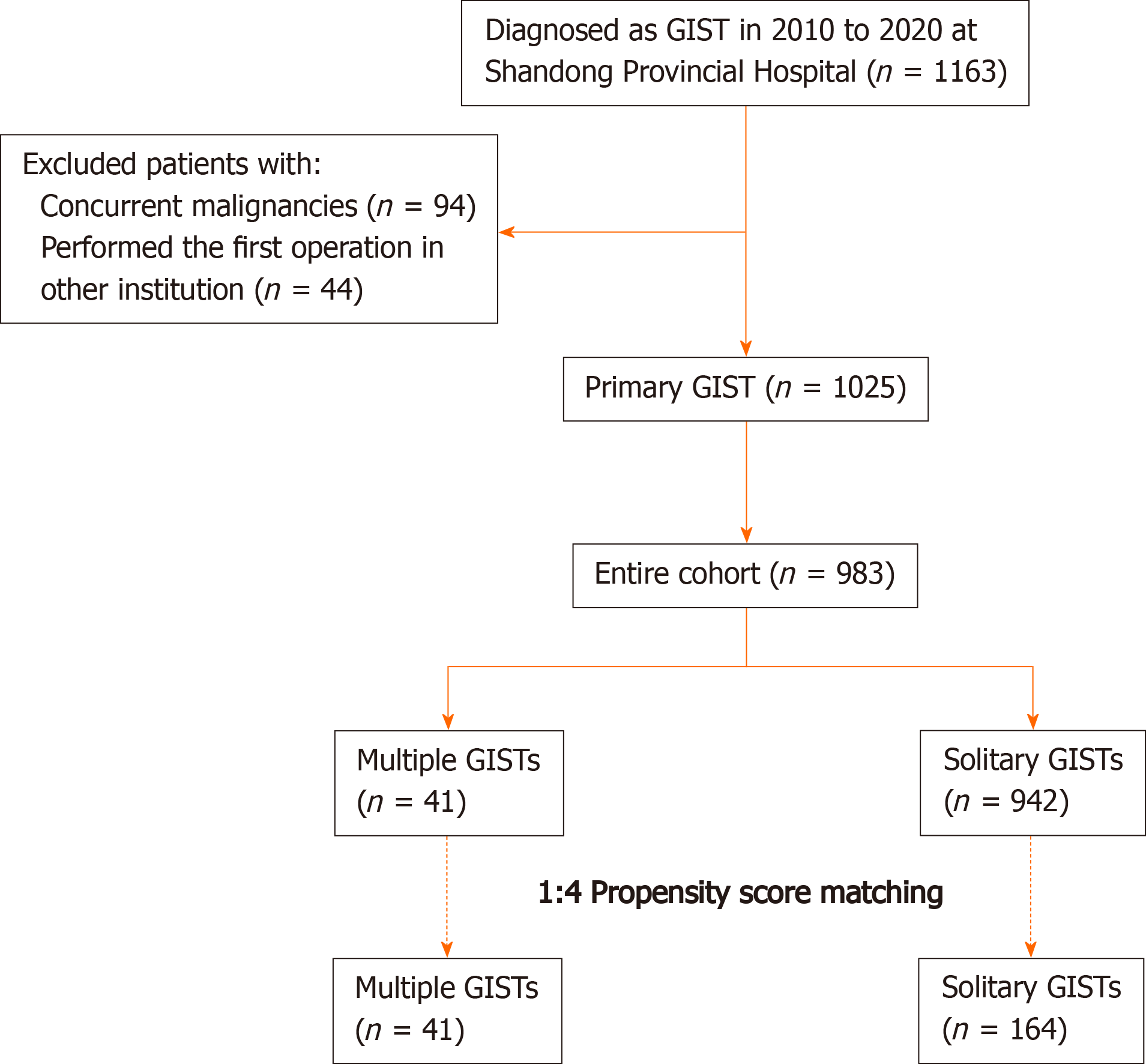
A total of 1163 consecutive patients diagnosed with GISTs and undergoing resection at our hospital between March 2010 and January 2020 were initially pooled; of whom, 1054 were classified as having primary GISTs (Figure 1). All oncological resections with curative intent were performed by senior surgeons specialized in achieving the rigorous standard at our institution. The inclusion criteria were: (1) Age > 18 years; (2) Pathological diagnosis of GIST; (3) No evidence of recurrent GIST or distant metastasis before treatment; and (4) Physiological status based on an Eastern Cooperative Oncology Group score < 3 points. The exclusion criteria were: (1) Any previous or concurrent malignancies; (2) First operation performed in other institutions; (3) Missing or illegible baseline information; and (4) Missing follow-up data. Finally, 983 patients with regular follow-up were included and analyzed. The follow-up was performed every 3 mo for the first 3 years, then every 6 mo up to 5 years, and then every year or until death in the following years. The latest follow-up date was December 2020.
The following clinicopathological characteristics were routinely collected from the GIST database: Age, sex, tobacco and alcohol use, body mass index (BMI), comorbidities, chief complaint, tumor location, tumor size, mitotic index (per 50 high power fields), American Society of Anesthesiologists score, modified National Institutes of Health risk category, surgical approach, intraoperative blood transfusion, operation time, postoperative complications, hospitalization time, postoperative imatinib, immunohistochemistry results and hematological indices. BMI was classified into the following categories: < 18.5, 18.5-24.9 and ≥ 25 kg/m2, based on the World Health Organization classification. The comorbidities analyzed comprised hypertension, diabetes mellitus, anemia, pulmonary disease (asthma, pneumonia, chronic obstructive pulmonary disease, etc.), heart disease (arrhythmia, coronary atherosclerotic heart disease, etc.), liver disease (hepatitis, cirrhosis, etc.), renal disease (nephritis, chronic kidney disease, etc.) and central nervous system disease (cerebrovascular disease, neurodegenerative disease, etc.).
