Published online Jul 21, 2021. doi: 10.3748/wjg.v27.i27.4371
Peer-review started: January 28, 2021
First decision: May 2, 2021
Revised: May 10, 2021
Accepted: June 22, 2021
Article in press: June 22, 2021
Published online: July 21, 2021
Processing time: 171 Days and 20 Hours
Pancreatic ductal adenocarcinoma is an aggressive tumor with poor long-term outcomes. Chronic pancreatitis (CP) is considered a risk factor for the development of pancreatic cancer (PC). Persistent pancreatic inflammation and activation of pancreatic stellate cells play a crucial role in the pathogenesis of CP-related PC by activating the oncogene pathway. While genetic mutations increase the possibility of recurrent and persistent pancreatic inflammation, they are not directly associated with the development of PC. Recent studies suggest that early surgical intervention for CP might have a protective role in the development of CP-related PC. Hence, the physician faces the clinical question of whether early surgical intervention should be recommended in patients with CP to prevent the development of PC. However, the varying relative risk of PC in different subsets of CP underlines the complex gene-environment interactions in the disease pathogenesis. Hence, it is essential to stratify the risk of PC in each individual patient. This review focuses on the complex relationship between CP and PC and the impact of surgical intervention on PC risk. The proposed risk stratification based on the genetic and environmental factors could guide future research and select patients for prophylactic surgery.
Core Tip: Chronic pancreatitis (CP) is a significant risk factor for pancreatic cancer (PC). However, the relative risk is not as high as previously reported. Persistent pancreatic inflammation plays an important role in pancreatic carcinogenesis, and genetic mutations implicated in the pathogenesis of CP are not directly involved in the development of PC. Although surgery for CP reduces the risk of PC, the evidence is not strong enough to recommend routine prophylactic surgery in CP patients. The risk stratification proposed in this review considers the genetic and environmental factors that could guide future prospective studies and select patients for prophylactic surgery.
- Citation: Kalayarasan R, Narayanan S, Sahoo J, Mohan P. Impact of surgery for chronic pancreatitis on the risk of pancreatic cancer: Untying the Gordian knot. World J Gastroenterol 2021; 27(27): 4371-4382
- URL: https://www.wjgnet.com/1007-9327/full/v27/i27/4371.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i27.4371
Chronic pancreatitis (CP) is a complex fibro-inflammatory disease of the pancreas characterized by destruction and fibrotic replacement of the pancreatic parenchyma[1]. Pain is the dominant symptom of CP, followed by clinical manifestations of exocrine and endocrine dysfunction[2]. Surgery and endoscopic interventions are primarily indicated in symptomatic CP patients to provide pain relief and may prevent disease progression if done early in the disease course[3,4]. Pancreatic ductal adenocarcinoma, the most common type of pancreatic cancer (PC), is an aggressive tumor with a dismal prognosis[5]. Epidemiological studies have identified CP as a risk factor for PC[6-8]. Recurrent or persistent pancreatic inflammation in the setting of CP has been implicated in pancreatic carcinogenesis[9,10]. Recent studies have shown that surgery for CP reduces the risk of PC[11,12]. This evidence raises the question of whether early surgical intervention should be considered in patients with CP to prevent the development of PC. The proposed mechanistic definition for CP highlights the complex gene-gene and gene-environmental interactions in the disease pathophysiology[13]. As not all patients with CP develop PC, and a high proportion of patients with PC do not have a history of CP, this review focuses on the complex relationship between CP and PC and the impact of surgical intervention on the risk of PC.
A PubMed search of relevant articles was performed by three authors The reference lists of the articles also were searched for additional appropriate studies. The keywords and combinations included in the search were: “pancreatitis”; ”chronic pancreatitis”; “hereditary pancreatitis”; “pancreatic cancer” and “chronic pancreatitis”; “pancreatic cancer” and “hereditary pancreatitis”; “pancreatitis” and “pancreatic cancer”; “genetics“ and ”pancreatic cancer“; ”pancreatic ductal adenocarcinoma“ and ”pancreatitis“; ”genetics“ and ”chronic pancreatitis“ and ”pancreatic cancer“; ”surgery“ and ”chronic pancreatitis“ and ”pancreatic cancer“. The search was limited to publications in English and stressed the latest evidence. All the authors agreed that the articles selected for review were relevant.
It is essential to understand the incidence of PC in CP patients to determine the impact of surgery for CP on the risk of PC. In the 19th century, Rudolph Virchow observed white blood cells within tumor tissue and suggested a relationship between inflammatory pathology and cancer[14]. Well known associations that support the hypothesis of inflammation-induced metaplasia and carcinogenesis include inflammatory bowel disease and colorectal cancer, gallstones and gallbladder cancer, Barrett’s esophagus, and esophageal cancer[15]. The earliest report of an association between CP and PC dates to the 1950s, when an autopsy study identified CP changes in 49% of 100 patients who died of PC[16]. Since then, multiple studies have documented an increased risk of PC in CP patients[17-19]. Raimondi et al[6], in a meta-analysis of 22 case-control and cohort studies, reported a relative risk (95%CI) of 13.3 (6.1-28.9), which means that CP patients had 13.3 times more risk of developing PC compared with the general population. However, the included studies did not consider the lag period, i.e. the interval between CP and PC diagnosis. To be labeled as CP-related PC, it is recommended to exclude PC cases diagnosed within 2 years of CP diagnosis to avoid potential misclassification. After considering the lag period, the relative risk of CP-related PC dropped to 11.8 with a 1-year lag period and to 5.8 with a 2-year lag period (Table 1). Tong et al[7] in a meta-analysis of 17 studies with acute, chronic, and unspecified pancreatitis, reported that CP patients had a 10.3-fold risk of developing PC compared with the general population. When the association between CP and PC was stratified by time, they reported risk estimates (95%CI) of 23.3 (14.0-38.9), 3.03 (2.41-3.81), 2.82 (2.12-3.76), and 2.25 (1.59-3.19) for lag periods of less than 1, 2, 5, and 10 years, respectively. While the estimated risk was less than reported in the previous meta-analysis, the inclusion of acute and unspecified pancreatitis along with CP might have underestimated the association between CP and PC, as acute pancreatitis is less likely to progress to PC compared with CP. Kirkegård et al[8], in a recent meta-analysis of 13 CP studies, estimated 8-fold and 3.5-fold increased risks of PC at 5- and 9-year lag periods, respectively, from CP diagnosis to PC diagnosis (Table 1). The meta-analyses suggest that patients with CP are at increased risk of developing PC compared with the general population. However, the estimated risk was much lower than that reported in some individual studies, especially when a 2-year lag period was included. While the increased duration of CP-related inflammation is expected to increase PC risk, the decrease in PC risk between the 2- and 5-year lag periods in the meta-analyses suggests initial misclassification of PC as CP in some of the included studies. Also, most studies did not evaluate the impact of confounding risk factors like smoking or alcohol intake on the risk estimate of CP-related PC.
| Ref. | Number of studies | Number of patients | Risk estimate | Remarks |
| Raimondi et al[6] | 22 (12 case-control, 10 cohort) | 16124 | RR, 11.8 (95%CI: for 1-yr lag period; RR, 5.8 (95%CI: 2.1-15.9) for 2-yr lag period; RR, 69.0 (95%CI: 56.4-84.4) for hereditary pancreatitis; RR, 100 (95%CI: 37.0-218.0) for tropical pancreatitis | Details of seven studies included for risk estimate calculation not mentioned; Details of number of patients with pancreatic cancer in included studies not mentioned; High relative risk reported for tropical pancreatitis based on a single study |
| Tong et al[7] | 17 (14 case-control, 3 cohort) | 14667 pancreatic cancer, 17587 pancreatitis | Pooled OR, 10.35 (95%CI: 9.13-11.75) for chronic pancreatitis; Pooled OR, 6.41 (95%CI: 4.93-8.34) for unspecified type of pancreatitis - | Inclusion of studies with both acute and chronic pancreatitis limits generalizability; Inclusion of acute, chronic and unspecified pancreatitis to calculate the temporality of the association might underestimate the relative risk |
| Kirkegård et al[8] | 13 (4 case-control, 9 cohort) | 674 pancreatic cancer, 25,329 chronic pancreatitis | EE, 16.16 (95%CI:12.59-20.73) for 2-yr lag period; EE, 7.90 (95%CI: 4.26-14.66) for 5-yr lag period; EE, 3.53 (95%CI: 1.69-7.38) for 9-yr lag period | Some of the included studies had a smaller number of patients with pancreatic cancer or chronic pancreatitis which might inflate the estimated risk; In some of the included studies more than one-third of patients were lost to follow-up |
The current evidence suggests that the incidence of PC in CP patients is variable (2.7- to 13.3-fold increased risk) and influenced by multiple factors[20,21]. Hence, identifying demographic and clinical risk factors for PC in CP patients will help to determine the subgroup of CP patients who might benefit from surgical intervention. A retrospective analysis of 1415 patients from an Indian center reported that a smoking and nonalcoholic CP etiology was a risk factor for developing PC[22] (Table 2). Among the nonalcoholic CP etiologies, idiopathic senile chronic pancreatitis (ISCP), defined as an age of onset of CP of more than 35 years, had a greater risk of PC than idiopathic juvenile CP (5% vs 0.2% at 10 years of follow-up). An inverse association between exocrine insufficiency and malignancy risk indicated that steatorrhea and malignant transformation occurred independently. A retrospective analysis of 2037 CP cases in a patient database in China did not find a significant difference in PC risk between alcoholic and idiopathic pancreatitis[23]. However, the analysis did not include a separate risk analysis of patients with IJSP and ISCP. A single-center European study by Müllhaupt et al[24] prospectively evaluated the risk of PC in 332 CP patients. The risk of PC was greater in idiopathic juvenile chronic pancreatitis (IJCP) compared with other CP etiological subtypes. The authors concluded that early onset and prolonged disease was a significant risk factor for CP-related PC. In another European study from Sweden, Vujasinovic et al[25] evaluated the risk of PC in a retrospective analysis of prospectively collected pancreatic clinical data. In contrast to other studies, they subclassified the CP patients into eight subgroups and calculated the PC risk. Among CP patients, those with diabetes mellitus and a high body mass index (BMI) at diagnosis or patients with a low BMI and pancreatic exocrine insufficiency at diagnosis were identified as groups at risk of developing CP-related cancer. However, relatively few patients (6/581) developed PC 2 years after the CP diagnosis. Studies conducted in different regions of the globe highlight differences in the demographic and clinical risk factors for PC in different CP populations. To evaluate the reason for the diverse predictive factors, the pathogenesis of CP and pathways for PC in those patients have to be well understood.
| Ref. | Number of patients | Etiological subtype, n (%) | Pancreatic cancer risk | Specific risk group |
| Agarwal et al[22] | 1415 | ACP, 540 (38.1); IJCP, 668 (47.2); ISCP, 207 (14.6) | 5-yr risk of pancreatic cancer: ACP, 0.5%; IJCP, 0.2%; ISCP, 3.7%. 10-yr risk of pancreatic cancer: ACP, 0.9%; IJCP, 0.2%; ISCP, 5.2% | Age of onset > 35 yr (HR 12.1; CI: 4.7-31.2; P < 0.001). Smoking (HR 6.48; CI: 2.2-19.0; P < 0.001). Nonalcoholic etiology (HR for alcohol use 0.14; CI: 0.04-0.42; P < 0.001); Absence of steatorrhea (HR for steatorrhea 0.19; 95%CI: 0.04-0.80; P < 0.023) |
| Hao et al[23] | 2037 | ACP, 404 (19.8); ICP, 1633 (80.2) | 5-yr risk of pancreatic cancer: ACP, 0.2%; ICP, 0.7%. 10-yr risk of pancreatic cancer: ACP, 0.9%; ICP, 1.1% | No significant difference in pancreatic cancer development between ACP and ICP patients |
| Vujasinovic et al[25] | 581 | Idiopathic, 47 (8.1); Alcohol and nicotine, 220 (37.9); Nicotine, 81 (13.9); Alcohol, 49 (8.4); Hereditary, 38 (6.5) Immunological, 59 (10.2) Efferent duct 62 (10.7) Miscellaneous/other, 25 (4.3) | Rate per 100 yr; Idiopathic, 0.00; Alcohol and nicotine, 0.07; Nicotine, 0.44; Alcohol, 0.32; Hereditary, 0.00; Immunological, 0.00; Efferent duct, 0.28; Miscellaneous/other, 0.81 | Low BMI and pancreatic exocrine insufficiency. Patients with diabetes mellitus and high BMI |
| Müllhaupt et al[24] | 332 | ACP, 265; IJCP, 21; ISCP, 46 | ACP, 1.5% over 16 yr; IJCP, 4.8% over 22 yr; ISCP, 2.2% over 14 yr | Early onset and prolonged duration of CP (rather than the specific type of CP) |
An accepted explanation of the pathogenesis of CP involves a sentinel acute pancreatitis event that initiates an inflammatory cascade in a susceptible individual[26,27] (Figure 1). Of the various predisposing factors, smoking and alcohol intake are the important modifiable risk factors relevant to pancreatic carcinogenesis in a genetically susceptible individual. The genes implicated in the pathogenesis of pancreatitis are protease serine 1 (PRSS1), serine peptidase inhibitor Kazal type 1 (SPINK 1), Cystic fibrosis transmembrane conductance regulator (CFTR), chymotrypsin C (CTRC) and calcium-sensing receptor (CASC)[28-30]. Recently mutations in the genes encoding carboxypeptidase A1 (CPA1), carboxyl ester lipase, and tight-junction protein claudin-2 (CLDN2) have also been postulated to initiate pancreatitis[29]. Gain-of-function mutations in the PRSS1 gene, which encodes cationic trypsinogen, facilitate intrapancreatic trypsin activation[31]. Mutation of the SPINK 1 gene that codes for trypsin inhibitor, together with CTRC and CASC mutations, promotes abnormal trypsin activation[32]. While PRSS1, SPINK1, CTRC and CASC initiate pancreatitis through a trypsin-dependent pathway, CFTR mutation affects the anion channel for chloride and bicarbonate[30]. Disturbed alkalinization of the pancreatic juice results in injury to the duct epithelium followed by protein plug and pancreatic stone formation[29,33]. In addition to the initiation of pancreatic inflammation, these genetic factors play an important role in the progression of acute inflammation to CP[30]. Recent studies have shown that the clinical manifestations of CP can be grouped into three complication clusters[34]. One group of patients has dominant inflammatory complications like pseudocyst, pancreatic ascites, and vascular (splenic or portal vein) thrombosis. The second group has predominant pancreatic exocrine (steatorrhea) and endocrine (diabetes) insufficiency. The third group has fibrotic complications characterized by biliary stricture, duodenal stenosis, and pancreatic duct lesions. Individual patients can have more than one complication cluster, but most have a single dominant complication cluster. As patients with predominant fibrotic complications are at increased risk of PC, understanding the inflammatory and gene pathways can help in the risk stratification of individual patients[22,34].
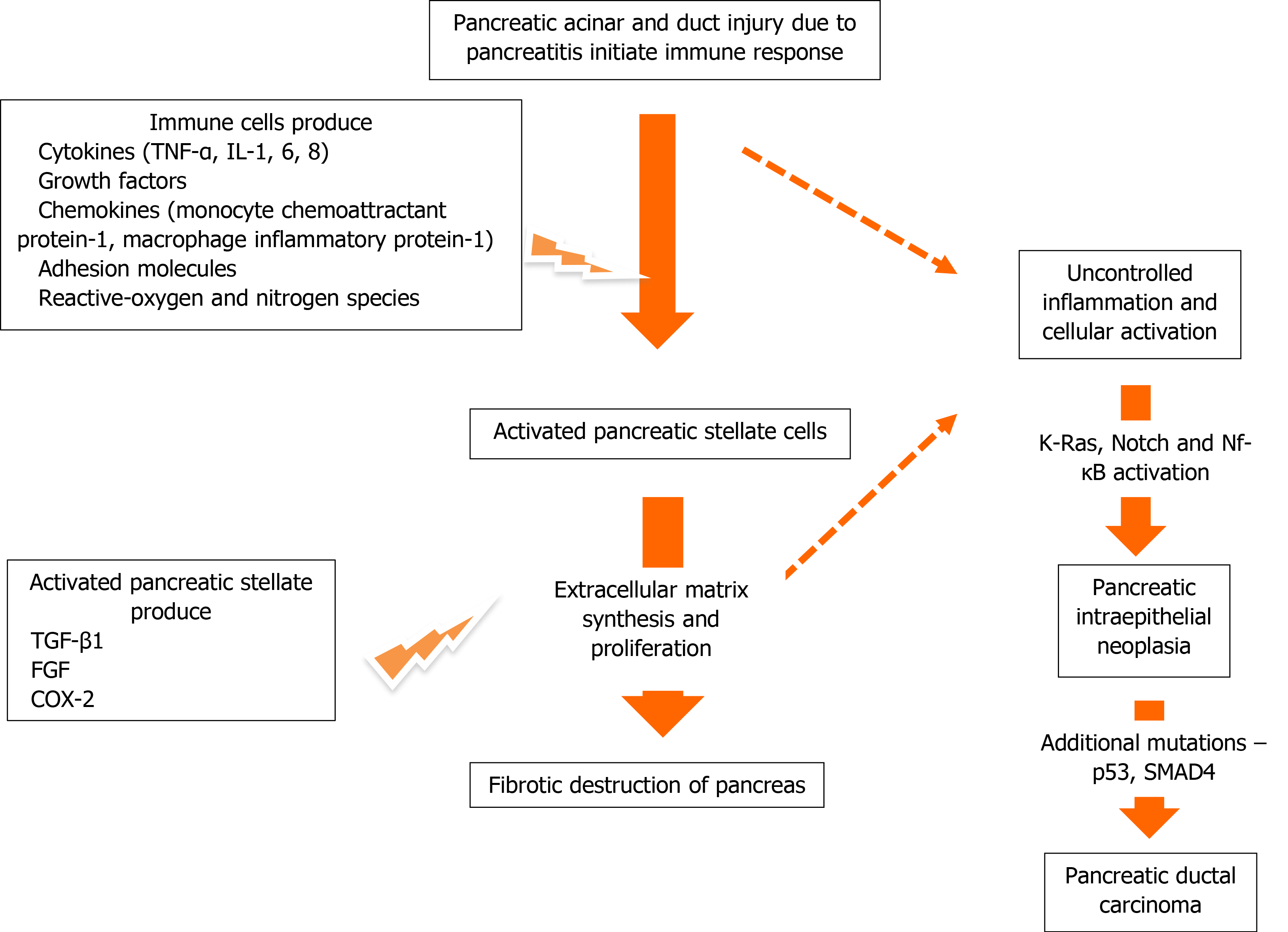
It is well known that PC tissue includes malignant duct cells surrounded by stroma that accounts for more than 90% of the tumor mass[35]. The histology of CP tissue includes fibrotic destruction of the pancreatic parenchyma, infiltration of inflammatory cells, and ductal and exocrine atrophy[2]. As pancreatic stellate cells are the principal source of fibrosis, their activation has been implicated in the pathogenesis of CP and CP-related PC[36-38] (Figure 2). Activation of the immune system following injury of the pancreatic duct and acini results in the release of multiple proinflammatory cytokines including tumor necrosis factor (TNF)-α and interleukin (IL)-1, IL-6, and IL-8, growth factors, chemokines including monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1, adhesion molecules, reactive oxygen and reactive nitrogen species that activate pancreatic stellate cells and induce proliferation[39,40]. Transforming growth factor (TGF) β1 and fibroblast growth factor produced by pancreatic stellate cells stimulate extracellular matrix synthesis and cyclooxygenase-2 mediates extracellular matrix proliferation[36-38]. The benign process of inflammation-related proliferation and fibrosis can turn malignant in the background of uncontrolled inflammation. This is supported by the observation that mutation of the K-Ras proto-oncogene stimulates tumor development only in the presence of inflammatory milieu; inflammatory mediators are required for its activation[41-44]. Approximately 30% of CP patients with CP have K-Ras mutations[44]. K-Ras mutation itself is not sufficient, the pathological activity necessary for PC development is achieved only in the presence of persistent inflammation[41]. Additionally, activation of the Notch signaling pathway and the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) transcription factor promotes carcinogenesis against an inflammatory background[45]. The current understanding of the inflammatory and oncogene pathways of PC in CP patients is that CP significantly increases the risk of PC (relative risk > 2) compared with other risk factors, and that inflammation plays a crucial role in carcinogenesis[20].
Genetic mutations responsible for hereditary pancreatitis are not directly associated with the development of PC. However, genetic susceptibility increases the possibility of recurrent and persistent inflammation, especially in the presence of environmental risk factors like smoking and alcohol intake, thereby increasing the risk of PC[46]. Among the genetic causes of pancreatitis, PRSS1 mutation carries the maximum risk for PC followed by other mutations. The difference in risk primarily results from the ability to initiate and sustain an inflammatory cascade with or without other environmental factors[47]. Differences in the risk of PC in IJCP in Indian and European studies are caused by differences in the underlying genetic mutations. IJCP is predominantly associated with CFTR and SPINK 1 mutations in the Indian population and with PRSS1 mutation in European populations[48].
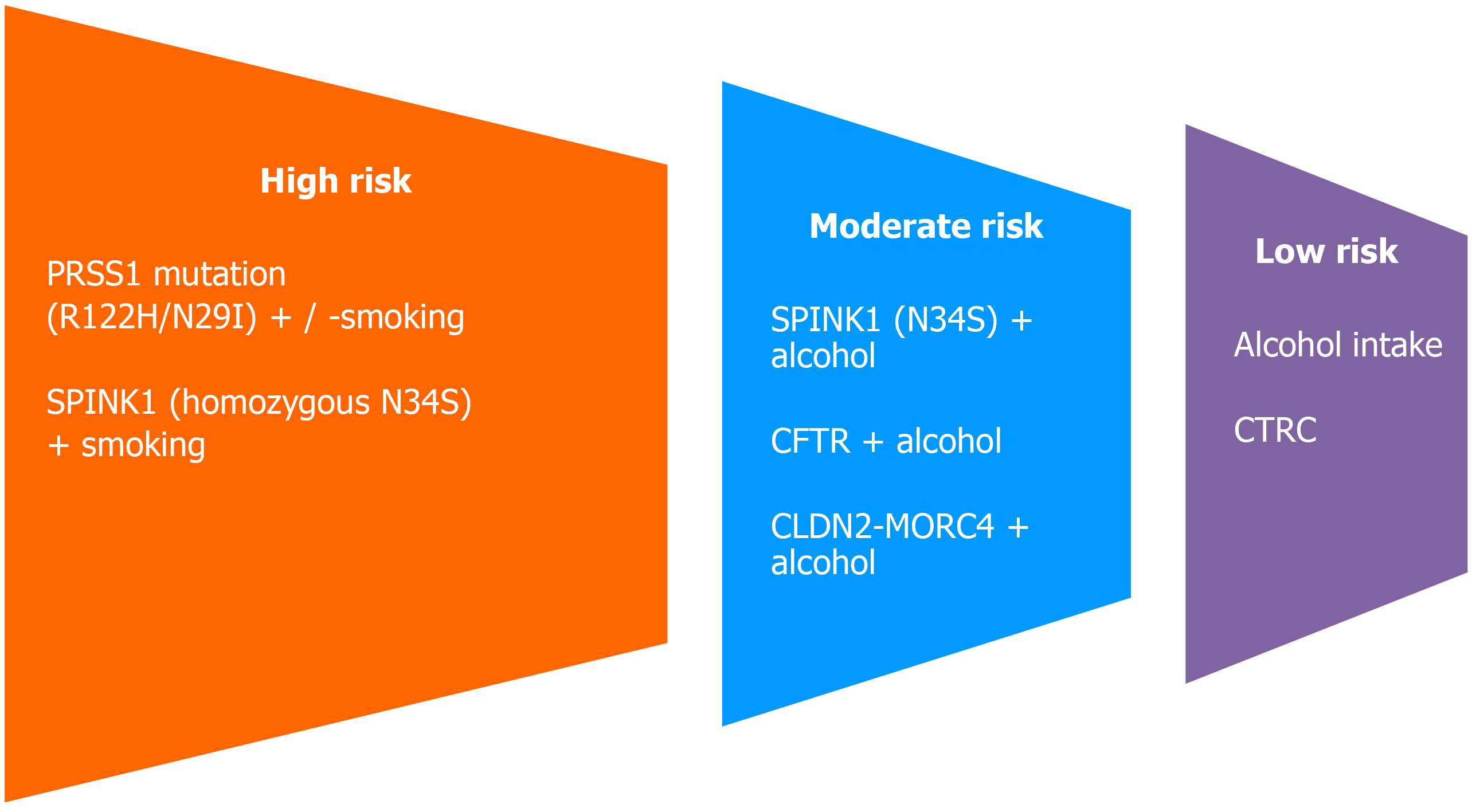
Prospective cohort studies of patients with hereditary pancreatitis have reported that avoidance of smoking and alcohol intake, ensuring a smoke-free environment in the home and workplace, maintaining normal BMI, and eating a healthy diet reduces the cumulative risk of PC[20,50]. The success of cancer preventive measures suggests that medical, endoscopic, and surgical interventions to minimize ongoing pancreatic inflammation can reduce the risk of PC. Alcohol consumption increases the risk of pancreatitis and smoking increases the risk of fibrotic complications in CP and related PC. Experimental studies have shown that aryl hydrocarbon receptor ligands in cigarette smoke induce production of interleukin-22 and pancreatic fibrosis. Hence, lifestyle modifications like smoking cessation can minimize the risk of PC[51]. Risk stratification of CP patients is proposed based on the current understanding of the inflammatory and oncogene pathways of CP-induced PC (Figure 3). The proposed risk stratification underlines the need for genetic testing in most patients with CP, as the combination of genetic and environmental factors determines the risk of PC.
Although CP is considered a risk factor for PC, the impact of surgery for CP on the risk of PC has not been well studied. To investigate the relationship between surgery for CP and PC, Ueda et al[12] performed a multicenter retrospective analysis of 893 CP patients at 22 institutions who fulfilled the 2001 Japan Pancreas Society diagnostic criteria for CP. They excluded 387 patients with less than 2 years of follow-up or a lag period of less than 2 years between CP diagnosis and PC. Of the 506 patients included in the study, 19 (3.7%) developed PC (Table 3). The standardized incidence ratio (SIR), defined as the ratio of observed to expected PC cases, was 11.8 (95%CI, 7.1-18.4). The cumulative incidence (95%CI) of PC was 2.0% (1.0-4.1) at 5; 4.6% (2.6-8.2) at 10; 7.5% (4.3-12.9) at 15; 10.7% (4.3-18.4) at 20, and 14.0% (7.5%-25.3%) at 25 years after the CP diagnosis. At a median follow-up of more than 5 years, the PC incidence was significantly lower in patients who had undergone surgery (1/147, 0.7%0) than in those who did not undergo surgical intervention (18/352, 5.1%) for CP (P = 0.03]. Based on the results, the authors concluded that surgery for CP decreased the risk of PC[12].
| Ref. | Number of patients | Etiological subtype, n (%) | Type of surgery, n (%) | Pancreatic cancer risk |
| Ueda et al[12] | 506 | Alcoholic pancreatitis, 345 (68.0); Idiopathic pancreatitis, 109 (22.0); Autoimmune pancreatitis, 16 (3); Hereditary pancreatitis, 9 (2); Miscellaneous, 27 (5) | Drainage operation, 60 (40.8); Pancreatic resection, 87 (59.2) | Surgery, 1/147 (0.7%); No surgery, 18/352 (5.1%) |
| Zheng et al[11] | 650 | Alcoholic pancreatitis, 361 (55.5); Idiopathic pancreatitis, 227 (34.9); Hereditary pancreatitis, 62 (9.5) | Drainage operation, 189 (29.1); Pancreatic resection, 461 (70.9) | Surgery, 12/650 (1.8%); Time interval to surgery, HR, 1.005 (95%CI: 1.002-1.008); De novo endocrine insufficiency, HR, 10.672 (95%CI: 2.567-44.372) |
Zheng et al[11] performed a retrospective analysis of 650 surgically managed CP patients to determine the incidence and risk factors for PC after surgery for CP. At a median follow-up of 4.4 years, 12 patients (1.8%) developed PC (Table 3). The SIR (95%CI) for PC in CP patients was 68.12 (35.20-118.99). The cumulative incidence (95%CI) of PC was 1.48% (0.46-2.51) at 3; 2.63% (0.93-4.32) at 6; and 3.71% (1.05-6.37%) at 9 years after CP surgery. The time interval to surgery, defined as the lag time between the initial CP diagnosis and operative intervention, was significantly longer in CP patients who developed PC (96.6 ± 164.4 mo vs 36.4 ± 74.2 mo, P = 0.016), with a hazard ratio (95%CI) of 1.005 (1.002-1.008, P = 0.002). Based on the results, the authors concluded that early surgical intervention for CP prevented the development of CP-related PC[11].
The proposed mechanisms by which surgery for CP reduces PC incidence are relief of pancreatic compartment syndrome and inflammation by resection of fibrotic parenchyma and surgical drainage[11]. As mentioned earlier, parenchymal fibrosis plays an essential role in pancreatic carcinogenesis, and its removal reduces the volume of pancreatic tissue at risk of malignancy. This hypothesis is supported by studies in which none of the patients who underwent pancreatoduodenectomy for CP developed PC during the follow-up period[11,12]. As persistent uncontrolled inflammation is a significant risk factor for developing PC, relief of ductal obstruction and pancreatic compartment syndrome attenuates pancreatic inflammation.
The standard indications for surgery in CP are intractable pain, suspicion of malignancy, and local pancreatic or extrapancreatic complications, such as biliary stricture, duodenal stenosis, pseudocysts, and vascular complications (e.g., splenic/portal vein thrombosis and arterial pseudoaneurysms)[2,4], and intractable pain is the most common indication. Traditionally, patients are managed by a step-up approach that begins with medical management (analgesics) followed by endoscopic intervention[52,53]. Surgery is generally considered as the last option after multiple endoscopic interventions fail to provide adequate symptomatic relief. However, based on the findings of numerous observational studies and a recent randomized controlled trial, international consensus guidelines for surgery and the timing of intervention in CP recommend early surgery[4]. Early surgery, i.e. performed within 3 years of the onset of CP symptoms, is associated with better pain relief and preservation of pancreatic function[3,54]. Considering recent evidence for early surgery in CP and its potential protective role in the development of PC, the clinician faces the question of prophylactic surgery in CP patients. Prophylactic surgery for CP is performed with the primary aim of reducing the risk of PC in the absence of typical surgical indications like pain and local complications. The dilemma is significant in light of the current availability of digitized clinical information for patients and increased litigation. Is the clinician is ethically right or legally justified in not offering a treatment that can prevent a deadly complication of CP? To answer that question, a critical analysis of the current evidence is essential. Many studies have evaluated the risk of PC, but only a few have investigated the protective effect of surgery for CP on reducing the risk of PC[11,12]. Limitations of previous studies are the inclusion of small numbers of patients and retrospective analysis, which make the accuracy and completeness of the study data difficult to control. Confounding risk factors like smoking and alcohol intake were not accurately quantified in most studies, and the proportion of patients who underwent resection procedures was relatively high, in at least in one of the studies, which contrasts with the drainage procedures commonly performed worldwide for CP patients[55]. Another limitation is the lack of objective evidence to document whether pancreatic inflammation was reduced after surgery. As persistent pancreatic inflammation is considered a significant risk factor for pancreatic carcinogenesis, a reliable biomarker to monitor the presence or absence of persistent pancreatic inflammation after surgery could help us better understand the role of surgery in reducing the risk of PC. Hence, to confirm the protective role of surgical procedures for CP, additional prospective studies involving large populations are required. While the risk of PC is increased in patients with CP, it is still too low to recommend routine active screening or prophylactic surgery in all patients[20]. With the available evidence, routine surveillance and prophylactic surgery are recommended only in subgroups of hereditary pancreatitis with a high risk of PC. In contrast to environmental risk factors, genetic factors are not modifiable. Hence, prophylactic surgery will have a greater role in this group of patients. Until we have more evidence, surgery for CP is currently recommended for only the described standard indications.
The mechanistic definition of CP is a “pathologic fibroinflammatory syndrome of the pancreas in individuals with genetic, environmental and/or other risk factors who develop persistent pathologic responses to parenchymal injury or stress”[13]. The current definition highlights the disease heterogeneity in terms of individual susceptibility, disease onset, modifying factors, rate of progression, and long-term outcomes. Detailed evaluation of an individual patient often identifies a genetic factor that predisposes to pancreatitis in the presence of environmental risk factors[29,30]. The complex interaction of genetic and environmental factors determines the clinical phenotype and risk of PC. Hence, the concept of precision medicine used in various complex benign and malignant gastrointestinal disorders should be employed in CP patients. Molecular pathways leading to CP-related PC should be better defined so that complication clusters in an individual patient and the role of interventions in mitigating the cancer risk can be well studied.
Advances in instrumentation and technology allow increasing use of endoscopic interventions in CP patients. However, long-term studies focusing on the risk of PC in patients undergoing endoscopic intervention are lacking. As previously mentioned, reliable markers for pancreatic inflammation would help to evaluate the success of endoscopic and surgical treatment in individual patients. Hence future prospective studies evaluating the role of surgical and endoscopic intervention in reducing PC should include accurate risk stratification of the study patients and preferably incorporate biomarkers of pancreatic inflammation.
CP is a significant risk factor for PC. However, the relative risk is as not high as previously reported. Persistent pancreatic inflammation plays a key role in pancreatic carcinogenesis, and genetic mutations implicated in the pathogenesis of CP are not directly involved in the development of PC. Activation of pancreatic stellate cells and enhanced K-Ras oncogene activity in the inflammatory milieu play a key role in the pathogenesis of CP-related PC. Although surgery for CP reduces the risk of PC, the evidence is not strong enough to recommend routine prophylactic surgery in CP patients. The proposed risk stratification considers the genetic and environmental factors that could guide future prospective studies and select patients for prophylactic surgery.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: International Hepato-Pancreato-Biliary Association, No. XHNLZQFPWCQ; Indian Association of Surgical Gastroenterology, No. K-65; IHPBA India, No. 563; and Asia-Pacific Hepato-Pancreato-Biliary Association, No. XHNLZQFPWCQ.
Specialty type: Surgery
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Egorov V S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Xing YX
| 1. | Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet. 2011;377:1184-1197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 338] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 2. | Majumder S, Chari ST. Chronic pancreatitis. Lancet. 2016;387:1957-1966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 237] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 3. | Willner A, Bogner A, Müssle B, Teske C, Hempel S, Kahlert C, Distler M, Weitz J, Welsch T. Disease duration before surgical resection for chronic pancreatitis impacts long-term outcome. Medicine (Baltimore). 2020;99:e22896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Kempeneers MA, Issa Y, Ali UA, Baron RD, Besselink MG, Büchler M, Erkan M, Fernandez-Del Castillo C, Isaji S, Izbicki J, Kleeff J, Laukkarinen J, Sheel ARG, Shimosegawa T, Whitcomb DC, Windsor J, Miao Y, Neoptolemos J, Boermeester MA; Working group for the International (IAP – APA – JPS – EPC) Consensus Guidelines for Chronic Pancreatitis. International consensus guidelines for surgery and the timing of intervention in chronic pancreatitis. Pancreatology. 2020;20:149-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 906] [Cited by in F6Publishing: 1075] [Article Influence: 179.2] [Reference Citation Analysis (31)] |
| 6. | Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24:349-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 408] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 7. | Tong GX, Geng QQ, Chai J, Cheng J, Chen PL, Liang H, Shen XR, Wang DB. Association between pancreatitis and subsequent risk of pancreatic cancer: a systematic review of epidemiological studies. Asian Pac J Cancer Prev. 2014;15:5029-5034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Kirkegård J, Mortensen FV, Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am J Gastroenterol. 2017;112:1366-1372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 300] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 9. | Bhanot UK, Möller P. Mechanisms of parenchymal injury and signaling pathways in ectatic ducts of chronic pancreatitis: implications for pancreatic carcinogenesis. Lab Invest. 2009;89:489-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Talamini G, Falconi M, Bassi C, Mastromauro M, Salvia R, Pederzoli P. Chronic pancreatitis: relationship to acute pancreatitis and pancreatic cancer. JOP. 2000;1:69-76. [PubMed] [Cited in This Article: ] |
| 11. | Zheng Z, Chen Y, Tan C, Ke N, Du B, Liu X. Risk of pancreatic cancer in patients undergoing surgery for chronic pancreatitis. BMC Surg. 2019;19:83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Ueda J, Tanaka M, Ohtsuka T, Tokunaga S, Shimosegawa T; Research Committee of Intractable Diseases of the Pancreas. Surgery for chronic pancreatitis decreases the risk for pancreatic cancer: a multicenter retrospective analysis. Surgery. 2013;153:357-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Whitcomb DC, Frulloni L, Garg P, Greer JB, Schneider A, Yadav D, Shimosegawa T. Chronic pancreatitis: An international draft consensus proposal for a new mechanistic definition. Pancreatology. 2016;16:218-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 302] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 14. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5245] [Cited by in F6Publishing: 5533] [Article Influence: 240.6] [Reference Citation Analysis (0)] |
| 15. | Thun MJ, Henley SJ, Gansler T. Inflammation and cancer: an epidemiological perspective. Novartis Found Symp. 2004;256:6-21; discussion 22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | MIKAL S, CAMPBELL AJ. Carcinoma of the pancreas; diagnostic and operative criteria based on 100 consecutive autopsies. Surgery. 1950;28:963-969. [PubMed] [Cited in This Article: ] |
| 17. | Bansal P, Sonnenberg A. Pancreatitis is a risk factor for pancreatic cancer. Gastroenterology. 1995;109:247-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 169] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Andrén-Sandberg A, Dervenis C, Lowenfels B. Etiologic links between chronic pancreatitis and pancreatic cancer. Scand J Gastroenterol. 1997;32:97-103. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Ekbom A, McLaughlin JK, Karlsson BM, Nyrén O, Gridley G, Adami HO, Fraumeni JF Jr. Pancreatitis and pancreatic cancer: a population-based study. J Natl Cancer Inst. 1994;86:625-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 135] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Greenhalf W, Lévy P, Gress T, Rebours V, Brand RE, Pandol S, Chari S, Jørgensen MT, Mayerle J, Lerch MM, Hegyi P, Kleeff J, Castillo CF, Isaji S, Shimosegawa T, Sheel A, Halloran CM, Garg P, Takaori K, Besselink MG, Forsmark CE, Wilcox CM, Maisonneuve P, Yadav D, Whitcomb D, Neoptolemos J; Working group for the International (IAP – APA – JPS – EPC) Consensus Guidelines for Chronic Pancreatitis. International consensus guidelines on surveillance for pancreatic cancer in chronic pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the International Association of Pancreatology, the American Pancreatic Association, the Japan Pancreas Society, and European Pancreatic Club. Pancreatology. 2020;20:910-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Duell EJ, Lucenteforte E, Olson SH, Bracci PM, Li D, Risch HA, Silverman DT, Ji BT, Gallinger S, Holly EA, Fontham EH, Maisonneuve P, Bueno-de-Mesquita HB, Ghadirian P, Kurtz RC, Ludwig E, Yu H, Lowenfels AB, Seminara D, Petersen GM, La Vecchia C, Boffetta P. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2012;23:2964-2970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 22. | Agarwal S, Sharma S, Gunjan D, Singh N, Kaushal K, Poudel S, Anand A, Gopi S, Mohta S, Sonika U, Saraya A. Natural course of chronic pancreatitis and predictors of its progression. Pancreatology. 2020;20:347-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Hao L, Wang LS, Liu Y, Wang T, Guo HL, Pan J, Wang D, Bi YW, Ji JT, Xin L, Du TT, Lin JH, Zhang D, Zeng XP, Zou WB, Chen H, Xie T, Li BR, Liao Z, Cong ZJ, Xu ZL, Li ZS, Hu LH. The different course of alcoholic and idiopathic chronic pancreatitis: A long-term study of 2,037 patients. PLoS One. 2018;13:e0198365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Müllhaupt B, Truninger K, Ammann R. Impact of etiology on the painful early stage of chronic pancreatitis: a long-term prospective study. Z Gastroenterol. 2005;43:1293-1301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Vujasinovic M, Dugic A, Maisonneuve P, Aljic A, Berggren R, Panic N, Valente R, Pozzi Mucelli R, Waldthaler A, Ghorbani P, Kordes M, Hagström H, Löhr JM. Risk of Developing Pancreatic Cancer in Patients with Chronic Pancreatitis. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Whitcomb DC. Hereditary pancreatitis: new insights into acute and chronic pancreatitis. Gut. 1999;45:317-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 203] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol. 2010;7:131-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 28. | Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK Jr, Amann ST, Toskes PP, Liddle R, McGrath K, Uomo G, Post JC, Ehrlich GD. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1123] [Cited by in F6Publishing: 1000] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 29. | Shimosegawa T. A New Insight into Chronic Pancreatitis. Tohoku J Exp Med. 2019;248:225-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Whitcomb DC. Genetic risk factors for pancreatic disorders. Gastroenterology. 2013;144:1292-1302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 31. | Gorry MC, Gabbaizedeh D, Furey W, Gates LK Jr, Preston RA, Aston CE, Zhang Y, Ulrich C, Ehrlich GD, Whitcomb DC. Mutations in the cationic trypsinogen gene are associated with recurrent acute and chronic pancreatitis. Gastroenterology. 1997;113:1063-1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 277] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Solomon S, Whitcomb DC. Genetics of pancreatitis: an update for clinicians and genetic counselors. Curr Gastroenterol Rep. 2012;14:112-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Kim Y, Jun I, Shin DH, Yoon JG, Piao H, Jung J, Park HW, Cheng MH, Bahar I, Whitcomb DC, Lee MG. Regulation of CFTR Bicarbonate Channel Activity by WNK1: Implications for Pancreatitis and CFTR-Related Disorders. Cell Mol Gastroenterol Hepatol. 2020;9:79-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Olesen SS, Nøjgaard C, Poulsen JL, Haas SL, Vujasinovic M, Löhr M, Lindkvist B, Bexander L, Gulbinas A, Kalaitzakis E, Ebrahim M, Erchinger F, Engjom T, Roug S, Novovic S, Hauge T, Waage A, Laukkarinen J, Parhiala M, Pukitis A, Ozola-Zālīte I, Drewes AM; Scandinavian Baltic Pancreatic Club. Chronic Pancreatitis Is Characterized by Distinct Complication Clusters That Associate With Etiological Risk Factors. Am J Gastroenterol. 2019;114:656-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 35. | Xu Z, Pothula SP, Wilson JS, Apte MV. Pancreatic cancer and its stroma: a conspiracy theory. World J Gastroenterol. 2014;20:11216-11229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 97] [Cited by in F6Publishing: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Apte MV, Wilson JS, Lugea A, Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210-1219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 334] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 37. | Haber PS, Keogh GW, Apte MV, Moran CS, Stewart NL, Crawford DH, Pirola RC, McCaughan GW, Ramm GA, Wilson JS. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol. 1999;155:1087-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 319] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 38. | Zimnoch L, Szynaka B, Puchalski Z. Mast cells and pancreatic stellate cells in chronic pancreatitis with differently intensified fibrosis. Hepatogastroenterology. 2002;49:1135-1138. [PubMed] [Cited in This Article: ] |
| 39. | Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1033] [Cited by in F6Publishing: 1116] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 40. | Dima SO, Tanase C, Albulescu R, Herlea V, Chivu-Economescu M, Purnichescu-Purtan R, Dumitrascu T, Duda DG, Popescu I. An exploratory study of inflammatory cytokines as prognostic biomarkers in patients with ductal pancreatic adenocarcinoma. Pancreas. 2012;41:1001-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Logsdon CD, Lu W. The Significance of Ras Activity in Pancreatic Cancer Initiation. Int J Biol Sci. 2016;12:338-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Rashid S, Singh N, Gupta S, Rashid S, Nalika N, Sachdev V, Bal CS, Datta Gupta S, Chauhan SS, Saraya A. Progression of Chronic Pancreatitis to Pancreatic Cancer: Is There a Role of Gene Mutations as a Screening Tool? Pancreas. 2018;47:227-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Guerra C, Schuhmacher AJ, Cañamero M, Grippo PJ, Verdaguer L, Pérez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 872] [Cited by in F6Publishing: 902] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 44. | Arvanitakis M, Van Laethem JL, Parma J, De Maertelaer V, Delhaye M, Devière J. Predictive factors for pancreatic cancer in patients with chronic pancreatitis in association with K-ras gene mutation. Endoscopy. 2004;36:535-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | De La O JP, Murtaugh LC. Notch and Kras in pancreatic cancer: at the crossroads of mutation, differentiation and signaling. Cell Cycle. 2009;8:1860-1864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Cazacu IM, Farkas N, Garami A, Balaskó M, Mosdósi B, Alizadeh H, Gyöngyi Z, Rakonczay Z Jr, Vigh É, Habon T, Czopf L, Lazarescu MA, Erőss B, Sahin-Tóth M, Hegyi P. Pancreatitis-Associated Genes and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Pancreas. 2018;47:1078-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 47. | Hegyi P, Párniczky A, Lerch MM, Sheel ARG, Rebours V, Forsmark CE, Del Chiaro M, Rosendahl J, de-Madaria E, Szücs Á, Takaori K, Yadav D, Gheorghe C, Rakonczay Z Jr, Molero X, Inui K, Masamune A, Fernandez-Del Castillo C, Shimosegawa T, Neoptolemos JP, Whitcomb DC, Sahin-Tóth M; Working Group for the International (IAP – APA – JPS – EPC) Consensus Guidelines for Chronic Pancreatitis. International Consensus Guidelines for Risk Factors in Chronic Pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the International Association of Pancreatology, the American Pancreatic Association, the Japan Pancreas Society, and European Pancreatic Club. Pancreatology. 2020;20:579-585. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Rebours V, Boutron-Ruault MC, Schnee M, Férec C, Le Maréchal C, Hentic O, Maire F, Hammel P, Ruszniewski P, Lévy P. The natural history of hereditary pancreatitis: a national series. Gut. 2009;58:97-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 49. | Midha S, Khajuria R, Shastri S, Kabra M, Garg PK. Idiopathic chronic pancreatitis in India: phenotypic characterisation and strong genetic susceptibility due to SPINK1 and CFTR gene mutations. Gut. 2010;59:800-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Shelton CA, Umapathy C, Stello K, Yadav D, Whitcomb DC. Hereditary Pancreatitis in the United States: Survival and Rates of Pancreatic Cancer. Am J Gastroenterol. 2018;113:1376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 51. | Xue J, Zhao Q, Sharma V, Nguyen LP, Lee YN, Pham KL, Edderkaoui M, Pandol SJ, Park W, Habtezion A. Aryl Hydrocarbon Receptor Ligands in Cigarette Smoke Induce Production of Interleukin-22 to Promote Pancreatic Fibrosis in Models of Chronic Pancreatitis. Gastroenterology. 2016;151:1206-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 52. | Bouwense SA, Olesen SS, Drewes AM, Frøkjær JB, van Goor H, Wilder-Smith OH. Is altered central pain processing related to disease stage in chronic pancreatitis patients with pain? PLoS One. 2013;8:e55460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Beyer G, Habtezion A, Werner J, Lerch MM, Mayerle J. Chronic pancreatitis. Lancet. 2020;396:499-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 234] [Article Influence: 58.5] [Reference Citation Analysis (1)] |
| 54. | Issa Y, Kempeneers MA, Bruno MJ, Fockens P, Poley JW, Ahmed Ali U, Bollen TL, Busch OR, Dejong CH, van Duijvendijk P, van Dullemen HM, van Eijck CH, van Goor H, Hadithi M, Haveman JW, Keulemans Y, Nieuwenhuijs VB, Poen AC, Rauws EA, Tan AC, Thijs W, Timmer R, Witteman BJ, Besselink MG, van Hooft JE, van Santvoort HC, Dijkgraaf MG, Boermeester MA; Dutch Pancreatitis Study Group. Effect of Early Surgery vs Endoscopy-First Approach on Pain in Patients With Chronic Pancreatitis: The ESCAPE Randomized Clinical Trial. JAMA. 2020;323:237-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 55. | Gopalakrishnan G, Kalayarasan R, Gnanasekaran S, Pottakkat B. Frey's plus vs Frey's procedure for chronic pancreatitis: Analysis of postoperative outcomes and quality of life. Ann Hepatobiliary Pancreat Surg. 2020;24:496-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Dumonceau JM, Delhaye M, Tringali A, Arvanitakis M, Sanchez-Yague A, Vaysse T, Aithal GP, Anderloni A, Bruno M, Cantú P, Devière J, Domínguez-Muñoz JE, Lekkerkerker S, Poley JW, Ramchandani M, Reddy N, van Hooft JE. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Updated August 2018. Endoscopy. 2019;51:179-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |