Published online Jul 14, 2021. doi: 10.3748/wjg.v27.i26.3984
Peer-review started: January 27, 2021
First decision: March 7, 2021
Revised: March 19, 2021
Accepted: June 22, 2021
Article in press: June 22, 2021
Published online: July 14, 2021
Processing time: 165 Days and 5.4 Hours
Acute kidney injury (AKI) in cirrhosis, including hepatorenal syndrome (HRS), is a common and serious complication in cirrhotic patients, leading to significant morbidity and mortality. AKI is separated into two categories, non-HRS AKI and HRS-AKI. The most recent definition and diagnostic criteria of AKI in cirrhosis and HRS have helped diagnose and prognosticate the disease. The patho
Core Tip: This review paper is a comprehensive review of acute kidney injury in cirrhosis as well as hepatorenal syndrome. We review the most current topics including diagnosis, current definitions, pathophysiology, novel biomarkers, treatment, pharmacology, nonpharmacologic treatment, and topics of further research.
- Citation: Gupta K, Bhurwal A, Law C, Ventre S, Minacapelli CD, Kabaria S, Li Y, Tait C, Catalano C, Rustgi VK. Acute kidney injury and hepatorenal syndrome in cirrhosis. World J Gastroenterol 2021; 27(26): 3984-4003
- URL: https://www.wjgnet.com/1007-9327/full/v27/i26/3984.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i26.3984
Acute kidney injury (AKI) is a relative decrease in a kidney’s glomerular kidney function (GFR) and frequently occurs in patients. The incidence of AKI ranges from 20%-50% in cirrhotic patients when hospitalized for acute decompensation[1-6]. AKI imparts significant morbidity and mortality in patients with liver cirrhosis. Hospitalized cirrhotic patients have a high mortality rate, both inpatient and post-discharge[7]. Cirrhosis itself is a complex disease process that causes significant morbidity due to substantial volume shifts and increased vasodilation. Renal dysfunction, therefore, imparts another layer of complexity to those with cirrhosis and must be considered when a patient is being evaluated for liver transplantation (LT)[8].
Renal function is a weighted parameter in the Model for End-Stage Liver disease (MELD) score[9,10]. By accounting for creatinine, the MELD score allows patients with renal failure (acute or chronic) to receive liver transplants promptly[9,10]. Renal disease is an increasing health care burden in the United States as there has been a rise in the prevalence and incidence of type II DM and obesity along with chronic liver disease. Rustgi et al[11] calculated the additional cost of chronic kidney disease (CKD) in chronic liver disease patients by stage[11].
In the 1960s, Hecker and Sherlock described the process of renal dysfunction with the presence of ascites in advanced cirrhosis and defined it as hepatorenal syndrome (HRS)[12,13]. HRS is renal dysfunction resulting from systemic hemodynamic effects of portal hypertension secondary to liver cirrhosis[12], AKI in liver cirrhosis has been separated into non-HRS-AKI and HRS. The latter has been subdivided into type 1 HRS, known more recently as HRS-AKI, or type 2 HRS, known as HRS-CKD. The current recommendations and literature involving AKI and HRS in patients with liver cirrhosis are reviewed here.
The definition of HRS relies first and foremost on the definition of AKI. The definition of AKI has evolved. The first challenge has been determining the most accurate and available renal function measurement, which is the calculation of GFR. There is, however, no consensus on the most accurate method to measure GFR. Traditionally, the definition of AKI has been based on urine output and serum creatinine (sCr). The diagnosis of AKI is dependent on the patient’s baseline sCr. The International Club of Ascites (ICA) defines a baseline sCr as the last sCr within three months of current sCr[14].
The definition of AKI historically has gone through many updates as enumerated in Table 1[14-17]: Given the complexity of cirrhosis, AKI in cirrhosis needed its definition with specific criteria. In 2004, AKI was defined by the Acute Dialysis Quality Initiative (ADQI) group using the RIFLE criteria and divided into the three stages (stage 1 or R, stage 2 or I, or stage 3 or F)[15]. Further updates by the AKI Network (AKIN) and Kidney Disease Improving Global Outcomes (KDIGO), which labeled the stages 1-3[14-17]. Numerous consensus definitions have defined AKI. KDIGO is the most recent consensus definition for AKI that was updated in 2012[17]. In 2010, the ADQI with the ICA defined criteria for AKI in liver cirrhosis as shown in Table 2[18-20].
| Criteria | Stage | Definition |
| RIFLE criteria/ADQI in 2004[15] | At least 1.5 × baseline serum creatinine within 7 d, decrease in urine output of 0.5 mL/kg/h for 6 h, decrease in GFR of at least 25% | |
| Stage 1 (R) | 1.5 × baseline Cr, GFR decrease of 25%, UOP < 0.5 mL/kg/h for 6-12 h. | |
| Stage 2 (I) | 2 × baseline serum creatinine, decrease of GFR < 50%, UOP < 0.5 mL/kg/h for 12 h | |
| Stage 3 (F) | 3 × baseline serum creatinine, decrease of GFR of 75%, UOP < 0.3 mL/kg/h for 24 h, anuria for 12 h, or on RRT acutely | |
| Acute Kidney Injury Network (AKIN) in 2007[16] | Definition: increase of at least 0.3 mg/dL in last 48 h, 1.5 × baseline creatinine in last 48 h, or UOP < 0.5 mL/kg/h for at least 6 h | |
| Stage 1 | Increase of 0.3 mg/dL w/in 2 d, 1.5-2 × baseline serum creatinine within 2 d, or UOP < 0.5 mL/kg/h for 6-12 h | |
| Stage 2 | 2-3 × baseline serum Cr, UOP < 0.5 mL/kg/h for at least 12 h | |
| Stage 3 | 3 × baseline serum Cr, UOP < 0.3 mL/kg/h for 24 h, anuria for 12 h, on RRT | |
| Kidney Disease Improving Global Outcomes (KDIGO) in 2012[17] | Increase in sCr of at least 0.3 mg/dL within 48 h, increase of at least 1.5 × baseline in the last 7 d, or urine output < 0.5 mL/kg/h for at least 6 h | |
| Stage 1 | Increase of 0.3 mg/dL, 1.5-2 × baseline Cr, UOP < 0.5 mL/kg/h for 6-12 h | |
| Stage 2 | 2-3 × baseline serum Cr or UOP < 0.5 mL/kg/h for at least 12 h | |
| Stage 3 | 3 × baseline serum Cr, increase of 0.5 mg/dL above absolute level of 4.0 mg/dL, on RRT, UOP < 0.3 mL/kg/h for 24 h, or 12 h of anuria |
| Criteria | Stage | Definition |
| ADQI/ICA in 2010[19] | The absolute increase in serum Cr of at least 0.3 mg/dL or 1.5 × baseline serum creatinine | |
| Stage 1 | Increase of 0.3 mg/dL within 48 h or 1.5-2 × baseline serum creatinine | |
| Stage 2 | Increase of 2-3 × baseline serum Cr | |
| Stage 3 | At least 3 × baseline serum Cr with an increase of 0.5 mg/dL or currently on RRT | |
| ICA-AKI in 2015[14] | An absolute increase in serum Cr of at least 0.3 mg/dL within 48 h or 1.5 × baseline Cr level within the last 7 d | |
| Stage 1A | Increase of 0.3 mg/dL from baseline in 48 h, 1.5-2 × baseline serum creatine. Absolute value of serum Cr < 1.5 mg/dL | |
| Stage 1B | Increase of 0.3 mg/dL from baseline in 48 h, 1.5-2 × baseline serum creatine. Absolute value of serum Cr > 1.5 mg/dL | |
| Stage 2 | Increase of 2-3 × baseline | |
| Stage 3 | Greater than 3 × baseline Cr, Cr > 4 mg/dL with rise of > 0.5, or on RRT |
The guidelines were again updated in 2015 by the ICA to adopt the 2012 KDIGO definition of AKI. The benefit of the KDIGO criteria over the AKIN criteria for AKI is removing the absolute creatinine value of at least 1.5 mg/dL as a requirement, sCr in patients with cirrhosis may underestimate renal dysfunction due to low baseline muscle mass[14]. However, in staging AKI, as stressed by Angeli et al[14], the absolute level of 1.5 mg/dL was used to differentiate between stage 1-A and stage 1-B[14], as shown in Table 2. The new ICA criteria emphasize the importance of having a baseline sCr for making the diagnosis and allow for a prior sCr within three months to be considered a baseline[14].
HRS is defined as renal dysfunction in chronic liver disease (usually severe or advanced cirrhosis) or acute liver failure[1,8,14]. HRS has primarily considered a diagnosis of exclusion with specific criteria explained in Table 3, and its two types are generally differentiated by disease course. However, it may be challenging to differentiate from acute tubular necrosis (ATN). Table 3 lists the definitions of HRS types 1 and 2[21]. Type 1 and 2 HRS were renamed HRS-AKI and HRS-CKD in 2015. The most significant difference between the prior diagnosis of HRS type 1 and HRS-AKI has been eliminating an absolute sCr level of 2.5 mg/dL[21-23].
| Previous and current definition and nomenclature | |
| Criteria to confirm of HRS vs other etiology of renal dysfunction | To diagnose HRS, patients must have: (1) The presence of ascites; (2) No improvement of creatinine after holding diuretics; (3) No improvement after 48 h of albumin supplementation (1 g/kg/d); (4) No signs of shock; (5) No recent nephrotoxic medications (antibiotics, contrast, NSAIDs); and (6) No signs of kidney disease (proteinuria, microhematuria, no findings on renal ultrasound) |
| HRS type 1 (most recent definition in 2007) | Rapid renal injury (within two weeks) defined by 2 × baseline serum creatinine to a value > 2.5 mg/dL or 50% reduction in creatinine clearance |
| HRS type 2 | Moderate renal failure with creatinine ranging from 1.5 to 2.5 mg/dL that occurs progressively |
| Definition of HRS-AKI | Patients with the criteria above and ICA-AKI 2015 definition for AKI |
| Definition of HRS-CKD | Patients who meet the criteria in row 1 and the rise of serum creatinine and changes in urine output are all progressive (> 1 wk) |
| Patients with HRS-CKD are known to have decreased urine output over weeks to months |
HRS has been theorized to be caused by various mechanisms. The most well-understood hypothesis evokes splanchnic vasodilation changes, leading to increased peripheral vasoconstriction[24,25]. Additionally, there is evidence for other processes. Hepatocytes and stellate cells are known to produce vasodilatory mediators, including nitric oxide, prostacyclin, carbon monoxide, endogenous cannabinoids, adreno
The typical forms of non-HRS-AKI include prerenal azotemia (PRA), parenchymal renal disease, and drug-induced kidney injury. Prerenal AKI accounts for up to 60% of all AKI cases in patients with cirrhosis[2,34]. The most common causes of AKI in cirrhosis are hypovolemia, SBP, bacterial infections (other than SBP), sepsis, upper gastrointestinal bleeding, and shock. Infections and sepsis (urinary tract infections, pneumonia, skin infections, or SBP) cause decreased blood flow to the renal vasculature and cause kidney injury for cirrhosis patients who are already susceptible to volume shifts[3-5,36,37]. Frequent large-volume paracentesis can cause hypo
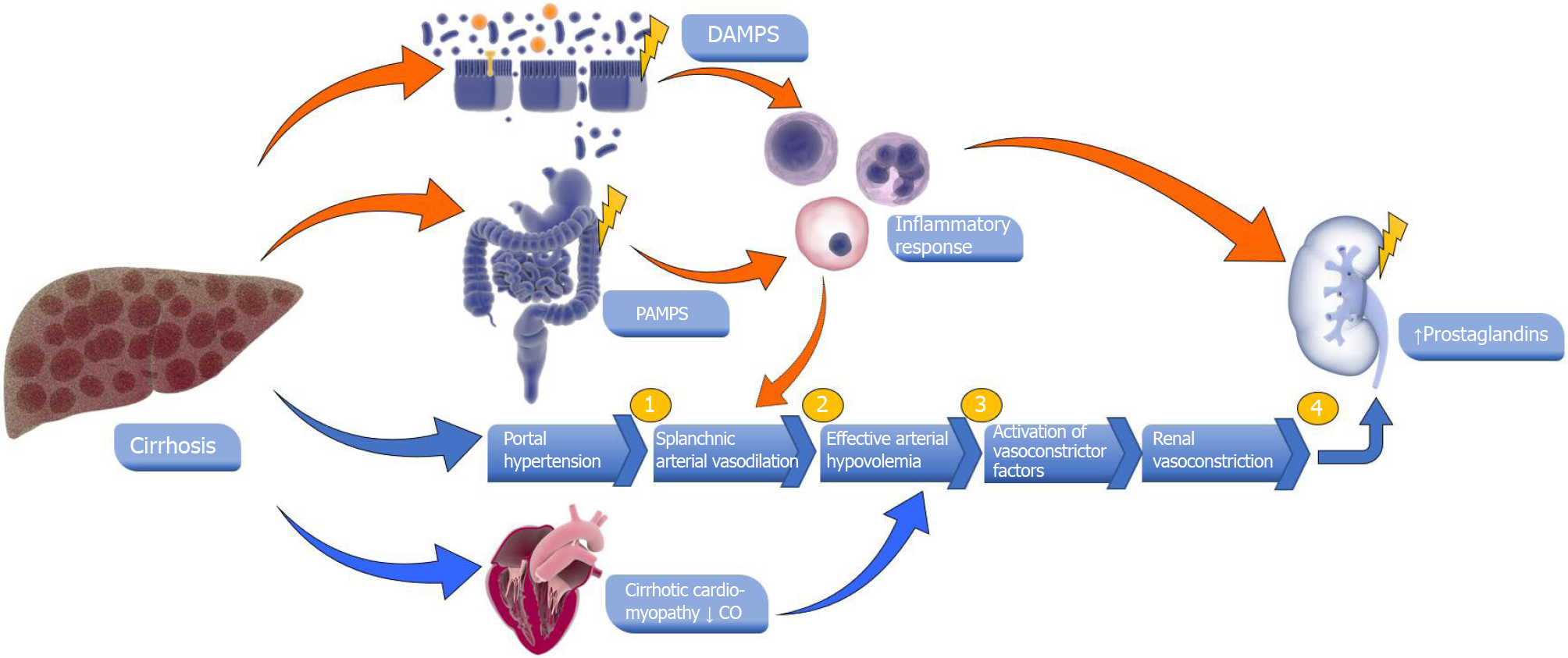
There are cirrhosis-specific mechanisms that also contribute to non-HRS AKI. Hepatic inflammation has been well-described in the literature for contributing to non-HRS AKI[12,38]. In the setting of cirrhosis or chronic liver disease, inflammation may be the result of damage-associated molecular patterns (DAMPs) in hepatocytes and gut immunity weakening from pathogen-associated molecular patterns (PAMPs)[12,39]. DAMPs specific to the liver include interleukin (IL)-1, IL-33, and bile acids recognized by the Kupfer cells’ toll-like receptors[12,40]. Gut bacterial translocation has been associated with the release of PAMPs (e.g., lipopolysaccharide), or DAMPs (e.g., heat shock proteins), from a cirrhotic liver leading to a systemic inflammatory response which can lead to the development of non-HRS AKI[12,41-45] (Figure 1).
Adrenal insufficiency is also frequently present in patients with cirrhosis. A retrospective study by Moini et al[46] evaluated 105 cirrhotic patients and reported that 15% of cirrhotic patients had some degree of adrenal insufficiency and identified hyponatremia and elevated international normalized ratio as risk factors for its development[46,47]. These processes can decrease glucocorticoids’ synthesis and result in adrenal insufficiency[48]. Inadequate adrenal response subsequently alters cardiovascular hemodynamics through vascular tone changes and cardiac output leading to decreased renal perfusion[46].
In patients with nonalcoholic steatohepatitis (NASH), studies have shown that around 28% have worsened renal function[46,49]. Patients with NASH/nonalcoholic fatty liver disease (NAFLD) and CKD have been shown to alter the renin-angiotensin system[46,50]. In patients with metabolic syndrome and NAFLD, alterations in the renin-angiotensin system with increased renin/angiotensin II receptor activation (from increased activation of angiotensin-converting enzyme-2) have been linked to hepatic steatosis, fibrosis and leading to NASH cirrhosis. This same process is well established to cause physiologic changes in the kidney, such as efferent artery vasoconstriction, which initially causes glomerular hyperfiltration and leads to hypertrophy with eventual scarring[46,50]. Other mechanisms in patients with NASH cirrhosis include 5’AMP-activated protein kinase activation, lipoprotein dysmetabolism, and oxidative damage through downregulation of sirtuin-1[46,51,52]. Patients with NAFLD/NASH will have comorbidities such as hypertension and diabetes mellitus and are highly susceptible to AKI[34,35].
In viral hepatitis, the most common kidney injury mechanism involves creating immune complexes with the virus, antibodies against infected hepatocytes, or direct cytopathic impact[46,53]. Hepatitis B infection is associated with polyarteritis nodosa (PAN), membranous nephropathy, and membranoproliferative glomerulonephritis[54,55]. Pathologically, renal biopsies generally reveal immune complex deposition, particularly hepatitis B envelope antigen in membranous nephropathy[55]. Chronic hepatitis C infections are also often linked with glomerular disease. The most common renal dysfunction causes include mixed cryoglobulinemia, PAN, and membranous nephropathy[56].
Early recognition of AKI and accurate measurement of renal function in cirrhosis is crucial when treating patients. Still, AKI can often be missed due to the baseline abnormalities present in patients with cirrhosis. Urine output is not an accurate measurement of a patient’s renal function or GFR in cirrhosis. Third-spacing causes urine output to drop, which underestimates renal function. At the same time, diuretic use may lead to an overestimation of renal function.
The most frequently used laboratory value to measure GFR is sCr because it is readily available, inexpensive, and accurate[57-60]. However, sCr has many factors that influence its value, such as race, age, gender, and muscle mass[18,60]. In cirrhosis, patients are malnourished, cachectic, and sarcopenic, leading to a deficiency in protein intake and is associated with muscle wasting[61]. These patient-specific factors are why creatinine may be lower in cirrhotic patients leading to an overestimation of GFR and renal function. Another factor leading to inaccuracy in creatinine correlating with GFR is that hyperbilirubinemia affects Jaffe’s kinetic assay that measures sCr and leads to an inaccurately low measurement[18,59].
sCr remains the primary measurement of renal function in cirrhosis because the use of novel biomarkers remains experimental[59]. Urinary sodium and the fractional excretion of sodium (FeNa) have only been used as an adjunct to sCr to help diagnose HRS and PRA[23].
Given that sCr may not evaluate the degree or the timing of AKI promptly, novel biomarkers with promise are being evaluated[59,62]. Cystatin C is a low-molecular-weight protein that is produced by all nucleated cells. It is filtered by the glomerulus and mainly reabsorbed by the proximal tubule[63]. Cystatin C testing is less readily available and is more expensive. Despite the limitations, cystatin C is not affected by age, muscle mass, malignancy, or inflammation[64,65]. The assay, unlike sCr, is not affected by high levels of serum bilirubin[66]. Prior studies have not had sufficient evidence of superiority for cystatin C in comparison to Cr. However, combination equations of Cr and cystatin C are superior to sCr[64,65]. Cystatin C is an independent predictor of AKI and outcomes, including mortality[67,68]. Other biomarkers of interest include neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), IL-18, and liver-type fatty acid-binding protein (L-FABP)[18,59,69]. The biomarkers’ clinical benefits and limitations are described in Table 4.
| Novel biomarker | Source | Benefits/Clinical uses | Limitations |
| Cystatin C[62-68] | Plasma, urine | Early biomarker of AKI, potential benefit with severity of disease. Unaffected with age, sarcopenia, gender, or sepsis. Unaffected by malignancy and serum bilirubin level. Multiple studies found it to be an independent risk factor of AKI and mortality | Increased levels in CKD. Influenced by low levels of albumin. Potentially influenced by elevated WBC and CRP. Takes longer time to result when compared to sCr |
| NGAL[18,67-79] | Urine | Found in kidney tubular cell that is released during damage or injury. Elevated in AKI in cirrhosis and potential predictor of mortality. Markedly elevated in ATN, mildly elevated in prerenal azotemia/CKD/HRS-AKI | Increased levels in CKD. Increased levels in infections, particularly urinary tract infections. Overlap with values in PRA, HRS, and other AKI types of AKI. Small quantities are made in the liver |
| IL-18[75,78,82-84] | Urine | Very similar to urinary NGAL. Markedly elevated in cirrhotic patients with ATN, in comparison to other AKI types. Found in monocytes and macrophages. A notable proinflammatory marker. Not confounded by CKD, sepsis or UTI | There are increased levels in PRA and HRS but significant overlap in values with limited clinical utility. Levels are increased in levels of inflammation in the kidney other than AKI |
| Kidney Injury Molecule-1[18,73,84-86] | Urine | Originally found in kidney tubular transmembrane protein. Not expressed in normal kidney tissue. Noted with increased levels in ATN in cirrhosis when compared to the other types of AKI in cirrhosis. High specificity for ischemic or nephrotoxic kidney injury | Elevated from inflammatory conditions. Found to have overlap between different forms of AKI. Confounded by presence of infection |
| L-FABP[87-93] | Urine | Found in kidney proximal tubule. Levels may be increased in AKI or AKI 2/2 sepsis. Potential utility in predictor in adverse outcomes including AKI in patients with chronic liver disease and other liver disease | Limited studies in cirrhosis. Found to be increased in CKD. Increased in acute liver injury and liver failure as well |
NGAL is a small protein made by the kidney, lung, stomach, and colon[70,71]. Using mouse and rat models, Mishra et al[70] in 2003 demonstrated that NGAL was upregulated in prerenal AKI and ATN setting and that increased urinary NGAL could be detected within 2 h of initial renal injury[70]. Multiple studies have evaluated the efficacy and utility of urinary NGAL in cirrhotic patients with AKI. When urinary NGAL was used to define and predict morbidity in AKI, the authors concluded that urinary NGAL levels were elevated in ATN compared to PRA or HRS-AKI. However, the most significant confounder in its utility is the overlap between ATN’s lower values and HRS’s upper values or PRA[18,72-75]. Two studies had found that urinary NGAL was superior to cystatin C in utility for diagnosis of AKI or ATN[75,76]. In contrast, Barreto et al[74] studied 132 cirrhotic patients hospitalized with infections. The authors found that among patients with persistent AKI, HRS-AKI could be accurately predicted with urinary NGAL values lower than 86 μg/g creatinine in 88% of patients[74]. In a study with 55 patients, Lee et al[77] found that urinary NGAL levels were significantly higher in ATN than HRS and PRA. Also, median urinary NGAL levels in HRS were markedly different from PRA levels, and the authors found that NGAL was an independent risk factor for mortality with AKI[77]. Jaques et al[67] studied multiple biomarkers in AKI in 55 decompensated cirrhosis patients. Compared to the non-AKI patients, they found that urinary NGAL levels are higher in ATN than PRA and HRS. However, HRS urinary NGAL levels had an intermediate pattern[67]. Urinary NGAL predicted poor outcomes in patients as well[67]. Kim et al[68] studied urinary NGAL and cystatin C in 328 decompensated cirrhosis patients (41 patients with AKI). The authors found that urinary NGAL is a predictor of AKI and outcomes (including mortality)[68]. Recently, Huelin et al[78] studied urinary NGAL and IL-18 on 320 cirrhosis patients with AKI. Urinary NGAL was elevated in AKI progression during hospitalization and was predictive of AKI progression in conjunction with MELD score. Urinary NGAL was significantly elevated in ATN when compared to hypovolemia-induced AKI and HRS-AKI[78]. Currently, there are no definitive diagnostic thresholds for differentiation between these types of AKI[79-81]. Urinary NGAL does not have an established role in the diagnosis, prediction, or prognosis of AKI in cirrhosis, but more promising results in extensive studies may change that. Another significant limitation is the expense of the test.
IL-18 is a proinflammatory cytokine expressed in the proximal tubule. It is released in urine when the cells are damaged in AKI[75]. Urinary IL-18 is elevated in patients with AKI, especially from ischemic injury, but urinary IL-18 is not elevated in conditions such as urinary tract infections, nephrotoxic injury, and CKD[75,82,83]. Tsai et al[84] in 2013 evaluated the clinical outcomes of 168 cirrhotic patients with AKI and severe sepsis. They found that urinary IL-18 was significantly higher in patients with ATN than patients with functional AKI, proposing a cutoff of 708.5 pg/mg creatinine to differentiate between the two groups. Urinary IL-18 was found to be a stronger predictor of ATN than serum IL-18. However, the authors were unable to conclude if urinary IL-18 could distinguish ATN from HRS-AKI. Clinically, they found that elevated urinary IL-18 was associated with higher hospital mortality[84]. Huelin et al[78], a study previously mentioned, studied IL-18 compared to urinary NGAL and found that it had a lower accuracy to predict ATN vs other forms of AKI[78].
KIM-1 is elevated in AKI from ischemic injury to the proximal tubule[83,84]. Belcher et al[73] evaluated KIM-1 in patients with AKI with other etiologies (PRA, ATN, and HRS) and found that ATN was the most elevated with overlap with HRS[73]. Other studies found that in patients with cirrhosis, elevations in urinary KIM-1 levels were increased mainly in ATN compared to other AKI presentations and could serve as a prognostic indicator[73,85,86].
L-FABP is a small protein found in the proximal tubular epithelium and binds to free fatty acids when reabsorbed in the proximal tubule[87]. L-FABP may be elevated in sepsis and specific etiologies of CKD (diabetic nephropathy or glomerulonephritis)[88]. Yamamoto et al[89] studied L-FABP in animal and human models (12 kidney transplant patients) in response to AKI[89]. The authors reported an increase in levels of L-FABP in mice models with prolonged exposure to ischemia to the kidneys, particularly during ischemic reperfusion injury. Doi et al[90] evaluated urinary L-FABP in 145 mice and 145 septic shock patients with AKI. L-FABP was high in septic shock patients with AKI and higher in the patients who did not survive[90]. L-FABP has been studied in acute liver failure and chronic liver disease and not just HRS and AKI in cirrhosis[91]. In patients with acetaminophen included acute liver failure, serum L-FABP levels were lower in survivors when compared to patients who passed away[92]. Eguchi et al[93] studied L-FABP in 242 chronic liver disease patients (chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma). The authors found that serum L-FABP increased in liver cirrhosis compared to chronic hepatitis and is higher in the presence of hepatocellular carcinoma. L-FABP correlates with kidney function markers, especially BUN, creatinine, and GFR[93]. This study does show the potential for L-FABP in chronic liver disease and other complications, including AKI. Serum L-FABP may have many clinical utilities in acute and chronic liver disease, including AKI; however, more large-scale studies should be performed to ascertain exact clinical utility.
Two new biomarkers being studied for potential benefits are insulin-like growth factor binding protein-7 and tissue matrix metalloproteinase inhibitor-2. However, there is not enough evidence to note potential utility. They are only approved for evaluating AKI in patients with intensive care unit (ICU) and need further evaluation[94]. Novel biomarkers can differentiate both the degree of renal dysfunction and possible etiology, but the data are not substantial enough to currently recommend utility. Additionally, these tests are not readily available and are expensive methods to evaluate renal function.
In AKI injury, clinicians must recognize and intervene as soon as possible. In patients with cirrhosis, all factors possibly contributing to AKI must be recognized promptly[14,20,37,95]. All unnecessary nephrotoxic medications such as Non-steroidal anti-inflammatory drugs should be discontinued and avoided altogether. Beta-blockers for variceal prophylaxis or other comorbidities should be evaluated for risk vs benefits[96,97]. In patients with PRA or dehydration, diuretics should first be discontinued as excessive diuresis is a common cause of kidney dysfunction in cirrhosis patients[20]. Excessive diarrhea from high doses of lactulose is another potential cause[20]. Patients with gastrointestinal bleeding should be transfused if indicated. Patients should have screening for infectious etiology, and patients should be placed on antibiotics immediately along with appropriate volume supplementation if an infection is diagnosed[98-100].
Clinicians should attempt a trial of volume expansion for the patients, but crystalloid, colloid, or blood products are dependent on etiology and clinical judgment. If a patient requires large-volume paracentesis, 6-8 g of albumin per liter of fluid removed after 5 L should be administered.
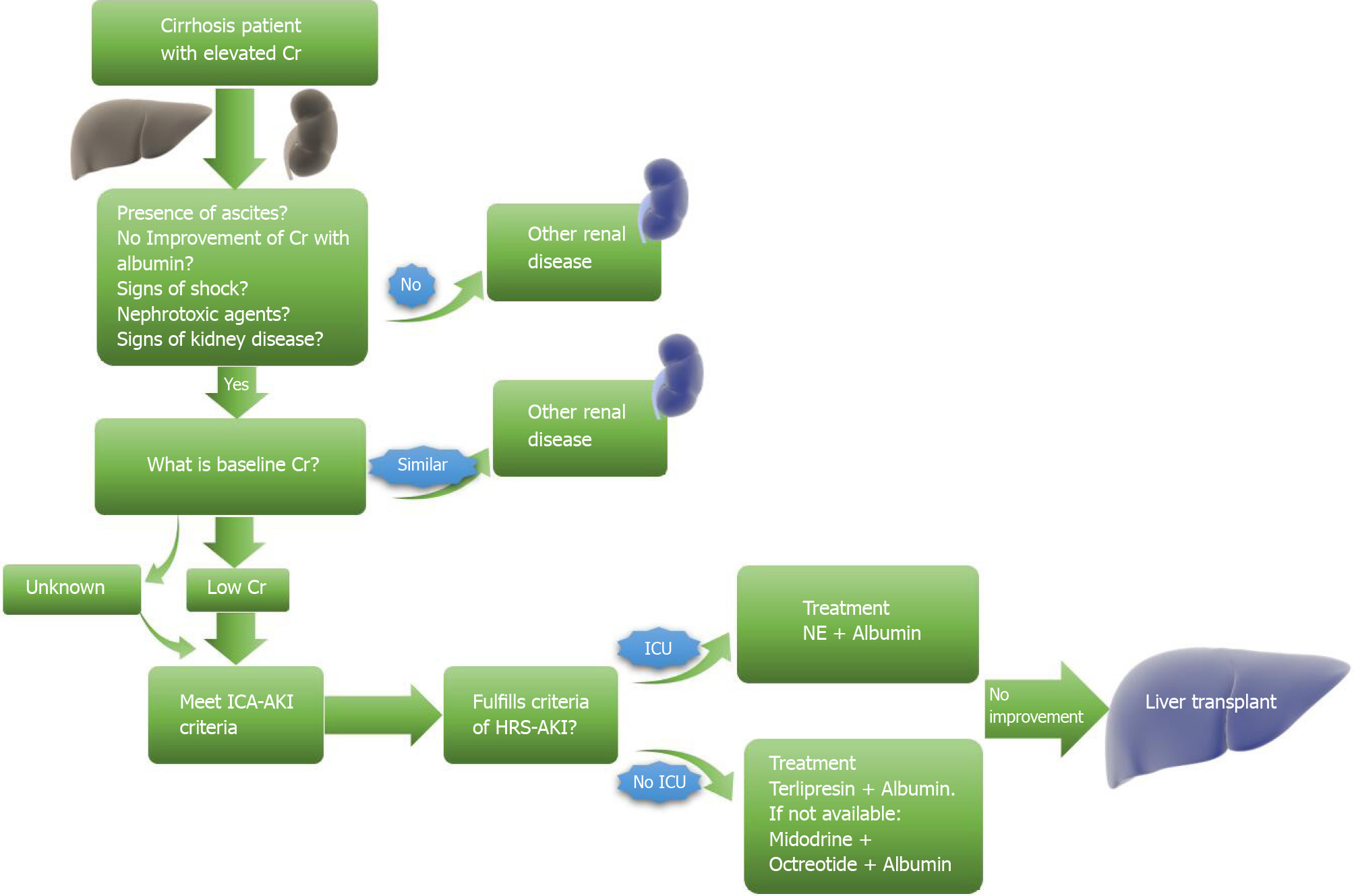
Therapeutic response is defined as improving serum creatine to at least 0.3 mg/dL near the baseline. However, even with adequate improvement, patients should be screened frequently to prevent a recurrence. Recommendations currently include an initial screen 2 to 4 d after discharge with a 2-4 wk follow-up for the first six months after discharge[14,36]. Patients with stage 2 or 3 AKI should be suspected of HRS-AKI, and HRS-AKI management should be initiated. Figure 2 provides a brief algorithm that can be used when first approaching AKI in a cirrhotic patient.
The patient meets the HRS criteria if there is no creatinine improvement after the withdrawal of all nephrotoxic agents and volume expansion with 1 g/kg/24 h for 48 h[14]. The patient should receive prompt pharmacologic therapy, which entails starting vasoconstrictor therapy with albumin supplementation to avoid cardiac output loss or loss of effective circulating volume[1,101]. The vasoconstrictors utilized for treatment are terlipressin, noradrenaline, octreotide, and midodrine[102-106]. The treatment goal is cited to be a goal sCr of 1.5 mg/dL or less with a reduction of at least 50%.
Terlipressin has been the most extensively studied and has the most robust evidence of efficacy in treating HRS-AKI of the three vasoconstrictor therapies with known superiority to octreotide and midodrine[101-108]. Terlipressin is more effective with fewer adverse effects when given in continuous infusions than bolus administration[99-108]. Over the years, multiple trials proved the efficacy of terlipressin with albumin as an effective treatment of HRS type 1[101,103-105,108-113]. A recent phase 3 trial by Wong et al[114] studied 300 patients using terlipressin and albumin compared to the placebo group. They found a significant improvement of HRS versal and renal function but was significantly associated with adverse events, including respiratory failure[114]. Serious adverse effects include angina, dysrhythmia, hypertension, and peripheral ischemia (intestines, fingers, scrotum). Patients with ischemic cardiomyopathy or peripheral vascular disease should not be treated with terlipressin[110]. Currently, it is not available in the Unoited States.
Noradrenaline has alpha-adrenergic properties that promote vasoconstriction with fewer effects on contractility[111,115]. Patients treated with noradrenaline require central venous access and require close, frequent monitoring in the ICU[116]. In their prospective study, Gupta et al[117] found norepinephrine to be an effective treatment for HRS reversal in 30 patients[117]. Multiple randomized controlled trials (RCTs) have compared noradrenaline to terlipressin[102,111,118-121]. Alessandria et al[118], in their pilot unblinded RCT, evaluated 22 patients comparing terlipressin and noradrenaline. The difference in HRS reversal was 83% and 70%, respectively, but there was no mortality difference[118]. Singh et al[119], Sharma et al[102], and Goyal et al[121] evaluated noradrenaline vs terlipressin and found them to have comparable efficacy and safety to improve HRS renal function[102,119,121]. Liu et al[122], in a randomized, double-blinded trial with 617 patients with septic shock found no significant difference in 28-d mortality between terlipressin compared to noradrenaline[122]. These studies have bolstered the use of noradrenaline, which is less expensive and more readily available in most countries. Consequently, Arora et al[123] in an open-label RCT, found that terlipressin, when compared to noradrenaline, showed significant improvement in the reversal of HRS (40% vs 16.7%), day 4 response (26.1% vs 11.7%), day 7 response (41.7% vs 20%) and in 28-d survival (48.3% vs 20%)[123].
The third vasoconstrictor therapy that is commonly used is midodrine in conjunction with albumin and octreotide. Midodrine is an alpha-adrenergic agonist that is frequently used in patients with orthostatic hypotension, and octreotide is a somatostatin analog that physiologically is meant to antagonize the primary pathophysiology of HRS[124,125]. In a pilot study, Angeli et al[124] evaluated the efficacy of octreotide, and midodrine found it to reverse HRS in around 40% of the patients with type 1 HRS[124]. It is recommended to utilize the regimen if terlipressin and noradrenaline are contraindicated or unavailable[116]. In 2009, Skagen et al[126], in a retrospective study, evaluated the use of octreotide, midodrine, and albumin in 75 patients and found that it improved short-term renal function and survival compared to the group who did not receive them[126].
Many patients, unfortunately, do not respond appropriately to pharmacologic therapy. After 14 d, all medications should be discontinued, and further nonpharmacologic treatment options must be considered.
Transjugular intrahepatic portosystemic shunt (TIPS) has been considered for the treatment of HRS, particularly HRS-AKI. Physiologically, treating portal hypertension should improve renal function in HRS; however, in practice, TIPS can cause transient ischemia to the liver, which can lead to acute on chronic liver failure. This may precipitate and worsen renal function in HRS, leading to increased mortality[1]. While several prospective studies have shown a significant benefit in renal function and mortality, they are limited by small size, lack of control groups, selection bias, and strict inclusion/exclusion criteria. The most extensive prospective study compared 31 transplant-ineligible patients with HRS (14 with HRS-AKI and 17 with HRS-NAKI) who underwent TIPS to 10 transplant-ineligible patients who did not undergo TIPS. The 3-mo survival rates were 81% for the group undergoing TIPS and 10% for the TIPS-ineligible group[127]. A 2018 meta-analysis of studies including 128 patients with HRS who underwent TIPS showed pooled 1-year survival rates of 47% in HRS-AKI patients and 64% in HRS-NAKI and renal improvement in 83% of patients[128]. While these results are certainly encouraging, randomized trials with adequate control groups are still lacking. Therefore, TIPS may be appropriate in specific clinical contexts but, at this time, is not routinely recommended in the treatment of HRS.
Renal replacement therapy (RRT) (hemodialysis) is not a treatment for HRS-AKI and is only meant to be a bridge for recovery of liver function or LT. RRT recommendations for cirrhosis patients are the same as for the general population (refractory volume overload, refractory electrolyte imbalance, refractory acidosis, uremia, or intoxication)[116]. Zhang et al[129], in a retrospective study, evaluated RRT in patients with HRS type 1 who did not respond to pharmacologic therapy. The study concluded that it did not improve mortality (30-d or 180-d survival)[129]. Patients who are not deemed transplant candidates are not considered candidates for RRT[130].
A molecular adsorbent recirculating system (MARS) is a form of albumin dialysis which circulates albumin to remove cytokines and bacterial products to combat vasodilation[12]. A 2010 RCT with 189 patients with acute-on-chronic liver failure (50% had HRS AKI) revealed a statistically significant reduction in sCr compared to medical management. However, overall mortality in 28 d was not significantly different in patients with HRS AKI[131]. In 2013, a trial by Lavayssière et al[132] studied MARS and found that compared to a control, MARS was able to lower bilirubin and sCr compared to the control group[132]. However, many studies did not show any significant improvement in creatinine or GFR after MARS. The RELIEF trial failed to show a statistically significant improvement in mortality compared to medical therapy[131]. Due to the equivocal results of all the trials evaluating MARS, the European Association for the Study of the Liver (EASL) does not recommend MARS for HRS treatment but suggested a further investigation into its potential benefits.
Another approach studied to bridge patients with cirrhosis to transplant or recovery includes bioartificial liver support systems. Several types exist, but all generally involve integrating animal or human hepatocytes into a bioreactor to filter toxins. These technologies continue to be studied in both clinical and preclinical trials, showing some promise in acute liver failure[133]. However, large-controlled trials are needed to understand better their role in the treatment of AKI in patients with acute on chronic liver failure.
Multiple studies have evaluated possible mechanisms to prevent HRS in patients from common causes. When treating infections in cirrhotic patients, there is evidence that albumin administration may have a protective role against HRS. The current recommendation to prevent HRS in SBP is albumin administration at a dosage of 1.5 g per kg on day 1 and 1 g per kg on day 3[134,135]. This albumin administration regimen has been found to reduce the incidence of HRS and overall mortality in SBP[134,136]. However, these results have not been replicated in other infections[136-138]. An RCT by Guevara et al[137] reported that renal function and circulatory function were significantly improved in the treatment group compared to the control with fewer cases of HRS type 1[137]. Another RCT by Thévenot et al[138] reported that albumin therapy delayed renal failure, but the 3-mo renal failure rate was not significantly improved. The authors cautioned using large amounts of albumin in critically ill cirrhotic patients[138]. SBP prophylaxis with norfloxacin has been studied and found to lower HRS incidence and improve survival[136,139].
The only definitive treatment of HRS refractory to pharmacologic therapy is LT. The use of creatinine in the MELD score has demonstrated the increased importance for patients with renal dysfunction (HRS-AKI or HRS-CKD) to undergo LT. In the setting of HRS, Boyer et al[140] reported a survival advantage of 100% vs 34% in patients with HRS treated with terlipressin and LT compared to patients treated with terlipressin alone[140]. Although LT remains the only definitive treatment of HRS-AKI, the role of the liver and even simultaneous liver-kidney transplant (SLK) remains unclear in the setting of non-HRS-AKI. In a large retrospective study comparing survival in HRS-AKI patients after undergoing SLK vs cirrhotic patients with non-HRS-AKI undergoing the same, HRS-AKI patients’ survival post-transplant was significantly superior to those in the non-HRS-AKI group[141].
The percentage of liver transplant recipients undergoing SLKs has substantially increased over the last 18 years. The increase in SLK is likely partly due to the adoption of the MELD score by the Unified Network for Organ Sharing in 2002. The MELD score places significant weight on sCr and imparts a high and increasingly higher transplant priority to progressive renal dysfunction patients. Guidelines for SLK, developed in 2012, were modified in 2017. For patients with cirrhosis and CKD, SLK was recommended for patients with epidermal GFR (eGFR) less than 60 mL/min for at least 90 d before listing or eGFR less than 35 mL/min during the time of listing or inherited metabolic disease[142]. In patients with cirrhosis and AKI, there must be a combination of dialysis and eGFR < 25 mL/min for six weeks[143].
AKI in cirrhosis has a high mortality rate, with 26% of patients dying before discharge[7]. Multiple studies show that the disease course and prognosis of AKI in cirrhosis depend on numerous factors-etiology of kidney injury, multiorgan dysfunction, stage of AKI upon diagnosis and progression of AKI, and lack of response to treatment[7]. Jenq et al[144], using the RIFLE criteria, found mortality of 134 cirrhotic patients admitted to the ICU to be 32.1% without AKI, 68.8% with RIFLE-R, 71.4% with RIFLE-I, and 94.8% with RIFLE-F[144]. However, the results were not reliable as patients admitted to the ICU usually have multiorgan dysfunction. The AKI stage directly correlates with in-hospital mortality and post-transplant mortality. Wong et al[145] found that the 30-d mortality of patients who do not recover from AKI was 80% vs 15% for those who recover[145]. Huelin et al[146] in a cohort of 547 patients, found a 90-d transplant-free survival to be 84% with stage 1A AKI, 58% with stage 1B AKI, 48% with stage 2 AKI, and 43% with stage 3 AKI compared to 89% with patients without AKI[1,146]. Bucsics et al[147], in a 239-patient retrospective study in 2015, also found that the 30-d mortality increased with increased stage of AKI on diagnosis or progression[147]. Mortality with AKI is markedly increased with complications of cirrhosis, including hepatic encephalopathy and ascites. In a retrospective study, Mindikoglu et al[148] reviewed 6917 cirrhotic patients between 2004 to 2014 who developed AKI during hospitalization and were subsequently discharged, and the authors calculated a 32% 90-d mortality and 48% 1-year mortality with higher rates in patients with pre-existing renal disease[148]. Although their study population was primarily male, this was one of the very few studies that studied post-discharge outcomes for patients, as most studies involved inpatient mortality only. Makar et al[149] studied the National Inpatient Sample data of 2016 and concluded that of the 6733 hospitalized cirrhosis patients who had AKI that patients with AKI had increased risk of mortality (OR: 8.09; 95%CI: 6.68-9.79; P < 0.0001) and prolonged hospital stay by 3.68 d (95%CI: 3.42-3.93; P < 0.0001)[149]. Another study found that community-acquired AKI had increased morbidity (progression to CKD) and mortality rates compared to hospital-acquired AKI[150]. In 2020, Tariq et al[151], in a meta-analysis of 18747 patients with cirrhosis (from 30 selected studies), found an in-hospital morality up to 6-fold higher in patients with AKI. Important risk factors were noted to be MELD score, Child-Pugh Turcotte stage C, presence of ascites, and sepsis (with or without shock)[151].
Once HRS of either type is diagnosed, it imparts a grave prognosis with median survival for HRS-AKI and HRS-NAKI determined to be about 1 and 6.7 mo, respectively[152]. Importantly, in all the studies evaluating AKI mortality in cirrhosis, the two types of AKI with the highest mortality were AKI-HRS and ATN[4,6,146,153]. Piano et al[6] also studied hospitalized patients with cirrhosis and ascites and AKI using the AKIN stage and found that patients who met the ICA criteria for HRS-AKI had the highest mortality[6]. Fagundes et al[4] found that patients with HRS or infection-related AKI had the highest mortality[4].
Regardless of type, AKI remains a severe complication to cirrhosis patients and a significant challenge for physicians tasked with treating it. Its incidence has increased as definitions shift to recognize and account for the unique clinical and laboratory abnormalities present in cirrhosis. Differentiating HRS-AKI from non-HRS-AKI is essential as the treatments vary, and early interventions may improve outcomes. Transplantation continues to be the only definitive therapy for HRS-AKI as more data are needed to support the use of less invasive strategies such as TIPS and liver replacement therapy. As our understanding of these diseases’ pathophysiology and progression evolve, novel biomarkers and directed therapies will hopefully evolve as well.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bramhall SR, Wu ZQ S-Editor: Fan JR L-Editor: A P-Editor: Liu JH
| 1. | Ginès P, Solà E, Angeli P, Wong F, Nadim MK, Kamath PS. Hepatorenal syndrome. Nat Rev Dis Primers. 2018;4:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (3)] |
| 2. | Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 3. | de Carvalho JR, Villela-Nogueira CA, Luiz RR, Guzzo PL, da Silva Rosa JM, Rocha E, Moraes Coelho HS, de Mello Perez R. Acute kidney injury network criteria as a predictor of hospital mortality in cirrhotic patients with ascites. J Clin Gastroenterol. 2012;46:e21-e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Fagundes C, Barreto R, Guevara M, Garcia E, Solà E, Rodríguez E, Graupera I, Ariza X, Pereira G, Alfaro I, Cárdenas A, Fernández J, Poch E, Ginès P. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. 2013;59:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 5. | Warner NS, Cuthbert JA, Bhore R, Rockey DC. Acute kidney injury and chronic kidney disease in hospitalized patients with cirrhosis. J Investig Med. 2011;59:1244-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 6. | Piano S, Rosi S, Maresio G, Fasolato S, Cavallin M, Romano A, Morando F, Gola E, Frigo AC, Gatta A, Angeli P. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013;59:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 7. | DellaVolpe J, Al-Khafaji A. Acute Kidney Injury Before and After Liver Transplant. J Intensive Care Med. 2019;34:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 541] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 9. | Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R; United Network for Organ Sharing Liver Disease Severity Score Committee. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 1863] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 10. | Gonwa TA, McBride MA, Anderson K, Mai ML, Wadei H, Ahsan N. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant. 2006;6:2651-2659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 11. | Rustgi VK, Li Y, John T, Catalano C, Elsaid MI. Health Care Resource Use and Cost Burden of Chronic Kidney Disease in Patients With Chronic Liver Disease: A Real-World Claims Analysis. Hepatol Commun. 2020;4:1404-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Chancharoenthana W, Leelahavanichkul A. Acute kidney injury spectrum in patients with chronic liver disease: Where do we stand? World J Gastroenterol. 2019;25:3684-3703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (4)] |
| 13. | Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif. 2002;20:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 222] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 14. | Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S, Moore K, Lee SS, Durand F, Salerno F, Caraceni P, Kim WR, Arroyo V, Garcia-Tsao G. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62:968-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 527] [Article Influence: 52.7] [Reference Citation Analysis (1)] |
| 15. | Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-R212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4448] [Cited by in RCA: 4697] [Article Influence: 223.7] [Reference Citation Analysis (0)] |
| 16. | Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4846] [Cited by in RCA: 4984] [Article Influence: 276.9] [Reference Citation Analysis (0)] |
| 17. | Kellum JA, Lameire N; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1192] [Cited by in RCA: 1845] [Article Influence: 153.8] [Reference Citation Analysis (0)] |
| 18. | MacDonald AJ, Nadim MK, Durand F, Karvellas CJ. Acute kidney injury in cirrhosis: implications for liver transplantation. Curr Opin Crit Care. 2019;25:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Nadim MK, Kellum JA, Davenport A, Wong F, Davis C, Pannu N, Tolwani A, Bellomo R, Genyk YS; ADQI Workgroup. Hepatorenal syndrome: the 8th International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2012;16:R23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Wong F. Acute kidney injury in liver cirrhosis: new definition and application. Clin Mol Hepatol. 2016;22:415-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Postgrad Med J. 2008;84:662-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 356] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 22. | Wong F. The evolving concept of acute kidney injury in patients with cirrhosis. Nat Rev Gastroenterol Hepatol. 2015;12:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, Reynolds TB, Ring-Larsen H, Schölmerich J. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1020] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 24. | Martín-Llahí M, Guevara M, Torre A, Fagundes C, Restuccia T, Gilabert R, Solá E, Pereira G, Marinelli M, Pavesi M, Fernández J, Rodés J, Arroyo V, Ginès P. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology 2011; 140: 488-496. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 25. | Fagundes C, Ginès P. Hepatorenal syndrome: a severe, but treatable, cause of kidney failure in cirrhosis. Am J Kidney Dis. 2012;59:874-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 26. | Martin PY, Ginès P, Schrier RW. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Engl J Med. 1998;339:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 272] [Article Influence: 10.1] [Reference Citation Analysis (33)] |
| 27. | Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121-S131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 404] [Article Influence: 21.3] [Reference Citation Analysis (14)] |
| 28. | Ros J, Clària J, To-Figueras J, Planagumà A, Cejudo-Martín P, Fernández-Varo G, Martín-Ruiz R, Arroyo V, Rivera F, Rodés J, Jiménez W. Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat. Gastroenterology. 2002;122:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Acevedo JG, Cramp ME. Hepatorenal syndrome: Update on diagnosis and therapy. World J Hepatol. 2017;9:293-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (3)] |
| 30. | Henriksen JH, Møller S, Ring-Larsen H, Christensen NJ. The sympathetic nervous system in liver disease. J Hepatol. 1998;29:328-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 120] [Article Influence: 4.4] [Reference Citation Analysis (34)] |
| 31. | Bernardi M, Trevisani F, Gasbarrini A, Gasbarrini G. Hepatorenal disorders: role of the renin-angiotensin-aldosterone system. Semin Liver Dis. 1994;14:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (34)] |
| 32. | Wong F. Cirrhotic cardiomyopathy. Hepatol Int. 2009;3:294-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 33. | Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Ginès P, Moreira V, Milicua JM, Jiménez W, Arroyo V. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 383] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 34. | Choi YJ, Kim JH, Koo JK, Lee CI, Lee JY, Yang JH, Ko SY, Choe WH, Kwon SY, Lee CH. Prevalence of renal dysfunction in patients with cirrhosis according to ADQI-IAC working party proposal. Clin Mol Hepatol. 2014;20:185-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Fang JT, Tsai MH, Tian YC, Jenq CC, Lin CY, Chen YC, Lien JM, Chen PC, Yang CW. Outcome predictors and new score of critically ill cirrhotic patients with acute renal failure. Nephrol Dial Transplant. 2008;23:1961-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Tsien CD, Rabie R, Wong F. Acute kidney injury in decompensated cirrhosis. Gut. 2013;62:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 37. | Bucsics T, Krones E. Renal dysfunction in cirrhosis: acute kidney injury and the hepatorenal syndrome. Gastroenterol Rep (Oxf). 2017;5:127-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 38. | Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 507] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 39. | Strnad P, Tacke F, Koch A, Trautwein C. Liver - guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 442] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 40. | Woolbright BL, Jaeschke H. The impact of sterile inflammation in acute liver injury. J Clin Transl Res. 2017;3:170-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 846] [Article Influence: 76.9] [Reference Citation Analysis (1)] |
| 42. | Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 432] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 43. | Cazzaniga M, Dionigi E, Gobbo G, Fioretti A, Monti V, Salerno F. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol. 2009;51:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 44. | Cervoni JP, Thévenot T, Weil D, Muel E, Barbot O, Sheppard F, Monnet E, Di Martino V. C-reactive protein predicts short-term mortality in patients with cirrhosis. J Hepatol. 2012;56:1299-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 45. | Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 561] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 46. | Moini M, Yazdani Sarvestani M, Shams M, Nomovi M. Evaluation of Adrenal Function in Nonhospitalized Patients with Cirrhosis. Can J Gastroenterol Hepatol. 2017;2017:2354253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Muciño-Bermejo MJ. Mechanisms of kidney dysfunction in the cirrhotic patient: Non-hepatorenal acute-on-chronic kidney damage considerations. Ann Hepatol. 2020;19:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Acevedo J, Fernández J, Prado V, Silva A, Castro M, Pavesi M, Roca D, Jimenez W, Ginès P, Arroyo V. Relative adrenal insufficiency in decompensated cirrhosis: Relationship to short-term risk of severe sepsis, hepatorenal syndrome, and death. Hepatology. 2013;58:1757-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 49. | Nampoothiri RV, Duseja A, Rathi M, Agrawal S, Sachdeva N, Mehta M, Dhaliwal HS, Dhiman RK, Chawla Y. Renal Dysfunction in Patients With Nonalcoholic Fatty Liver Disease is Related to the Presence of Diabetes Mellitus and Severity of Liver Disease. J Clin Exp Hepatol. 2019;9:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Mizuiri S, Ohashi Y. ACE and ACE2 in kidney disease. World J Nephrol. 2015;4:74-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 128] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 51. | Musso G, Gambino R, Cassader M. Emerging molecular targets for the treatment of nonalcoholic fatty liver disease. Annu Rev Med. 2010;61:375-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Ramis MR, Esteban S, Miralles A, Tan DX, Reiter RJ. Caloric restriction, resveratrol and melatonin: Role of SIRT1 and implications for aging and related-diseases. Mech Ageing Dev. 2015;146-148:28-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 53. | Lhotta K. Beyond hepatorenal syndrome: glomerulonephritis in patients with liver disease. Semin Nephrol. 2002;22:302-308. [PubMed] |
| 54. | Johnson RJ, Couser WG. Hepatitis B infection and renal disease: clinical, immunopathogenetic and therapeutic considerations. Kidney Int. 1990;37:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 140] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 55. | Lai KN, Ho RT, Tam JS, Lai FM. Detection of hepatitis B virus DNA and RNA in kidneys of HBV related glomerulonephritis. Kidney Int. 1996;50:1965-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Lai TS, Lee MH, Yang HI, You SL, Lu SN, Wang LY, Yuan Y, L'Italien G, Chien KL, Chen CJ; REVEAL-HCV Study Group. Hepatitis C viral load, genotype, and increased risk of developing end-stage renal disease: REVEAL-HCV study. Hepatology. 2017;66:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 57. | Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10672] [Cited by in RCA: 11005] [Article Influence: 224.6] [Reference Citation Analysis (1)] |
| 58. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11183] [Cited by in RCA: 11817] [Article Influence: 454.5] [Reference Citation Analysis (0)] |
| 59. | Francoz C, Nadim MK, Durand F. Kidney biomarkers in cirrhosis. J Hepatol. 2016;65:809-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 60. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20319] [Cited by in RCA: 20105] [Article Influence: 1256.6] [Reference Citation Analysis (0)] |
| 61. | Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis. 2003;41:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 233] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 62. | Herget-Rosenthal S, Trabold S, Pietruck F, Holtmann M, Philipp T, Kribben A. Cystatin C: efficacy as screening test for reduced glomerular filtration rate. Am J Nephrol. 2000;20:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 63. | Tenstad O, Roald AB, Grubb A, Aukland K. Renal handling of radiolabelled human cystatin C in the rat. Scand J Clin Lab Invest. 1996;56:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 316] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 64. | Shlipak MG, Coresh J, Gansevoort RT. Cystatin C versus creatinine for kidney function-based risk. N Engl J Med. 2013;369:2459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2913] [Cited by in RCA: 3153] [Article Influence: 242.5] [Reference Citation Analysis (0)] |
| 66. | Gerbes AL, Gülberg V, Bilzer M, Vogeser M. Evaluation of serum cystatin C concentration as a marker of renal function in patients with cirrhosis of the liver. Gut. 2002;50:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 67. | Jaques DA, Spahr L, Berra G, Poffet V, Lescuyer P, Gerstel E, Garin N, Martin PY, Ponte B. Biomarkers for acute kidney injury in decompensated cirrhosis: A prospective study. Nephrology (Carlton). 2019;24:170-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 68. | Kim TH, Seo YS, Kang SH, Kim MY, Kim SG, Lee HY, Lee JH, Lee YS, Kim JH, Jeong SW, Jang JY, Suk KT, Jung YK, An H, Yim HJ, Kim YS, Um SH; Korean Study Group of Portal Hypertension. Prognosis predictability of serum and urine renal markers in patients with decompensated cirrhosis: A multicentre prospective study. Liver Int. 2020;40:3083-3092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 69. | Puthumana J, Ariza X, Belcher JM, Graupera I, Ginès P, Parikh CR. Urine Interleukin 18 and Lipocalin 2 Are Biomarkers of Acute Tubular Necrosis in Patients With Cirrhosis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2017; 15: 1003-1013. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 70. | Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534-2543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1227] [Cited by in RCA: 1286] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 71. | Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Barasch J, Devarajan P. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004;15:3073-3082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 371] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 72. | Fagundes C, Pépin MN, Guevara M, Barreto R, Casals G, Solà E, Pereira G, Rodríguez E, Garcia E, Prado V, Poch E, Jiménez W, Fernández J, Arroyo V, Ginès P. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol. 2012;57:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 73. | Belcher JM, Sanyal AJ, Peixoto AJ, Perazella MA, Lim J, Thiessen-Philbrook H, Ansari N, Coca SG, Garcia-Tsao G, Parikh CR; TRIBE-AKI Consortium. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology. 2014;60:622-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 244] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 74. | Barreto R, Elia C, Solà E, Moreira R, Ariza X, Rodríguez E, Graupera I, Alfaro I, Morales-Ruiz M, Poch E, Guevara M, Fernández J, Jiménez W, Arroyo V, Ginès P. Urinary neutrophil gelatinase-associated lipocalin predicts kidney outcome and death in patients with cirrhosis and bacterial infections. J Hepatol. 2014;61:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 75. | Ariza X, Solà E, Elia C, Barreto R, Moreira R, Morales-Ruiz M, Graupera I, Rodríguez E, Huelin P, Solé C, Fernández J, Jiménez W, Arroyo V, Ginès P. Analysis of a urinary biomarker panel for clinical outcomes assessment in cirrhosis. PLoS One. 2015;10:e0128145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 76. | Jo SK, Yang J, Hwang SM, Lee MS, Park SH. Role of biomarkers as predictors of acute kidney injury and mortality in decompensated cirrhosis. Sci Rep. 2019;9:14508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 77. | Lee JH, Yoon EL, Park SE, Park JY, Choi JM, Jeon TJ, Shin WC, Choi WC. Clinical Significance of Urinary Neutrophil Gelatinase-associated Lipocalin Levels in Defining the Various Etiologies of Acute Kidney Injury in Liver Cirrhosis Patients. Korean J Gastroenterol. 2019;74:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 78. | Huelin P, Solà E, Elia C, Solé C, Risso A, Moreira R, Carol M, Fabrellas N, Bassegoda O, Juanola A, de Prada G, Albertos S, Piano S, Graupera I, Ariza X, Napoleone L, Pose E, Filella X, Morales-Ruiz M, Rios J, Fernández J, Jiménez W, Poch E, Torres F, Ginès P. Neutrophil Gelatinase-Associated Lipocalin for Assessment of Acute Kidney Injury in Cirrhosis: A Prospective Study. Hepatology. 2019;70:319-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 79. | Davenport A, Sheikh MF, Lamb E, Agarwal B, Jalan R. Acute kidney injury in acute-on-chronic liver failure: where does hepatorenal syndrome fit? Kidney Int. 2017;92:1058-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 80. | Ostermann M, Joannidis M. Biomarkers for AKI improve clinical practice: no. Intensive Care Med. 2015;41:618-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Glassford NJ, Schneider AG, Xu S, Eastwood GM, Young H, Peck L, Venge P, Bellomo R. The nature and discriminatory value of urinary neutrophil gelatinase-associated lipocalin in critically ill patients at risk of acute kidney injury. Intensive Care Med. 2013;39:1714-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 82. | Liu Y, Guo W, Zhang J, Xu C, Yu S, Mao Z, Wu J, Ye C, Mei C, Dai B. Urinary interleukin 18 for detection of acute kidney injury: a meta-analysis. Am J Kidney Dis. 2013;62:1058-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 83. | Wu H, Craft ML, Wang P, Wyburn KR, Chen G, Ma J, Hambly B, Chadban SJ. IL-18 contributes to renal damage after ischemia-reperfusion. J Am Soc Nephrol. 2008;19:2331-2341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 84. | Tsai MH, Chen YC, Yang CW, Jenq CC, Fang JT, Lien JM, Hung CC, Weng HH, Wu CS, Peng YS, Shen CH, Tung SY, Tian YC. Acute renal failure in cirrhotic patients with severe sepsis: value of urinary interleukin-18. J Gastroenterol Hepatol. 2013;28:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 85. | Qasem AA, Farag SE, Hamed E, Emara M, Bihery A, Pasha H. Urinary biomarkers of acute kidney injury in patients with liver cirrhosis. ISRN Nephrol. 2014;2014:376795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 86. | Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135-4142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 885] [Cited by in RCA: 934] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 87. | Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7:489-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1415] [Cited by in RCA: 1329] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 88. | Xu Y, Xie Y, Shao X, Ni Z, Mou S. L-FABP: A novel biomarker of kidney disease. Clin Chim Acta. 2015;445:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 89. | Yamamoto T, Noiri E, Ono Y, Doi K, Negishi K, Kamijo A, Kimura K, Fujita T, Kinukawa T, Taniguchi H, Nakamura K, Goto M, Shinozaki N, Ohshima S, Sugaya T. Renal L-type fatty acid--binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18:2894-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 271] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 90. | Doi K, Noiri E, Maeda-Mamiya R, Ishii T, Negishi K, Hamasaki Y, Fujita T, Yahagi N, Koide H, Sugaya T, Nakamura T. Urinary L-type fatty acid-binding protein as a new biomarker of sepsis complicated with acute kidney injury. Crit Care Med. 2010;38:2037-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 91. | Eguchi A, Iwasa M. The Role of Elevated Liver-Type Fatty Acid-Binding Proteins in Liver Diseases. Pharm Res. 2021;38:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 92. | Karvellas CJ, Speiser JL, Tremblay M, Lee WM, Rose CF; US Acute Liver Failure Study Group. Elevated FABP1 serum levels are associated with poorer survival in acetaminophen-induced acute liver failure. Hepatology. 2017;65:938-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 93. | Eguchi A, Hasegawa H, Iwasa M, Tamai Y, Ohata K, Oikawa T, Sugaya T, Takei Y. Serum Liver-Type Fatty Acid-Binding Protein Is a Possible Prognostic Factor in Human Chronic Liver Diseases From Chronic Hepatitis to Liver Cirrhosis and Hepatocellular Carcinoma. Hepatol Commun. 2019;3:825-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 94. | Allegretti AS, Solà E, Ginès P. Clinical Application of Kidney Biomarkers in Cirrhosis. Am J Kidney Dis. 2020;76:710-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 95. | Wong F. Diagnosing and treating renal disease in cirrhotic patients. Minerva Gastroenterol Dietol. 2016;62:253-266. [PubMed] |
| 96. | Mandorfer M, Reiberger T. Beta blockers and cirrhosis, 2016. Dig Liver Dis. 2017;49:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 97. | Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Kruzik M, Hagmann M, Blacky A, Ferlitsch A, Sieghart W, Trauner M, Peck-Radosavljevic M, Reiberger T. Nonselective β blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology 2014; 146: 1680-90. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 280] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 98. | Schwabl P, Bucsics T, Soucek K, Mandorfer M, Bota S, Blacky A, Hirschl AM, Ferlitsch A, Trauner M, Peck-Radosavljevic M, Reiberger T. Risk factors for development of spontaneous bacterial peritonitis and subsequent mortality in cirrhotic patients with ascites. Liver Int. 2015;35:2121-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 99. | Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R, Albillos A, Lammert F, Wilmer A, Mookerjee R, Vila J, Garcia-Martinez R, Wendon J, Such J, Cordoba J, Sanyal A, Garcia-Tsao G, Arroyo V, Burroughs A, Ginès P. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 642] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 100. | Arabi YM, Dara SI, Memish Z, Al Abdulkareem A, Tamim HM, Al-Shirawi N, Parrillo JE, Dodek P, Lapinsky S, Feinstein D, Wood G, Dial S, Zanotti S, Kumar A; Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Antimicrobial therapeutic determinants of outcomes from septic shock among patients with cirrhosis. Hepatology. 2012;56:2305-2315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 101. | Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, Blei A, Gülberg V, Sigal S, Teuber P; Terlipressin Study Group. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 446] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 102. | Sharma P, Kumar A, Shrama BC, Sarin SK. An open label, pilot, randomized controlled trial of noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. Am J Gastroenterol. 2008;103:1689-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 103. | Martín-Llahí M, Pépin MN, Guevara M, Díaz F, Torre A, Monescillo A, Soriano G, Terra C, Fábrega E, Arroyo V, Rodés J, Ginès P; TAHRS Investigators. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 401] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 104. | Cavallin M, Kamath PS, Merli M, Fasolato S, Toniutto P, Salerno F, Bernardi M, Romanelli RG, Colletta C, Salinas F, Di Giacomo A, Ridola L, Fornasiere E, Caraceni P, Morando F, Piano S, Gatta A, Angeli P; Italian Association for the Study of the Liver Study Group on Hepatorenal Syndrome. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: A randomized trial. Hepatology. 2015;62:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 259] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 105. | Boyer TD, Sanyal AJ, Wong F, Frederick RT, Lake JR, O'Leary JG, Ganger D, Jamil K, Pappas SC; REVERSE Study Investigators. Terlipressin Plus Albumin Is More Effective Than Albumin Alone in Improving Renal Function in Patients With Cirrhosis and Hepatorenal Syndrome Type 1. Gastroenterology 2016; 150: 1579-1589. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 106. | Cavallin M, Piano S, Romano A, Fasolato S, Frigo AC, Benetti G, Gola E, Morando F, Stanco M, Rosi S, Sticca A, Cillo U, Angeli P. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: A randomized controlled study. Hepatology. 2016;63:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 107. | Gifford FJ, Morling JR, Fallowfield JA. Systematic review with meta-analysis: vasoactive drugs for the treatment of hepatorenal syndrome type 1. Aliment Pharmacol Ther. 2017;45:593-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 108. | Solanki P, Chawla A, Garg R, Gupta R, Jain M, Sarin SK. Beneficial effects of terlipressin in hepatorenal syndrome: a prospective, randomized placebo-controlled clinical trial. J Gastroenterol Hepatol. 2003;18:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 109. | Neri S, Pulvirenti D, Malaguarnera M, Cosimo BM, Bertino G, Ignaccolo L, Siringo S, Castellino P. Terlipressin and albumin in patients with cirrhosis and type I hepatorenal syndrome. Dig Dis Sci. 2008;53:830-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 110. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1131] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 111. | Mattos ÂZ, Schacher FC, Mattos AA. Vasoconstrictors in hepatorenal syndrome - A critical review. Ann Hepatol. 2019;18:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 112. | Krishna R, Raj J, Dev D, Prasad SC, Reghu R, V SO. A study on clinical outcomes of combination of terlipressin and albumin in Hepatorenal Syndrome. Scand J Gastroenterol. 2020;55:860-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 113. | Kalambokis GN, Christaki M, Tsiakas I, Despotis G, Milionis HJ. Efficacy of treatment with terlipressin plus albumin in hepatorenal syndrome diagnosed with the new acute kidney injury versus the conventional criteria. Eur J Gastroenterol Hepatol. 2019;31:1292-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 114. | Wong F, Pappas SC, Curry MP, Reddy KR, Rubin RA, Porayko MK, Gonzalez SA, Mumtaz K, Lim N, Simonetto DA, Sharma P, Sanyal AJ, Mayo MJ, Frederick RT, Escalante S, Jamil K; CONFIRM Study Investigators. Terlipressin plus Albumin for the Treatment of Type 1 Hepatorenal Syndrome. N Engl J Med. 2021;384:818-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 306] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 115. | Duvoux C, Zanditenas D, Hézode C, Chauvat A, Monin JL, Roudot-Thoraval F, Mallat A, Dhumeaux D. Effects of noradrenalin and albumin in patients with type I hepatorenal syndrome: a pilot study. Hepatology. 2002;36:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 215] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 116. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1811] [Article Influence: 258.7] [Reference Citation Analysis (2)] |
| 117. | Gupta K, Rani P, Rohatgi A, Verma M, Handa S, Dalal K, Jain A. Noradrenaline for reverting hepatorenal syndrome: a prospective, observational, single-center study. Clin Exp Gastroenterol. 2018;11:317-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 118. | Alessandria C, Ottobrelli A, Debernardi-Venon W, Todros L, Cerenzia MT, Martini S, Balzola F, Morgando A, Rizzetto M, Marzano A. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. J Hepatol. 2007;47:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 215] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 119. | Singh V, Ghosh S, Singh B, Kumar P, Sharma N, Bhalla A, Sharma AK, Choudhary NS, Chawla Y, Nain CK. Noradrenaline vs. terlipressin in the treatment of hepatorenal syndrome: a randomized study. J Hepatol. 2012;56:1293-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 120. | Ghosh S, Choudhary NS, Sharma AK, Singh B, Kumar P, Agarwal R, Sharma N, Bhalla A, Chawla YK, Singh V. Noradrenaline vs terlipressin in the treatment of type 2 hepatorenal syndrome: a randomized pilot study. Liver Int. 2013;33:1187-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 121. | Goyal O, Sidhu SS, Sehgal N, Puri S. Noradrenaline is as Effective as Terlipressin in Hepatorenal Syndrome Type 1: A Prospective, Randomized Trial. J Assoc Physicians India. 2016;64:30-35. [PubMed] |
| 122. | Liu ZM, Chen J, Kou Q, Lin Q, Huang X, Tang Z, Kang Y, Li K, Zhou L, Song Q, Sun T, Zhao L, Wang X, He X, Wang C, Wu B, Lin J, Yuan S, Gu Q, Qian K, Shi X, Feng Y, Lin A; Study Group of investigators; Guan XD. Terlipressin versus norepinephrine as infusion in patients with septic shock: a multicentre, randomised, double-blinded trial. Intensive Care Med. 2018;44:1816-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 123. | Arora V, Maiwall R, Rajan V, Jindal A, Muralikrishna Shasthry S, Kumar G, Jain P, Sarin SK. Terlipressin Is Superior to Noradrenaline in the Management of Acute Kidney Injury in Acute on Chronic Liver Failure. Hepatology. 2020;71:600-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 124. | Angeli P, Volpin R, Gerunda G, Craighero R, Roner P, Merenda R, Amodio P, Sticca A, Caregaro L, Maffei-Faccioli A, Gatta A. Reversal of type 1 hepatorenal syndrome with the administration of midodrine and octreotide. Hepatology. 1999;29:1690-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 325] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 125. | Wong F, Pantea L, Sniderman K. Midodrine, octreotide, albumin, and TIPS in selected patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2004;40:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 227] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 126. | Skagen C, Einstein M, Lucey MR, Said A. Combination treatment with octreotide, midodrine, and albumin improves survival in patients with type 1 and type 2 hepatorenal syndrome. J Clin Gastroenterol. 2009;43:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 127. | Brensing KA, Textor J, Perz J, Schiedermaier P, Raab P, Strunk H, Klehr HU, Kramer HJ, Spengler U, Schild H, Sauerbruch T. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut. 2000;47:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 286] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 128. | Song T, Rössle M, He F, Liu F, Guo X, Qi X. Transjugular intrahepatic portosystemic shunt for hepatorenal syndrome: A systematic review and meta-analysis. Dig Liver Dis. 2018;50:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 129. | Zhang Z, Maddukuri G, Jaipaul N, Cai CX. Role of renal replacement therapy in patients with type 1 hepatorenal syndrome receiving combination treatment of vasoconstrictor plus albumin. J Crit Care. 2015;30:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 130. | Keller F, Heinze H, Jochimsen F, Passfall J, Schuppan D, Büttner P. Risk factors and outcome of 107 patients with decompensated liver disease and acute renal failure (including 26 patients with hepatorenal syndrome): the role of hemodialysis. Ren Fail. 1995;17:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 131. | Bañares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M, Saliba F, Sauerbruch T, Klammt S, Ockenga J, Pares A, Wendon J, Brünnler T, Kramer L, Mathurin P, de la Mata M, Gasbarrini A, Müllhaupt B, Wilmer A, Laleman W, Eefsen M, Sen S, Zipprich A, Tenorio T, Pavesi M, Schmidt HH, Mitzner S, Williams R, Arroyo V; RELIEF study group. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57:1153-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 377] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 132. | Lavayssière L, Kallab S, Cardeau-Desangles I, Nogier MB, Cointault O, Barange K, Muscari F, Rostaing L, Kamar N. Impact of molecular adsorbent recirculating system on renal recovery in type-1 hepatorenal syndrome patients with chronic liver failure. J Gastroenterol Hepatol. 2013;28:1019-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 133. | He YT, Qi YN, Zhang BQ, Li JB, Bao J. Bioartificial liver support systems for acute liver failure: A systematic review and meta-analysis of the clinical and preclinical literature. World J Gastroenterol. 2019;25:3634-3648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (3)] |
| 134. | Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, Ginès P, Rodés J. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1002] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 135. | Terg R, Gadano A, Cartier M, Casciato P, Lucero R, Muñoz A, Romero G, Levi D, Terg G, Miguez C, Abecasis R. Serum creatinine and bilirubin predict renal failure and mortality in patients with spontaneous bacterial peritonitis: a retrospective study. Liver Int. 2009;29:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 136. | de Mattos ÁZ, de Mattos AA, Méndez-Sánchez N. Hepatorenal syndrome: Current concepts related to diagnosis and management. Ann Hepatol. 2016;15:474-481. [PubMed] |
| 137. | Guevara M, Terra C, Nazar A, Solà E, Fernández J, Pavesi M, Arroyo V, Ginès P. Albumin for bacterial infections other than spontaneous bacterial peritonitis in cirrhosis. A randomized, controlled study. J Hepatol. 2012;57:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 138. | Thévenot T, Bureau C, Oberti F, Anty R, Louvet A, Plessier A, Rudler M, Heurgué-Berlot A, Rosa I, Talbodec N, Dao T, Ozenne V, Carbonell N, Causse X, Goria O, Minello A, De Ledinghen V, Amathieu R, Barraud H, Nguyen-Khac E, Becker C, Paupard T, Botta-Fridlung D, Abdelli N, Guillemot F, Monnet E, Di Martino V. Effect of albumin in cirrhotic patients with infection other than spontaneous bacterial peritonitis. A randomized trial. J Hepatol. 2015;62:822-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 139. | Fernández J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, Vila C, Pardo A, Quintero E, Vargas V, Such J, Ginès P, Arroyo V. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 450] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 140. | Boyer TD, Sanyal AJ, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, Gülberg V, Sigal S, Bexon AS, Teuber P; Terlipressin Study Group. Impact of liver transplantation on the survival of patients treated for hepatorenal syndrome type 1. Liver Transpl. 2011;17:1328-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 141. | Cannon RM, Jones CM, Davis EG, Eckhoff DE. Effect of Renal Diagnosis on Survival in Simultaneous Liver-Kidney Transplantation. J Am Coll Surg 2019; 228: 536-544. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 142. | Nadim MK, Sung RS, Davis CL, Andreoni KA, Biggins SW, Danovitch GM, Feng S, Friedewald JJ, Hong JC, Kellum JA, Kim WR, Lake JR, Melton LB, Pomfret EA, Saab S, Genyk YS. Simultaneous liver-kidney transplantation summit: current state and future directions. Am J Transplant. 2012;12:2901-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 143. | Hussain SM, Sureshkumar KK. Refining the Role of Simultaneous Liver Kidney Transplantation. J Clin Transl Hepatol. 2018;6:289-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 144. | Jenq CC, Tsai MH, Tian YC, Lin CY, Yang C, Liu NJ, Lien JM, Chen YC, Fang JT, Chen PC, Yang CW. RIFLE classification can predict short-term prognosis in critically ill cirrhotic patients. Intensive Care Med. 2007;33:1921-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 145. | Wong F, O'Leary JG, Reddy KR, Patton H, Kamath PS, Fallon MB, Garcia-Tsao G, Subramanian RM, Malik R, Maliakkal B, Thacker LR, Bajaj JS; North American Consortium for Study of End-Stage Liver Disease. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology 2013; 145: 1280-8. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 146. | Huelin P, Piano S, Solà E, Stanco M, Solé C, Moreira R, Pose E, Fasolato S, Fabrellas N, de Prada G, Pilutti C, Graupera I, Ariza X, Romano A, Elia C, Cárdenas A, Fernández J, Angeli P, Ginès P. Validation of a Staging System for Acute Kidney Injury in Patients With Cirrhosis and Association With Acute-on-Chronic Liver Failure. Clin Gastroenterol Hepatol 2017; 15: 438-445. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 147. | Bucsics T, Mandorfer M, Schwabl P, Bota S, Sieghart W, Ferlitsch A, Trauner M, Peck-Radosavljevic M, Reiberger T. Impact of acute kidney injury on prognosis of patients with liver cirrhosis and ascites: A retrospective cohort study. J Gastroenterol Hepatol. 2015;30:1657-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 148. | Mindikoglu AL, Hernaez R, Liu Y, Kramer JR, Taylor T, Rana A, Kanwal F. Renal Trajectory Patterns Are Associated With Postdischarge Mortality in Patients With Cirrhosis and Acute Kidney Injury. Clin Gastroenterol Hepatol 2020; 18: 1858-1866. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 149. | Makar M, Reja D, Chouthai A, Kabaria S, Patel AV. The impact of acute kidney injury on mortality and clinical outcomes in patients with alcoholic cirrhosis in the USA. Eur J Gastroenterol Hepatol. 2021;33:905-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 150. | Patidar KR, Shamseddeen H, Xu C, Ghabril MS, Nephew LD, Desai AP, Anderson M, El-Achkar TM, Ginès P, Chalasani NP, Orman ES. Hospital-Acquired Versus Community-Acquired Acute Kidney Injury in Patients With Cirrhosis: A Prospective Study. Am J Gastroenterol. 2020;115:1505-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 151. | Tariq R, Hadi Y, Chahal K, Reddy S, Salameh H, Singal AK. Incidence, Mortality and Predictors of Acute Kidney Injury in Patients with Cirrhosis: A Systematic Review and Meta-analysis. J Clin Transl Hepatol. 2020;8:135-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 152. | Alessandria C, Ozdogan O, Guevara M, Restuccia T, Jiménez W, Arroyo V, Rodés J, Ginès P. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology. 2005;41:1282-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 232] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 153. | Belcher JM, Garcia-Tsao G, Sanyal AJ, Bhogal H, Lim JK, Ansari N, Coca SG, Parikh CR; TRIBE-AKI Consortium. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013;57:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 286] [Article Influence: 23.8] [Reference Citation Analysis (0)] |