The primary outcome was progression-free survival (PFS), which was defined as the interval between the date of resection and the date of confirmed disease progression or death. The secondary outcome was overall survival (OS), which was calculated from the date of surgery until the date of death. Patients were censored at the date of the last follow-up without the above event.
There is currently no authoritative and recognized definition of MGISTs. With reference to the criteria for multiple other cancers[8-11], especially multiple gastric cancers[12], we defined MGISTs as follows: (1) Each lesion must be pathologically proven; (2) All lesions must be separated microscopically; and (3) The possibility that one of the lesions represents a local extension of a metastatic tumor must be ruled out beyond reasonable doubt. It is because of the above criteria that all patients with any GIST outside the digestive tract (such as the omentum) were excluded. We defined MGISTs as two or more GISTs in the digestive tract. When MGISTs were different in size and mitotic index, the tumor was recorded according to the most advanced tumor. Similarly, in clinical practice, a modified National Institutes of Health risk category was also defined and assessed according to the most advanced tumor.
Categorical variables were analyzed using Pearson’s χ2 test or Fisher’s exact test, according to the expected values. The Mann–Whitney U test was utilized to compare continuous variables, which are presented as medians and interquartile ranges and as the mean ± SD. The Kaplan–Meier method and log-rank test were performed to conduct survival analyses and evaluate differences in survival time, respectively. Univariate and multivariate analyses were performed using the Cox proportional hazards model. Univariate analysis was primarily performed, and variables with P < 0.2 were subsequently input into the multivariate analysis to determine the independent prognostic factors. Hazard ratios with their 95% confidence intervals were also derived. Statistical significance was defined as P < 0.05. SPSS version 26.0 (IBM, Armonk, NY, United States) and R version 3.5.3 (The R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analysis.
Due to the inhomogeneous distribution of several baseline characteristics and uneven group sizes between patients with MGISTs and SGISTs, propensity score matching was performed. First, a propensity score was calculated using a logistic regression model in which the MGIST group was regressed as a dependent variable on relevant baseline parameters. The propensity score matching ratio was set to a 1:4 ratio to minimize the differences due to comorbidities, BMI, tumor location, mitotic index, sex, age and American Society of Anesthesiologist score with the nearest neighbor method. The assessment of propensity score matching is shown in Supplementary Figure 1.
The flow chart for the study is shown in Figure 1. Of the 983 consecutive patients who were pooled into the entire cohort between March 2010 and January 2020 at our institution, 41 (4.17%) with MGISTs were identified.
The preoperative clinical characteristics are described in Table 1. Before matching, most of the baseline characteristics were similar between the two groups, with significant differences only in BMI (P = 0.010). Regarding surgical and postoperative pathological characteristics and treatment of the SGISTs and MGISTs (Table 2), there were significant differences in surgical approach (P = 0.028), tumor size (P = 0.007), mitotic index (P = 0.009) and modified National Institutes of Health risk category (P = 0.044).
| Parameters | Entire cohort, before matching | P value | Propensity score matched cohort | P value | ||
| SGIST, n (%) | MGIST, n (%) | SGIST, n (%) | MGIST, n (%) | |||
| All cases | 942 | 41 | 164 | 41 | ||
| Age (yr) | 0.450 | 0.889 | ||||
| ≤ 60 | 516 (54.78) | 20 (48.78) | 82 (50.00) | 20 (48.78) | ||
| > 60 | 426 (45.22) | 21 (51.22) | 82 (50.00) | 21 (51.22) | ||
| Gender | 0.060 | 0.596 | ||||
| Male | 479 (50.85) | 27 (65.85) | 115 (70.12) | 27 (65.85) | ||
| Female | 463 (49.15) | 14 (34.15) | 49 (29.88) | 14 (34.15) | ||
| BMI (kg/m2) | 0.010 | 0.193 | ||||
| BMI < 18.5 | 41 (4.35) | 6 (14.63) | 11 (6.71) | 6 (14.63) | ||
| 18.5 ≤ BMI < 25 | 501 (53.18) | 19 (46.34) | 94 (57.32) | 19 (46.34) | ||
| BMI ≥ 25 | 400 (42.46) | 16 (39.02) | 59 (35.98) | 16 (39.02) | ||
| Tobacco use | 0.899 | 0.093 | ||||
| No | 704 (74.73) | 31 (75.61) | 101 (61.59) | 31 (75.61) | ||
| Yes | 238 (25.27) | 10 (24.39) | 63 (38.41) | 10 (24.39) | ||
| Alcohol use | 0.514 | 0.768 | ||||
| No | 687 (72.93) | 28 (60.98) | 108 (65.85) | 28 (60.98) | ||
| Yes | 255 (27.07) | 13 (31.71) | 56 (34.15) | 13 (31.71) | ||
| ASA score | 0.443 | 0.868 | ||||
| I/II | 779 (82.70) | 32 (78.05) | 126 (74.83) | 32 (78.05) | ||
| III/IV | 163 (17.30) | 9 (21.95) | 38 (23.17) | 9 (21.85) | ||
| Comorbidities1 | 0.236 | 1.000 | ||||
| Present | 417 (44.27) | 22 (53.66) | 88 (53.66) | 22 (53.66) | ||
| Absent | 525 (55.73) | 19 (46.34) | 76 (46.34) | 19 (46.34) | ||
| Location | 0.450 | 0.884 | ||||
| Gastric | 650 (69.00) | 26 (63.41) | 106 (64.63) | 26 (63.41) | ||
| Non-gastric | 292 (31.00) | 15 (36.58) | 58 (35.37) | 15 (36.58) | ||
| Chief complaint | ||||||
| Abdominal pain | 303 (32.17) | 15 (36.58) | 0.554 | 54 (32.93) | 15 (36.58) | 0.657 |
| Abdominal distention | 149 (15.82) | 9 (21.95) | 0.295 | 23 (14.02) | 9 (21.95) | 0.211 |
| Hematemesis | 42 (4.46) | 3 (7.32) | 0.429 | 9 (5.49) | 3 (7.32) | 0.710 |
| Hematochezia | 167 (17.72) | 9 (21.95) | 0.490 | 34 (20.73) | 9 (21.95) | 0.864 |
| Medical examination | 275 (29.19) | 11 (26.83) | 0.744 | 42 (25.61) | 11 (26.83) | 0.906 |
| Other | 96 (10.19) | 2 (4.88) | 0.266 | 17 (10.37) | 2 (4.88) | 0.278 |
| Parameters | Entire cohort, before matching | P value | Propensity score matched cohort | P value | ||
| SGIST, n (%) | MGIST, n (%) | SGIST, n (%) | MGIST, n (%) | |||
| All cases | 942 | 41 | 164 | 41 | ||
| Surgical approach | 0.028 | 0.361 | ||||
| Open | 458 (48.62) | 27 (65.85) | 94 (57.32) | 27 (65.85) | ||
| Laparoscopic | 255 (27.07) | 11 (26.83) | 43 (26.22) | 11 (26.83) | ||
| Endoscopic | 229 (24.31) | 3 (7.32) | 27 (14.63) | 3 (7.32) | ||
| Blood transfusion | 0.161 | 1.000 | ||||
| Yes | 171 (18.15) | 11 (26.83) | 44 (26.83) | 11 (26.83) | ||
| No | 771 (81.85) | 30 (73.17) | 120 (73.17) | 30 (73.17) | ||
| Tumor size (cm) | 0.007 | 0.200 | ||||
| Length ≤ 5 | 575 (61.04) | 15 (36.59) | 74 (45.12) | 15 (36.59) | ||
| 5 < Length ≤ 10 | 239 (25.37) | 16 (39.02) | 41 (25.00) | 16 (39.02) | ||
| Length > 10 | 128 (13.59) | 10 (24.39) | 49 (29.88) | 10 (24.39) | ||
| Mitotic index (per 50 HPF) | 0.009 | 0.715 | ||||
| 0-5 | 724 (76.86) | 27 (65.85) | 101 (61.59) | 27 (65.85) | ||
| 6-10 | 127 (13.48) | 4 (9.76) | 24 (14.63) | 4 (9.76) | ||
| > 10 | 91 (9.66) | 10 (24.39) | 39 (23.78) | 10 (24.39) | ||
| Modified NIH risk | 0.044 | 0.486 | ||||
| Very low | 210 (22.29) | 4 (9.76) | 29 (17.68) | 4 (9.76) | ||
| Low | 291 (30.89) | 9 (21.95) | 30 (18.29) | 9 (21.95) | ||
| Intermediate | 147 (15.61) | 8 (19.51) | 22 (13.41) | 8 (19.51) | ||
| High | 294 (31.21) | 20 (48.78) | 83 (50.61) | 20 (48.78) | ||
| Operate time (min) | 0.336 | 0.627 | ||||
| mean ± SD | 139 ± 78 | 151 ± 63 | 145 ± 67 | 151 ± 63 | ||
| Median (IQR) | 120 (80-180) | 150 (120-180) | 140 (90-190) | 150 (120-180) | ||
| Intraoperative tumor rupture | 0.376 | 0.490 | ||||
| Yes | 10 (1.06) | 1 (2.44) | 2 (1.22) | 1 (2.44) | ||
| No | 932 (98.94) | 40 (97.56) | 162 (98.78) | 40 (97.56) | ||
| Hospitalization time in d | 0.226 | 0.442 | ||||
| mean ± SD | 13.55 ± 6.32 | 14.78 ± 7.66 | 13.96 ± 5.69 | 14.78 ± 7.66 | ||
| Median (IQR) | 12 (10-15) | 14 (9-16) | 13 (11-16) | 14 (9-16) | ||
| Postoperative complications | 0.391 | 0.610 | ||||
| Present | 179 (19.00) | 10 (24.39) | 34 (20.73) | 10 (24.39) | ||
| Absent | 763 (81.00) | 31 (73.61) | 130 (79.27) | 31 (73.61) | ||
| Imatinib treatment | 0.187 | 0.832 | ||||
| Yes | 298 (31.63) | 17 (41.46) | 71 (43.29) | 17 (41.46) | ||
| No | 644 (68.37) | 24 (58.54) | 93 (56.71) | 24 (58.54) | ||
After propensity score matching at a 1:4 ratio, all baseline characteristics of the 41 patients in the MGIST group were compared with those of the 164 patients in the SGIST group (Tables 1 and 2). Supplementary pathological characteristics and blood indicators are shown in Supplementary Table 1. We also listed the clinical characteristics of patients with multiple tumors in detail (Supplementary Table 2) and showed several pathological images from these patients (Supplementary Figure 2).
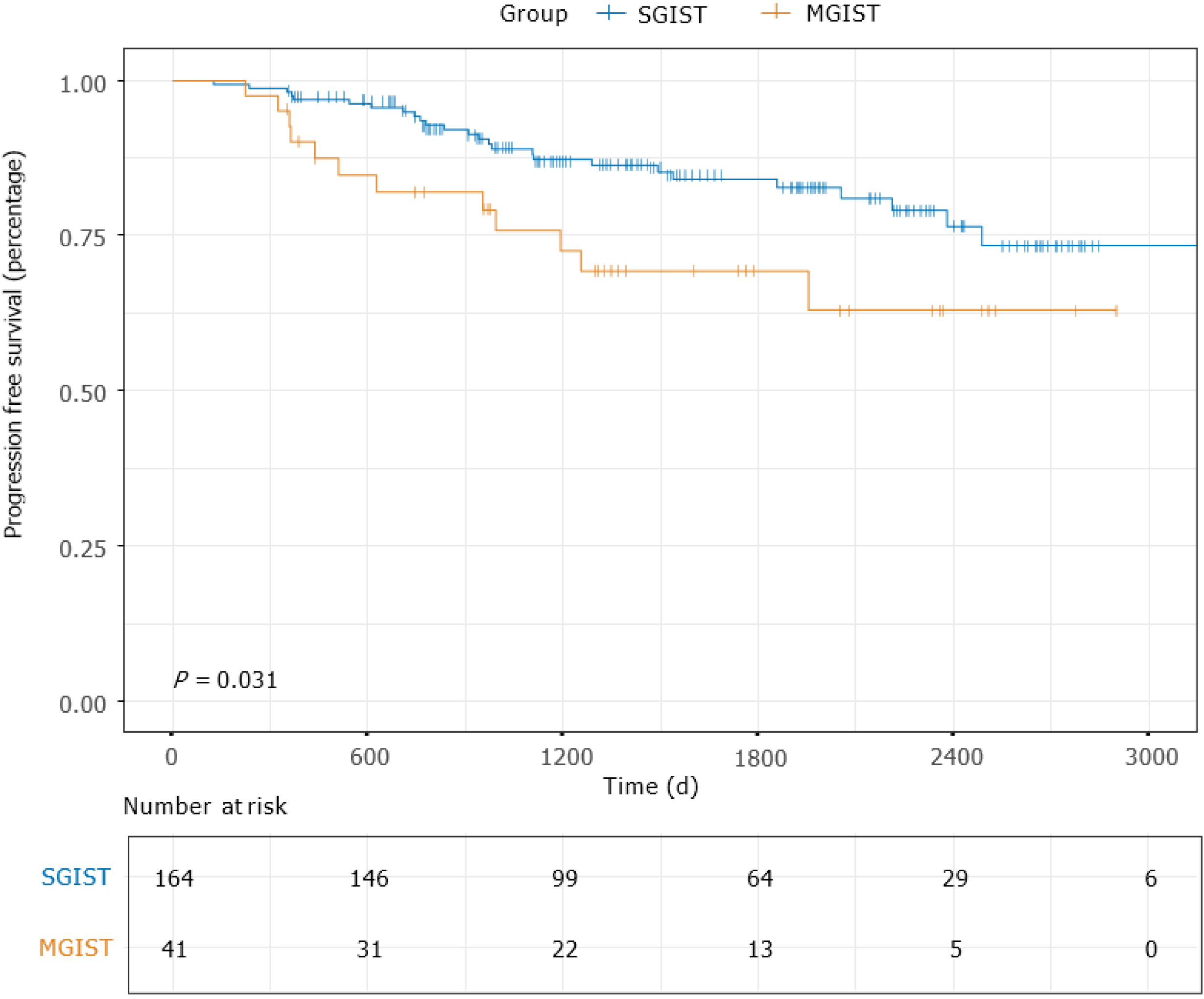
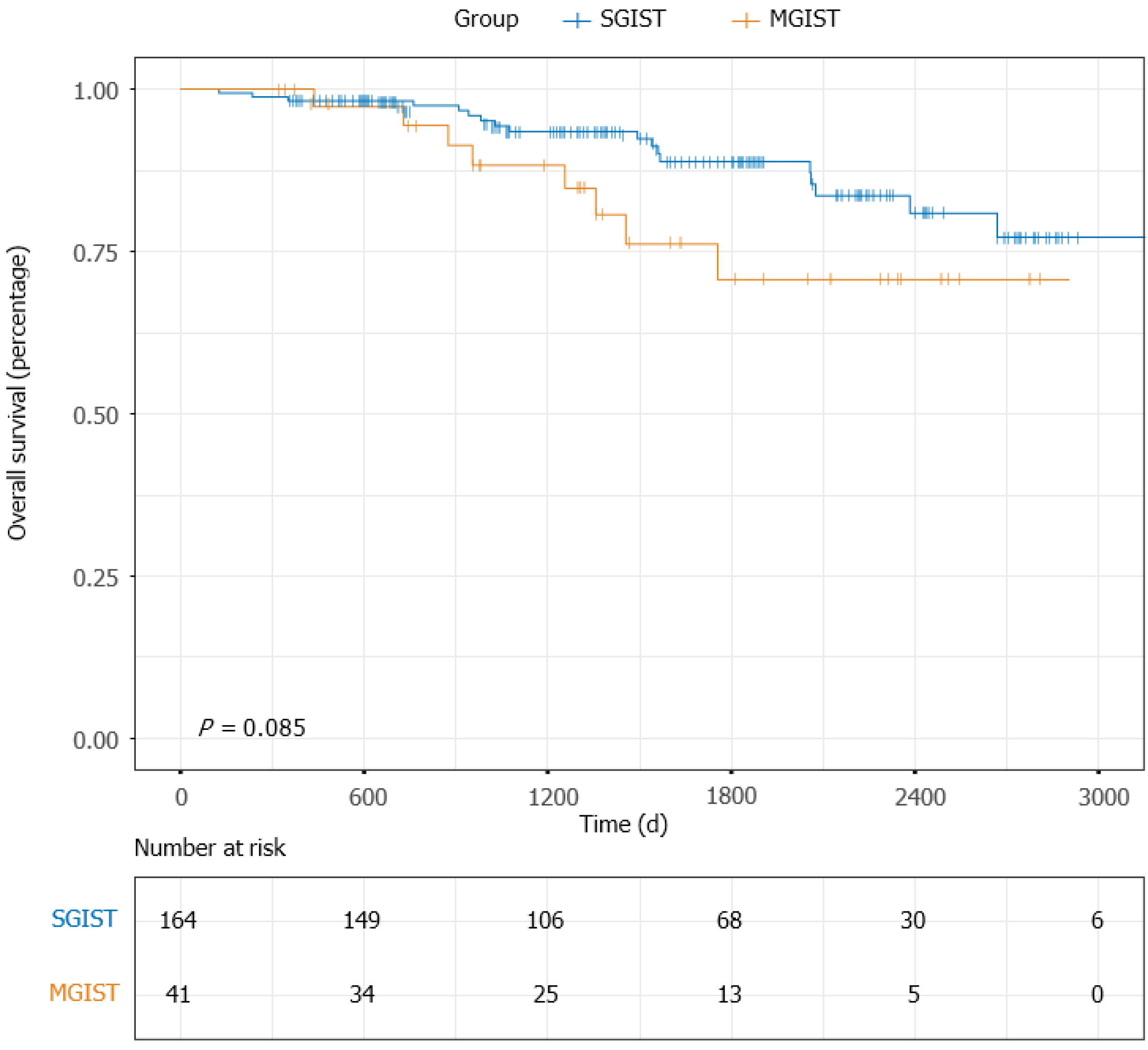
The median follow-up time of the entire matched cohort was 1468 d (IQR, 938-2225 d), and the 1-, 3- and 5-year PFS rates were 96.06%, 83.66% and 70.09%, respectively. For the patients with MGISTs, the 1-, 3- and 5-year PFS rates were 90.00%, 74.19% and 52.17%, respectively and compared with the 1-, 3- and 5-year PFS rates of 97.55%, 86.78% and 75.00% for the patients with SGISTs (P = 0.031) (Figure 2). We continued to explore the impact of MGISTs on OS. The 1-, 3- and 5-year OS rates were 98.16%, 92.50% and 83.75% for patients with SGISTs compared with 100%, 86.67% and 60.00% for patients with MGISTs, respectively, with no significant differences (P = 0.085) (Figure 3).
Univariate analysis identified the following prognostic factors for PFS: age (P = 0.069), tumor location (P < 0.001), tumor size (P = 0.001), mitotic index (P < 0.001), blood transfusion (P = 0.018), intraoperative tumor rupture (P = 0.011), imatinib treatment (P = 0.133) and MGISTs (P = 0.035). On multivariate analysis, tumor location (P = 0.002), tumor size (P = 0.035), mitotic index (P = 0.001), imatinib treatment (P < 0.001) and MGISTs (P = 0.029) were eventually identified as independent prognostic factors for PFS (Table 3).
| Characteristics | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age in yr | ||||
| ≤ 60 | Reference | Reference | ||
| > 60 | 1.845 (0.953-3.571) | 0.069 | 1.525 (0.740-3.143) | 0.252 |
| Sex | ||||
| Female | Reference | |||
| Male | 0.913 (0.460-1.809) | 0.793 | ||
| Comorbidities | ||||
| Absent | Reference | |||
| Present | 1.147 (0.606-2.172) | 0.673 | ||
| Tumor location | ||||
| Gastric | Reference | Reference | ||
| Non-gastric | 3.548 (1.847-6.818) | < 0.001 | 3.176 (1.526-6.607) | 0.002 |
| Tumor size in cm | 0.001 | 0.035 | ||
| Length ≤ 5 | Reference | Reference | ||
| 5 < Length ≤ 10 | 3.022 (1.117-8.179) | 0.029 | 2.180 (0.716-6.642) | 0.170 |
| Length > 10 | 5.848 (2.359-14.499) | < 0.001 | 4.071 (1.375-12.052) | 0.011 |
| Mitotic index, per 50 HPF | < 0.001 | 0.001 | ||
| 0-5 | Reference | Reference | ||
| 6-10 | 4.094 (1.555-10.777) | 0.004 | 4.108 (1.309-12.897) | 0.015 |
| > 10 | 7.859 (3.676-16.799) | < 0.001 | 6.577 (2.540-17.029) | < 0.001 |
| Surgical approach | 0.251 | |||
| Open | Reference | |||
| Laparoscopic | 0.449 (0.174-1.154) | 0.096 | ||
| Endoscopic | 0 (0-1.006E+230) | 0.961 | ||
| Blood transfusion | ||||
| No | Reference | Reference | ||
| Yes | 2.176 (1.143-4.145) | 0.018 | 1.569 (0.739-3.332) | 0.241 |
| Intraoperative tumor rupture | ||||
| No | Reference | Reference | ||
| Yes | 6.383 (1.524-26.726) | 0.011 | 4.458 (0.863-23.015) | 0.074 |
| Imatinib treatment | ||||
| Yes | Reference | Reference | ||
| No | 0.584 (0.290-1.178) | 0.133 | 4.608 (2.030-10.461) | < 0.001 |
| MGIST | ||||
| Absent | Reference | Reference | ||
| Present | 2.091 (1.055-4.145) | 0.035 | 2.431 (1.097-5.386) | 0.029 |
Univariate analysis revealed that tumor location (P < 0.001), tumor size (P = 0.021), mitotic index (P < 0.001), blood transfusion (P = 0.008), intraoperative tumor rupture (P = 0.113), imatinib treatment (P = 0.030) and MGISTs (P = 0.092) were correlated with OS. Subsequent multivariate analysis showed that tumor location (P = 0.003), mitotic index (P= 0.015) and imatinib treatment (P < 0.001) could be identified as independent risk factors for OS (Table 4). OS of patients with MGISTs (P = 0.106) was similar to that of patients with a single GIST.
| Characteristics | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age in yr | ||||
| ≤ 65 | Reference | |||
| > 65 | 1.665 (0.754-3.676) | 0.207 | ||
| Gender | ||||
| Female | Reference | |||
| Male | 1.099 (0.461-2.616) | 0.832 | ||
| Comorbidities | ||||
| Absent | Reference | |||
| Present | 1.061 (0.491-2.291) | 0.881 | ||
| Tumor location | ||||
| Gastric | Reference | Reference | ||
| Non-gastric | 4.428 (1.967-9.965) | < 0.001 | 3.812 (1.577-9.213) | 0.003 |
| Tumor size in cm | 0.021 | 0.556 | ||
| Length ≤ 5 | Reference | Reference | ||
| 5 < Length ≤ 10 | 2.279 (0.722-7.189) | 0.160 | 1.381 (0.346-5.519) | 0.648 |
| Length > 10 | 4.159 (1.498-11.549) | 0.006 | 2.070 (0.539-7.959) | 0.290 |
| Mitotic index, per 50 HPF | < 0.001 | 0.015 | ||
| 0-5 | Reference | Reference | ||
| 6-10 | 3.120 (0.913-10.669) | 0.070 | 4.129 (0.963-17.707) | 0.056 |
| > 10 | 7.515 (3.045-18.548) | < 0.001 | 6.051 (1.762-20.778) | 0.004 |
| Surgical approach | 0.684 | |||
| Open | Reference | |||
| Laparoscopic | 0.621 (0.213-1.815) | 0.384 | ||
| Endoscopic | 0 (0-3.609E+275) | 0.968 | ||
| Blood transfusion | ||||
| No | Reference | Reference | ||
| Yes | 2.832 (1.312-6.113) | 0.008 | 1.298 (0.547-3.082) | 0.554 |
| Intraoperative tumor rupture | ||||
| No | Reference | Reference | ||
| Yes | 5.094 (0.682-38.043) | 0.113 | 3.264 (0.355-30.022) | 0.296 |
| Imatinib treatment | ||||
| Yes | Reference | Reference | ||
| No | 2.952 (1.113-7.831) | 0.030 | 7.841 (2.677-22.693) | < 0.001 |
| MGIST | ||||
| Absent | Reference | Reference | ||
| Present | 2.050 (0.889-4.726) | 0.092 | 2.404 (0.830-6.966) | 0.106 |
MGISTs are often found in clinical treatment but ignored or misinterpreted as recurrence or metastasis. With the current lack of convincing results from large-scale studies based on demographics or clinicopathological characteristics, this is the first study to analyze the clinicopathological differences between patients with MGISTs and those with SGISTs. For accuracy and clarity, propensity score matching was used to balance the differences to explore the prognostic factors for patients with MGISTs.
A total of 983 patients were included in the cohort, including 41 with MGISTs. MGISTs accounted for approximately 4.17% of all GISTs in our study. With a median age of 60 years, patients with MGISTs had a similar age at initial diagnosis as patients with SGISTs. The incidence of GISTs is almost equal in men and women[13]; however, in our study, a male predisposition (M/F=27/14) was observed.
The BMI of patients with MGISTs was significantly lower than that of patients with SGISTs, which was pointed out for the first time, but the reason for this finding is not clear. Smoking and alcohol consumption did not affect the occurrence of MGISTs. There were no significant differences in American Society of Anesthesiologists classification or comorbidities, which means that the patients were in similar physical conditions at the time of diagnosis.
There was no significant difference in the chief complaint, which also implies difficulty in clinical diagnosis. MGISTs are often reported to be associated with type 1 neurofibromatosis[14], the Carney triad[15] and Carney–Stratakis syndrome[16]. In the records of our medical center, there was only one patient with neurofibromatosis, who has been reported in our previous study[17]. However, this patient was not included in this cohort because of concurrent colon cancer. Familial and pediatric GISTs are also associated with multicentric risk[18]. However, these types of MGISTs were not found in our study. This may be due to bias in the publication of case reports on these particular patients or the fact that the examination strategy has not been perfected so that such patients are missed by our colleagues. In addition, all cases of GISTs predominantly affected a single organ, and we should still pay attention to patients with multiorgan involvement to prevent misdiagnosis.
Imaging examination is an important basis for diagnosis[19]. Computed tomography or enhanced computed tomography is currently recommended but does not play an adequate role in the diagnosis of MGISTs. Of the 41 MGIST patients in our hospital, 38 underwent computed tomography examination, but only four were diagnosed accordingly. None of the MGISTs < 1 cm were detected, but this may be due to the large size of the major tumor or insufficient imaging evidence to diagnose the small tumor. For endoscopic examination, 8 of the 26 patients were suspected to have MGISTs before surgery, but endoscopy only revealed the tumor growing into the intestinal cavity, and none of the 15 cases of small intestinal MGISTs was detected. No case of MGISTs was diagnosed by B-ultrasonography or upper digestive tract radiography. Magnetic resonance imaging might be useful for diagnosis but is seldom used in ordinary examinations. Micro-GISTs, with low or no mitotic activity and little clinical significance, are common in the stomach (20%-35%)[20,21] and can transform to clinical GISTs by unknown mechanisms. Therefore, the development or modification of an examination method for preoperative screening of MGISTs can develop a more appropriate treatment plan for patients and obtain a greater survival benefit.
Heterogeneous morphology could be observed and the common growth patterns of MGISTs manifest a satellite phenomenon, that is, one or more main tumors surrounded by several small tumors. Of course, homogeneous morphology was still present in some tumors of MGISTs. Almost all gastric MGISTs consisted of two tumors and grew inside. On the contrary, small intestinal GISTs, especially in the jejunum, almost always had more than two tumors, and most of them grew outside. Unfortunately, the phenomenon has rarely been described in other studies, and it is difficult to determine whether it is a general finding. It is possible that MGISTs have different growth patterns and prognoses from SGISTs, and further research is needed.
The cellular types of the tumors were similar. Before propensity score matching, there was a significant difference in tumor necrosis. In addition, the proportion of calcification and cystic degeneration of MGISTs was higher than that of SGISTs, but the difference was not significant. In the propensity score matched cohort, all the above characteristics of MGISTs were compared with those of SGISTs.
For blood parameters, except for prealbumin, no significant differences were found. Immunohistochemical staining for Ki-67 antigen is often used, although this score is relatively subjective[22]. With regard to immunohistochemical markers, KIT (CD117) and ANO1 (DOG1) are two of the most sensitive and specific for GISTs[23]. S-100 and CD34 can also be used as auxiliary diagnostic indicators[24]. Unfortunately, there was no significant difference in these indicators between the patients with MGISTs and those with SGISTs.
The tumor mitotic rate is the most significant independent prognostic factor for GIST recurrence after surgery for both the stomach[25] and small intestine[26]. In our cohort, patients with MGISTs had larger tumors and a higher mitotic index, which also contributed to more advanced tumor stages.
The main treatment strategy for GISTs > 2 cm is surgical resection, and this is also the case for MGISTs. R0 resection, minimally invasive surgery and regular imaging surveillance are required to ensure perioperative and postoperative safety of patients. MGISTs may involve many segments of the gastrointestinal tract, so extensive resection is more common than with SGISTs. Therefore, consultation with experienced specialists is required to assess surgical extension and perioperative adjuvant therapy. In the practice of our medical center, the rate of open surgery for MGISTs is significantly higher than that for SGISTs. Imatinib was used for KIT/PDGFRA mutated GISTs[27] and is usually recommended for high-risk patients after surgery.
Of the 41 patients evaluated, 11 received intraoperative blood transfusions, which is higher than the rate for other operations and should compel surgeons to conduct a thorough preoperative evaluation and develop appropriate protocols. However, there was only a slight increase in operation time, intraoperative tumor rupture, postoperative hospital stays and postoperative complications in patients with MGISTs, and the difference was not significant.
After propensity score matching, all the above parameters were well balanced. We then tried to evaluate the clinical, perioperative and therapeutic factors associated with OS through Cox univariate and multivariate hazard ratio models.
There have been few studies related to the prognosis of patients with MGISTs[28]. Our study demonstrated that PFS of patients with MGISTs was significantly poorer than that of patients with SGISTs and that MGISTs were an independent risk factor for poor PFS. However, OS was similar between patients with SGISTs and those with MGISTs. This may be related to the characteristics of patients with MGISTs, suggesting that clinicians should closely monitor the condition of these patients, and it may be necessary to improve the risk classification of MGIST patients.
Given the lack of clinical trials, SGIST therapy has conflicting results in MGIST patients with regard to factors such as surgical excision and perioperative adjuvant therapy. This study suggests that more attention should be paid to such patients to explore more suitable treatment strategies. Gene detection and molecular biological experiments are also needed to explain specific manifestations[29,30].
There were some shortcomings in our research. First, as a single-center design with a small sample size, the statistical power of our findings might have been weakened. Moreover, due to the high cost, few of these patients had undergone gene detection. Last, there may have been a possibility of missed diagnoses, which could have led to an underestimation of the incidence of MGISTs.
To date, this study has the most detailed data and the largest number of patients, which may bring new insight to the diagnosis and treatment of MGISTs.
Patients with MGISTs may have demographic characteristics and immunohistochemical markers that are similar to those of patients with SGISTs, but MGIST patients also have unique tumor features. In this study, we found that MGISTs were an independent factor for PFS after propensity score matching analysis; however, OS was similar. The perioperative and long-term prognosis of patients remains of concern and requires multicenter, large sample, long-term follow-up, prospective studies. Most importantly, risk criteria, diagnostic strategies and treatment procedures suitable for this disease of lower morbidity should be developed to achieve personalized precision therapy and maximize the survival benefit of these patients.
Synchronous primary multiple gastrointestinal stromal tumors (MGISTs) are specific and rare. The diagnosis, treatment and follow-up strategies of MGISTs are not specifically described in guidelines.
Due to the low incidence, there is currently no large-scale demographic survey showing the incidence of MGISTs. Additionally, little is known about the impact of MGISTs on the survival of patients with gastrointestinal stromal tumors (GISTs).
This study aimed to compare the clinicopathological characteristics and prognoses of patients with MGISTs and patients with solitary GISTs (SGISTs).
Due to the inhomogeneous distribution of several baseline characteristics and uneven MGIST and SGIST group sizes, propensity score matching was performed according to comorbidities, body mass index, tumor location, mitotic index, sex, age and American Society of Anesthesiologists score.
Among the entire cohort, the incidence of MGISTs was 4.17%. Patients with MGISTs and those with SGISTs had disparities in body mass index, surgical approach, tumor size and mitotic index. Tumor location, tumor size, mitotic index, imatinib treatment and MGISTs were identified as independent prognostic factors of progression-free survival. However, overall survival was similar between the SGIST and MGIST groups.
Patients with MGISTs may have demographic characteristics and immunohistochemical markers that are similar to those of patients with SGISTs, but MGIST patients also have unique tumor features. Without specific diagnostic indicators and symptoms, patients with MGISTs were identified as having a poorer progression-free survival than patients with SGISTs.
Risk criteria, diagnostic strategies and treatment procedures suitable for these tumors of lower morbidity should be developed to achieve personalized precision therapy and maximize the survival benefit of these patients.
We extend our thanks to all patients involved in the study. We would also like to thank the Medical Records Department of Shandong Provincial Hospital for providing data support and the efforts of medical workers over the past decades.
Manuscript source: Unsolicited manuscript
Corresponding Author’s Membership in Professional Societies: Chinese Society of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vij M S-Editor: Yan JP L-Editor: Filipodia P-Editor: Li JH
| 1. | Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382:973-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 482] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 2. | Ma GL, Murphy JD, Martinez ME, Sicklick JK. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 183] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | Serrano C, George S. Gastrointestinal Stromal Tumor: Challenges and Opportunities for a New Decade. Clin Cancer Res. 2020;26:5078-5085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 4. | Nilsson B, Bümming P, Meis-Kindblom JM, Odén A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103:821-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 875] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 5. | Gasparotto D, Rossi S, Bearzi I, Doglioni C, Marzotto A, Hornick JL, Grizzo A, Sartor C, Mandolesi A, Sciot R, Debiec-Rychter M, Dei Tos AP, Maestro R. Multiple primary sporadic gastrointestinal stromal tumors in the adult: an underestimated entity. Clin Cancer Res. 2008;14:5715-5721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Zhang X, Ning L, Hu Y, Zhao S, Li Z, Li L, Dai Y, Jiang L, Wang A, Chu X, Li Y, Yang D, Lu C, Yao L, Cui G, Lin H, Chen G, Cui Q, Guo H, Zhang H, Lun Z, Xia L, Su Y, Han G, Hui X, Wei Z, Sun Z, Shen S, Zhou Y. Prognostic Factors for Primary Localized Gastrointestinal Stromal Tumors After Radical Resection: Shandong Gastrointestinal Surgery Study Group, Study 1201. Ann Surg Oncol. 2020;27:2812-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Yao XI, Wang X, Speicher PJ, Hwang ES, Cheng P, Harpole DH, Berry MF, Schrag D, Pang HH. Reporting and Guidelines in Propensity Score Analysis: A Systematic Review of Cancer and Cancer Surgical Studies. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 292] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 8. | He W, Zheng C, Wang Y, Dan J, Zhu M, Wei M, Wang J, Wang Z. Prognosis of synchronous colorectal carcinoma compared to solitary colorectal carcinoma: a matched pair analysis. Eur J Gastroenterol Hepatol. 2019;31:1489-1495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Bos ACRK, Matthijsen RA, van Erning FN, van Oijen MGH, Rutten HJT, Lemmens VEPP. Treatment and Outcome of Synchronous Colorectal Carcinomas: A Nationwide Study. Ann Surg Oncol. 2018;25:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Otowa Y, Nakamura T, Takiguchi G, Yamamoto M, Kanaji S, Imanishi T, Oshikiri T, Suzuki S, Tanaka K, Kakeji Y. Safety and benefit of curative surgical resection for esophageal squamous cell cancer associated with multiple primary cancers. Eur J Surg Oncol. 2016;42:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Yu YC, Hsu PK, Yeh YC, Huang CS, Hsieh CC, Chou TY, Hsu HS, Wu YC, Huang BS, Hsu WH. Surgical results of synchronous multiple primary lung cancers: similar to the stage-matched solitary primary lung cancers? Ann Thorac Surg. 2013;96:1966-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Moertel CG, Bargen JA, Soule EH. Multiple gastric cancers; review of the literature and study of 42 cases. Gastroenterology. 1957;32:1095-1103. [PubMed] |
| 13. | Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 441] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 14. | Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol. 2006;30:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 321] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 15. | Zhang L, Smyrk TC, Young WF Jr, Stratakis CA, Carney JA. Gastric stromal tumors in Carney triad are different clinically, pathologically, and behaviorally from sporadic gastric gastrointestinal stromal tumors: findings in 104 cases. Am J Surg Pathol. 2010;34:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 16. | Pasini B, McWhinney SR, Bei T, Matyakhina L, Stergiopoulos S, Muchow M, Boikos SA, Ferrando B, Pacak K, Assie G, Baudin E, Chompret A, Ellison JW, Briere JJ, Rustin P, Gimenez-Roqueplo AP, Eng C, Carney JA, Stratakis CA. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet. 2008;16:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 323] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 17. | Shang L, Fang Z, Liu J, Du F, Jing H, Xu Y, Dong K, Zhang X, Wu H, Jing C, Li L. Case report of ascending colon cancer and multiple jejunal GISTs in a patient with neurofibromatosis type 1 (NF1). BMC Cancer. 2019;19:1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Haller F, Schulten HJ, Armbrust T, Langer C, Gunawan B, Füzesi L. Multicentric sporadic gastrointestinal stromal tumors (GISTs) of the stomach with distinct clonal origin: differential diagnosis to familial and syndromal GIST variants and peritoneal metastasis. Am J Surg Pathol. 2007;31:933-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Vernuccio F, Taibbi A, Picone D, LA Grutta L, Midiri M, Lagalla R, Lo Re G, Bartolotta TV. Imaging of Gastrointestinal Stromal Tumors: From Diagnosis to Evaluation of Therapeutic Response. Anticancer Res. 2016;36:2639-2648. [PubMed] |
| 20. | Kawanowa K, Sakuma Y, Sakurai S, Hishima T, Iwasaki Y, Saito K, Hosoya Y, Nakajima T, Funata N. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37:1527-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 21. | Agaimy A, Wünsch PH, Hofstaedter F, Blaszyk H, Rümmele P, Gaumann A, Dietmaier W, Hartmann A. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol. 2007;31:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 22. | Liang YM, Li XH, Li WM, Lu YY. Prognostic significance of PTEN, Ki-67 and CD44s expression patterns in gastrointestinal stromal tumors. World J Gastroenterol. 2012;18:1664-1671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Novelli M, Rossi S, Rodriguez-Justo M, Taniere P, Seddon B, Toffolatti L, Sartor C, Hogendoorn PC, Sciot R, Van Glabbeke M, Verweij J, Blay JY, Hohenberger P, Flanagan A, Dei Tos AP. DOG1 and CD117 are the antibodies of choice in the diagnosis of gastrointestinal stromal tumours. Histopathology. 2010;57:259-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Riddle ND, Gonzalez RJ, Bridge JA, Antonia S, Bui MM. A CD117 and CD34 immunoreactive sarcoma masquerading as a gastrointestinal stromal tumor: diagnostic pitfalls of ancillary studies in sarcoma. Cancer Control. 2011;18:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 866] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 26. | Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30:477-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 437] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 27. | Li K, Tjhoi W, Shou C, Yang W, Zhang Q, Liu X, Yu J. Multiple gastrointestinal stromal tumors: analysis of clinicopathologic characteristics and prognosis of 20 patients. Cancer Manag Res. 2019;11:7031-7038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Li C, Yang KL, Wang Q, Tian JH, Li Y, Gao ZD, Yang XD, Ye YJ, Jiang KW. Clinical features of multiple gastrointestinal stromal tumors: A pooling analysis combined with evidence and gap map. World J Gastroenterol. 2020;26:7550-7567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Nguyen V, Banerjee S, Sicklick JK. Moving gastrointestinal stromal tumours towards truly personalised precision therapy. Lancet Oncol. 2020;21:865-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Li J, Shen L. The current status of and prospects in research regarding gastrointestinal stromal tumors in China. Cancer. 2020;126 Suppl 9:2048-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |